Abstract
Benign paroxysmal positional vertigo (BPPV) is the most frequent type of vertigo. The treatment of canalithiasis of the posterior semicircular canal consists in performing a particle-repositioning maneuver, such as the Epley maneuver (EM). However, the EM is not effective in all cases. The objective of this study is to identify risk factors, which predict the EM failure, among the clinical variables recorded in anamnesis and patient examination. This is an observational prospective multicentric study. All patients presenting with BPPV were recruited and applied the EM and appointed for a follow-up visit 7 days later. The following variables were recorded: sex, age, arterial hypertension, diabetes, hyperlipidemia, smoking habit, alcohol consumption, migraine, osteoporosis, diseases of the inner ear, previous ipsilateral BPPV, previous traumatic brain injury, previous sudden head deceleration, time of evolution, sulpiride or betahistine treatment, experienced symptoms, outcome of the Halmagyi maneuver, laterality, cephalic hyperextension of the neck, intensity of nystagmus, intensity of vertigo, duration of nystagmus, occurrence of orthotropic nystagmus, symptoms immediately after the EM, postural restrictions, and symptoms 7 days after the EM. Significant differences in the rate of loss of nystagmus were found for six variables: hyperlipidemia, previous ipsilateral BPPV, intensity of nystagmus, duration of nystagmus, post-maneuver sweating, and subjective status. The most useful significant variables in the clinical practice to predict the success of the EM are previous BPPV and intensity of nystagmus. In the other significant variables, no physiopathological hypothesis can be formulated or differences between groups are too small.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Benign paroxysmal positional vertigo (BPPV) is the most frequent type of vertigo [1]. It is produced by otoliths’ migration from the utricle macula into the semicircular canals. Migration may take place in two different ways: canalithiasis, where otoliths enter the canal through its non-ampullar end and freely move into the canal [2], or a less frequent form, cupulolithiasis, where otoliths attach to the cupula [3]. The most frequently involved canal is the posterior semicircular canal and it is mainly affected by the canalithiasis form [4].
A possible treatment for canalithiasis of the posterior semicircular canal is to perform a particle-repositioning maneuver. One of the most extensively used maneuvers is the Epley maneuver (EM) [5], which has a success rate of 75–89% [6]. The reason why it is not effective in some cases remains unknown. Studies suggest that certain risk markers may be identified at the moment of diagnosis, which help predicting those cases where the maneuver will probably not be effective.
Being able to predict failure of the EM would help optimize the clinical strategy, allocating more time and resources to those patients with a higher probability of failure.
Objective
The objective of this study was to identify risk factors that predict failure of the EM among the clinical variables recorded during anamnesis and examination of patients, who present with BPPV of the posterior semicircular canal in its canalithiasis form.
Materials and methods
All patients presenting with BPPV to the Otoneurology Units of five participating hospitals were prospectively recruited between April 1, 2015 and March 31, 2016. Recruitment was made according to the following protocol:
-
1.
Patients who met any of the three following conditions were provisionally recruited: patients reporting short vertigo attacks triggered by head movements; patients presenting with instability and reporting a history of vertigo with BPPV as the suspected cause or patients where BPPV was not initially suspected but incidentally discovered in routine examination.
-
2.
Spontaneous nystagmus—different from that associated with extreme positions of gaze—was evaluated and patients presenting such nystagmus were excluded.
-
3.
Patients were subjected to the Halmagyi maneuver (head impulse test) [7], left and right, to detect possible alterations of the vestibulo-ocular reflex. The result of this maneuver was not used as an exclusion criterion.
-
4.
All patients underwent left and right Pagnini–McClure maneuver (head supine test) [8, 9]. Patients presenting nystagmus compatible with canalithiasis or cupulolithiasis of the horizontal semicircular canal were excluded.
-
5.
All patients underwent left and right Dix–Hallpike maneuvers [10] before the EM. Only those, where nystagmus could be observed with the naked eye (without Frenzel glasses) were included in the study. Observed nystagmus had to be compatible with canalithiasis involvement of only one of the posterior semicircular canals. Patients, who reported vertigo symptoms in the Dix–Hallpike position, but did not present nystagmus, were excluded. Patients presenting nystagmus both in the right and left Dix–Hallpike maneuver were also excluded regardless the type of nystagmus. Finally, patients in whom the maneuver triggered atypical nystagmus were also excluded, either because it was not a combination of torsional and vertical nystagmus or because it lasted 60 s or more [11].
-
6.
Patient consent was required before the EM. Patients who did not consent were excluded from the study.
All patients fulfilling the inclusion criteria were subjected to the EM in the first visit. The physician advised them to sleep with a 30° elevated head of bed.
Variables 1–30 of Table 1 were recorded from each patient. These variables were selected from those which have been suspected to worsen the prognosis of EM in the literature.
Patients were appointed for a follow-up visit 7 days later. Those who failed to attend the follow-up visit were excluded from the study. In the follow-up visit, patients were again subjected to the Dix–Hallpike maneuver and then fields 31–33 of Table 1 were recorded.
Recovery of BPPV was defined as lack of nystagmus in the Dix–Hallpike position, regardless of the presence of vertigo symptoms in that position.
Between-group differences of nominal qualitative variables were analyzed with the Chi-square test or the Fisher’s test; ordinal qualitative and quantitative variables differences were analyzed with the Mann–Whitney test.
The protocol of this research study was accepted by the ethics committee of Complejo Hospitalario Universitario Insular Materno-Infantil, Las Palmas de Gran Canaria.
Results
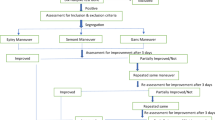
Data from 688 potentially eligible patients were recorded. However, 454 patients were excluded for different reasons. Therefore, the final sample size was 234 patients. Figure 1 sketches the selection process.
The studied population had a 68/166 men/women proportion, 62 years average age and 45 days median duration of current vertigo episode. In the follow-up visit, 67.1% of patients showed loss of nystagmus in the Dix–Hallpike maneuver.
Tables 2 and 3 show patient distribution according to variables potentially influencing the EM outcome. Six variables showed significant differences in the percentage of subjects with loss of nystagmus: hyperlipidemia, previous ipsilateral BPPV, intensity of nystagmus, duration of nystagmus, post-maneuver sweating, and subjective status 1 week after the maneuver.
Discussion
Some studies analyzing risk factors potentially influencing the EM outcome have been published, although authors used a different methodology in the three following aspects.
First, many of such studies analyzed the number of maneuvers necessary to achieve loss of nystagmus, whereas we only analyzed the outcome of the first maneuver.
Second, many published studies evaluated the efficacy of the EM 1 month later, while we evaluated it 1 week later. A 1-week follow-up period could theoretically reduce the amount of patients improving spontaneously.
Finally, recovery of BPPV has not been universally defined. In our study, recovery was defined as “loss of nystagmus” regardless of loss of symptoms to improve objectivity by disregarding the subjective component that patients could feel when again subjected to the Dix–Hallpike maneuver in a follow-up visit.
In a study where many hypothesis contrasts are planned, the probability of type 1 error (detecting significant differences that are not present) is high. However, despite this shortcoming, studies with the characteristics of this one have a strong point in preventing biases in publication derived from the fact that studies with non-significant results are usually not reported.
Below is a discussion of individual potential risk factors:
-
Variable 1: Sex. Like in other published studies [12, 13], significant differences were not found between the proportions of men and women, who achieved recovery.
-
Variable 2: Age. No significant difference was found in age distribution between patients who showed loss of nystagmus and patients who did not. Over the time, changes occur in the number and morphology of otoconia, which become more fragmented [14] and less abundant [15] as the patient grows older. However, such changes did not seem to be sufficient to change the efficacy of the EM in our population. Given that Babac et al. [12] reported significant differences between up-to-50-year-old patients and older patients, we split our population into two equivalent age groups; however, differences were still not significant. This finding could be due to the fact that Babac et al.’s follow-up visits took place 3 months after the maneuver, while ours were conducted 7 days after the maneuver.
-
Variable 3: High blood pressure. We found no significant differences in the rate of recovery, between patients with or without high blood pressure. No studies on a possible association between high blood pressure and the EM outcome have been published. However, it has been reported that subjects with high blood pressure were found to need more maneuvers to achieve the loss of nystagmus and remission of BPPV symptoms [16].
-
Variable 4: Diabetes. Diabetes has been described as a risk factor for BPPV [17]; however, our data suggested that the outcome of the EM was not influenced by this factor.
-
Variable 5: Hyperlipidemia. Our results indicated that hyperlipidemia could increase the probability of a positive response to the EM. No similar finding has been reported in the scientific literature. In theory, hyperlipidemia could cause vascular damage to the inner ear, which could in turn trigger BPPV [18]. No physiopathological reasons explain why hyperlipidemia significantly enhanced the prognosis in our sample, while no other vascular risk factors did so.
-
Variable 6: Smoking habit. Sunami et al. concluded that smoking was associated with lower risk of developing BPPV and with lower risk of relapse [19]. In our sample, no significant difference was found in the percentage of recovered subjects between smokers and non-smokers.
-
Variable 7: Alcohol consumption. The “buoyancy hypothesis” postulates that, due to their high capillary density, alcohol is easily spread into the cupulas [20]. Our data suggested that this phenomenon did not influence the EM outcome.
-
Variable 8: Migraine. In our sample, no significant differences were found in the rate of loss of nystagmus between migraine sufferers and non-sufferers. Other studies also reported no differences in recovery rates [21] or in the number of maneuvers necessary for loss of nystagmus and symptoms [22], between patients with and without migraine.
-
Variable 9: Osteoporosis. Patients with osteoporosis are at higher risk of both BPPV onset and relapse [23]. Rats with osteoporosis showed changes in the otoconia, which decreased in density and increased in size [24]. In our study, no significant association between osteoporosis and response to the EM was found; however, when bone density was analyzed as an ordinal qualitative variable, a non-significant tendency to a better response with a lower bone density was observed. More studies are needed to investigate this proposal since Babac et al. detected that osteoporosis was a risk factor for poorer response to maneuvers [12].
-
Variable 10: Concomitant disease of the inner ear. In agreement with other studies [12, 25], we found no significant differences in the EM outcome between patients who suffered from concomitant diseases of the inner ear and those who did not. This is, however, a controversial finding since other authors [13, 26] did report such differences.
-
Variable 11: Previous repositioning. According to our results, experiencing a previous BPPV attack in one ear and repositioning maneuvers with consequent loss of nystagmus in a subsequent Dix–Hallpike maneuver is a risk factor that significantly worsens the prognosis of an EM conducted in case of relapse in the same ear. Choi et al. [27] has reported significant differences in the number of maneuvers required to resolve BPPV nystagmus and symptoms in patients undergoing a first BPPV attack or a recurrent one.
-
Variable 12: Traumatic brain injury. In a recent review [28], it was concluded that up-to-the-moment available evidence does not support that patients with BPPV due to traumatic brain injury show poorer EM prognosis than patients with idiopathic BPPV. Our results do not support it either.
-
Variable 13: Traffic accident. No distinctions are made in the related literature between BPPV caused by direct traumatic brain injury or by sudden deceleration in a traffic accident. In our study, we found no differences in the prognosis of patients subjected to sudden deceleration as compared with the rest of patients. If variables 12 and 13 were clustered in one variable, they would not differ significantly.
-
Variables 14 and 15: Time from first attack and duration of current episode. Time of BPPV may refer to two different concepts: time from the first BPPV attack and time from the start of the current. Variable 14 is the time from the first vertigo attack in the patient’s life; variable 15 referred to the duration of the current episode, namely the time elapsed since the moment symptoms reappeared after a period free of vertigo. In our sample, no differences were found between patients with or without loss of nystagmus in the distribution of time in any of them. Other authors reported both significant [29] and non-significant [12, 13, 30] differences in the EM outcome depending on the time of evolution of the disease, but they did not separate the evolution time into two variables as we did in this study.
-
Variables 16 and 17: Sulpiride and betahistine. These drugs are frequently used to treat vertigo symptoms. Patients in our study were not recommended to take medication before the maneuver; however, some of them did so. Intake of one or the other drug was analyzed independently; no significant differences were found in the rate of loss of nystagmus between patients who were taking medication and those who were not. No references were found in the literature on the role of sulpiride in EM prognosis. However, three experimental studies reported that taking betahistine enhanced the EM prognosis [31,32,33]. Our study—simply observational—does not support those findings.
-
Variable 18: Symptoms. Although vertigo attack triggered by changes in the head’s position is the most frequent BPPV symptom, this entity may also be associated with instability and a recent history of vertigo episodes [11]. It can also appear as a variety of otoneurological symptoms, which may be incidentally found in routine examination. In our study, patients were clustered into three groups according to their symptoms, though no differences were found in the proportion of subjects with loss of nystagmus between such groups.
-
Variable 19: Halmagyi maneuver. No significant association was found between the outcome of the Halmagyi maneuver [7] and the EM. No studies up to now have related positive head thrust test with BPPV prognosis.
-
Variable 20: Laterality. In our study, no relationship was found between the laterality of the disease and the success of the EM.
-
Variable 21: Hyperextension. Data showed no significant differences in EM success depending on whether proper hyperextension of the neck could be done in the first phase of the maneuver or not. This finding led us to postulate that correct otolith repositioning depends more on the head turn in the yaw plane during the maneuver than on the hyperextension of the neck itself.
-
Variable 22: Intensity of nystagmus. In our study, subjective intensity of nystagmus, as observed by the physician during the Dix–Hallpike maneuver, was associated with success of the EM. Thus, higher observed intensity of nystagmus corresponded to lower probability of success. The intensity of nystagmus during the EM shows high inter-individual variability [34]. Our results could be explained by assuming that higher intensity of nystagmus corresponds to higher amount of moving otoliths, which are harder to be repositioned.
-
Variable 23: Intensity of vertigo. Although our data suggested a tendency to poorer maneuver’s prognosis with higher vertigo intensity in the Dix–Hallpike maneuver, such a tendency was not significant. This variable is related to variable 22 since higher nystagmus intensity corresponds to higher vertigo intensity.
-
Variable 24: Duration of nystagmus. Hypothesis contrast to compare the duration of nystagmus between groups yielded significant differences. However, the medians of each group (10 s for the group with loss of nystagmus and 12 for the group without it) did not show differences relevant to the clinical practice. Soto-Varela et al. [35] failed to find a relationship between nystagmus duration or latency and outcome of the Semont maneuver.
-
Variables 25, 26, and 27: Orthotropism during the second, third or fourth phase of the EM. We found no significant differences in the rate of EM success between patients presenting or not presenting orthotropic nystagmus in any of the maneuver’s phases. However, we observed a tendency to better prognosis when orthotropic nystagmus occurred in the second phase of the EM, as well as a tendency to poorer prognosis for inverted orthotropic nystagmus. Other authors [36, 37] reported a relationship between the occurrence of nystagmus in this phase and the maneuver’s prognostic, a finding that is physiopathologically consistent. However, these authors studied orthotropic nystagmus with Frenzel glasses while we studied it with the naked eye. The lack of significant differences in our sample could be due to the fact that orthotropic nystagmus occurred in only 28.7% of patients, a smaller percentage than those reported in studies with Frenzel glasses. It is worth noting that such a tendency was only observed in the second phase of the EM and not in the other phases. We postulate that this could be due to the fact that in the second EM phase, otoliths are separated from the cupula and move along that canal portion that is farthest from the end—a critical segment where it is most important that otoliths move away from the ampulla.
-
Variables 28, 29, 30, and 31: Post-maneuver nausea, sweating, instability or dizziness. After the EM, there are more patients experiencing symptoms like nausea, vomit, sweating, instability or dizziness than patients not experiencing them [38]. Regarding the maneuver’s success, when these four variables were analyzed separately, the percentage of loss of nystagmus was higher in patients without symptoms. The difference was only significant for sweating.
-
Variable 32: Elevated head of bed. A review of evidence on the improvement of the EM prognostic through postural restrictions showed that such restrictions do not play an important role in prognosis but they do so in post-maneuver subjective feeling of vertigo [39]. Our results supported those findings. However, when patients were asked about the intensity of vertigo in the Dix–Hallpike position 1 week after the EM, those who had slept the first night with an elevated head of bed showed significant symptom reduction than those who had not.
-
Variable 33: Subjective status 1 week afterwards. Patients who were asymptomatic showed significantly higher rate of loss of nystagmus than patients who reported symptoms. Among symptomatic patients, no significant differences were found between those reporting their subjective status to be better, the same or worse than before the EM.
In conclusion, six variables showed significant differences in the percentage of loss of nystagmus. These variables were not dependent between themselves, except for the intensity and duration of nystagmus.
However, no physiopathological hypothesis can be formulated to explain the relationship between hyperlipidemia and BPPV or between sweating and BPPV; the difference in duration of nystagmus between groups was too small to have practical usefulness and the subjective status 1 week after the maneuver cannot be assessed immediately.
Thus, we consider that the most useful variables in the clinical practice are: previous BPPV and intensity of nystagmus. These variables are easy to collect and will help physicians to identify patients with a higher risk for a bad response to EM.
Conclusion
In our study, six variables showed significant differences in the rate of loss of nystagmus in BPPV of the posterior semicircular canal after an EM; but we consider that only two of these variables, previous ipsilateral BPPV and intensity of nystagmus, are useful in the clinical practice to predict the success of the EM.
References
Lee SH, Ji SK (2010) Benign paroxysmal positional vertigo. J Clin Neurol (Seoul, Korea) 6(2):51–63
Hall SF, Ruby RR, McClure JA (1979) The mechanics of benign paroxysmal vertigo. J Otolaryngol 8:151–158
Schuknecht HF (1969) Cupulolithiasis. Arch Otolaryngol 90:765–778
Parnes LS, Agrawal SK, Atlas J (2003) Diagnosis and management of benign paroxysmal positional vertigo (BPPV). CMAJ 169:681–693
Epley JM (1992) The canalith repositioning procedure: for treatment of benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg 107:399–404
Fife TD, Iverson DJ, Lempert T et al (2008) Practice parameter: therapies for benign paroxysmal positional vertigo (an evidence-based review): report of the quality standards subcommittee of the American academy of neurology. Neurology 70(22):2067–2074
Halmagyi GM, Curthoys IS (1988) A clinical sign of canal paresis. Arch Neurol 45(7):737–739
Pagnini P, Nuti D, Vannucchi P (1989) Benign paroxysmal vertigo of the horizontal canal. ORL J Otorhinolaryngol Relat Spec 51(3):161–170
McClure JA (1985) Horizontal canal BPV. J Otolaryngol 14(1):30–35
Dix MR, Hallpike CS (1952) The pathology, symptomatology and diagnosis of certain common disorders of the vestibular system. Ann Otol Rhinol Laryngol 61(4):987–1016
von Brevern M, Bertholon P, Brandt T et al (2015) Benign paroxysmal positional vertigo: diagnostic criteria. J Vestib Res 25(3–4):105–117. doi:10.3233/VES-150553
Babac S, Dragoslava D, Petrovic-Lazic M, Arsovic N, Aleksandar M (2014) Why do treatment failure and recurrences of benign paroxysmal positional vertigo occur? Otol Neurotol 35(6):1105–1110
Korres S, Balatsouras DG, Ferekidis E (2006) Prognosis of patients with benign paroxysmal positional vertigo treated with repositioning manoeuvres. J Laryngol Otol 120(7):528–533
Jang YS, Hwang CH, Shin JY, Bae WY, Kim LS (2006) Age-related changes on the morphology of the otoconia. Laryngoscope 116(6):996–1001
Ross MD, Peacor D, Johnsson LG, Allard LF (1976) Observations on normal and degenerating human otoconia. Ann Otol Rhinol Laryngol 85(3 pt 1):310–326
Korkmaz M, Korkmaz H (2016) Cases requiring increased number of repositioning maneuvers in benign paroxysmal positional vertigo. Braz J Otorhinolaryngol 82(4):452–457
Cohen HS, Kimball KT, Stewart MG (2004) Benign paroxysmal positional vertigo and comorbid conditions. ORL J Otorhinolaryngol Relat Spec 66(1):11–15
von Brevern M, Radtke A, Lezius F et al (2007) Epidemiology of benign paroxysmal positional vertigo: a population based study. J Neurol Neurosurg Psychiatr 78(7):710–715
Sunami K, Tochino R, Tokuhara Y et al (2006) Effects of cigarettes and alcohol consumption in benign paroxysmal positioning vertigo. Acta Otolaryngol 126(8):834–838
Brandt T (1990) Positional and positioning vertigo and nystagmus. J Neurol Sci 95(1):3–28
Yetiser S, Gokmen MHA (2015) Clinical aspects of benign paroxysmal positional vertigo associated with migraine. Int Tinnitus J 19(2):64–68
Faralli M, Cipriani L, Del Zompo MR, Panichi R, Calzolaro L, Ricci G (2014) Benign paroxysmal positional vertigo and migraine: analysis of 186 cases. B-ENT 10(2):133–139
Yu S, Liu F, Cheng Z, Wang Q (2014) Association between osteoporosis and benign paroxysmal positional vertigo: a systematic review. BMC Neurol 14:110. doi:10.1186/1471-2377-14-110
Vibert D, Sans A, Kompis M et al (2008) Ultrastructural changes in otoconia of osteoporotic rats. Audiol Neurootol 13(5):293–301
Pollak L, Davies RA, Luxon LL (2002) Effectiveness of the particle repositioning maneuver in benign paroxysmal positional vertigo with and without additional vestibular pathology. Otol Neurotol 23(1):79–83
Lee NH, Ban JH, Lee KC, Kim SM (2010) Benign paroxysmal positional vertigo secondary to inner ear disease. Otolaryngol Head Neck Surg 143(3):413–417. doi:10.1016/j.otohns.2010.06.905
Choi SJ, Lee JB, Lim HJ et al (2012) Clinical features of recurrent or persistent benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg 147(5):919–924
Aron M, Lea J, Nakku D, Westerberg BD (2015) Symptom resolution rates of posttraumatic versus nontraumatic benign paroxysmal positional vertigo: a systematic review. Otolaryngol Head Neck Surg 153(5):721–730
Harvey SA, Hain TC, Adamiec LC (1994) Modified liberatory maneuver: effective treatment for benign paroxysmal positional vertigo. Laryngoscope 104(10):1206–1212
Balatsouras DG, Aspris A, Ganelis P et al (2015) Duration of Benign paroxysmal positional vertigo as a predictor for therapy. B-ENT 11(3):199–203
Zhang H, Geng M, Yan B, Xing L (2012) Epley’s manoeuvre versus Epley’s manoeuvre plus labyrinthine sedative in the management of benign paroxysmal positional vertigo: prospective, randomised study. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 26(16):750–752
Guneri EA, Kustutanv O (2012) The effects of betahistine in addition to Epley maneuver in posterior canal benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg 146(1):104–108
Cavaliere M, Mottola G, Iemma M (2005) Benign paroxysmal positional vertigo: a study of two manoeuvres with and without betahistine. Acta Otorhinolaryngol Ital 25(2):107–112
Squires TM, Weidman MS, Hain TC, Stone HA (2004) A mathematical model for top-shelf vertigo: the role of sedimenting otoconia in BPPV. J Biomech 37(8):1137–1146
Soto-Varela A, Rossi-Izquierdo M, Santos-Pérez S (2011) Can we predict the efficacy of the semont maneuver in the treatment of benign paroxysmal positional vertigo of the posterior semicircular canal? Otol Neurotol 32(6):1008–1011
Marques PS, Catillo R, Santos M, Pérez-Fernández N (2014) Repositioning nystagmus: prognostic usefulness?. Acta Otolaryngol 134(5):491–496
Oh HJ, Kim JS, Han BI, Lim JG (2007) Predicting a successful treatment in posterior canal benign paroxysmal positional vertigo. Neurology 68(15):1219–1222
Sridhar S, Panda N (2005) Particle repositioning manoeuvre in benign paroxysmal positional vertigo: is it really safe? J Otolaryngol 34(1):41–45
Fyrmpas G, Rachovitsas D, Haidich AB et al (2009) Are postural restrictions after an Epley maneuver unnecessary? First results of a controlled study and review of the literature. Auris Nasus Larynx 36(6):637–643
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was not funded.
Conflict of interest
None of the author had conflict of interest in relation to the study.
Human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Domínguez-Durán, E., Domènech-Vadillo, E., Álvarez-Morujo de Sande, M.G. et al. Analysis of risk factors influencing the outcome of the Epley maneuver. Eur Arch Otorhinolaryngol 274, 3567–3576 (2017). https://doi.org/10.1007/s00405-017-4674-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-017-4674-9
Keywords
- BPPV
- Epley maneuver
- Prognosis
- Sex
- Age
- Hypertension
- Diabetes mellitus
- Hyperlipidemia
- Smoking
- Alcohol-related disorders
- Migraine disorders
- Osteoporosis
- Labyrinth diseases
- Traumatic brain injury
- Whiplash injuries
- Time-to-treatment
- Sulpiride
- Betahistine
- Signs and symptoms
- Head impulse test
- Lateralization
- Cervical extension
- Nystagmus
- Orthotropic nystagmus