Abstract
Few data are available about the pattern of upper airway (UA) obstruction in children <2 years with obstructive sleep apnea syndrome (OSAS). Also, the role of adenoidectomy versus adenotonsillectomy (AT) is poorly defined in this age group. We performed drug-induced sedation endoscopy (DISE) in young OSAS children to investigate the pattern of UA obstruction and the value of DISE in therapeutic decision making. Retrospective analysis of ≤2-year-old children undergoing DISE-directed UA surgery. OSAS severity and the treatment outcomes were documented by polysomnography. Data are available for 28 patients, age 1.5 years (1.3–1.8), BMI-z score 0.5 (−0.7 to 1.3) with severe OSAS, obstructive apnea/hypopnea index (oAHI) 13.8/hr (7.5–28.3). All but 3 had (>50%) obstruction at the level of the adenoids, and all but 5 had (>50%) tonsillar obstruction. DISE-directed treatment consisted of adenoidectomy (n = 4), tonsillectomy (n = 1), and AT (n = 23). There was a significant improvement in respiratory parameters. Twenty children (71.4%) had a postoperative oAHI <2/hr. None had palatal or tongue base obstruction. Five children had a circumferential UA narrowing (hypotonia), 2 of them had residual OSAS. DISE showed a collapse of the epiglottis in 6 and late-onset laryngomalacia in 4. These findings did not affect surgical outcome. Adenotonsillar hypertrophy is the major cause of UA obstruction, and DISE-directed UA surgery was curative in 71,4% of children ≤2 years. We suggest that DISE may be helpful in surgical decision making. Circumferential UA narrowing may result in less favorable surgical outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Adenotonsillectomy (AT) is the first-line treatment in ≥2-year-old children with obstructive sleep apnea syndrome [1]. In children <2 years of age and infants, both OSAS definitions and management strategies are less clear. Based upon a literature review, Katz et al. suggested that an apnea/hypopnea index (AHI) >2/hr indicates the presence of OSAS in infants because this value represents the upper limit of normality in infants [2]. De Haan et al. performed a retrospective analysis of 232 infants undergoing clinical polysomnography (PSG) at a tertiary referral center [3]. Not only were respiratory parameters such as AHI, obstructive and mixed apnea/hypopnea index, oxygen desaturation index, and minimum saturation different in infants <6 months compared to the older age groups (6–12 and 12–24 months). Also, treatment recommendations were different according to age group: non-invasive ventilation was most commonly recommended in the <6 months of age group, no treatment for those between 6 and 12 months, and surgery was the most common treatment recommendation for infants 12–24 months of age.
Physiologic differences exist between the infant UA and the upper airway of older children. The infant’s upper airway is more prone to narrowing due to unique anatomic features such as a narrow UA diameter, relatively large tongue size, shape of the jaw and more close apposition of the tongue and larynx and a relatively high mean closing pressure of the passive larynx. When the child grows older, the airway becomes more mature and OSAS is more often caused by fixed obstructions such as adenotonsillar hypertrophy [4].
Croft and colleagues were the first to describe the value of endoscopic evaluation and treatment of sleep-associated upper airway obstruction in infants and young children [5]. These authors emphasized the importance of thorough upper airway evaluation when considering surgery in infants and young children with OSAS [5]. Despite this recommendation being formulated more than 30 years ago, a limited number studies performed an UA evaluation in young children with OSAS and related their findings to treatment outcome [5, 6]. Few data are available on the outcomes of surgical management for OSAS in children <2 years of age.
Only one paper was identified reporting on surgical outcomes in children <12 months of age with presence confirmed by PSG in all subjects [7]. In this study, objective outcome data are reported for adenoidectomy alone in children between 3 and 5 months and 6–11 months of age with a decrease in AHI of 86.3 and 56.6%, respectively. Objective outcomes on tonsillectomy or AT were not available for infants up to 11 months of age. In the paper by Cheng et al. where 21 of 25 children had PSG-proven OSAS, the lower limit for AT was 8 months [8]. The lower age limit for surgical treatment was 8.2 months in the study by Brigance et al. where all had PSG-proven OSAS and ATE was the most commonly performed procedure in 55 out of 61 patients [9].
Furthermore the role of adenoidectomy versus tonsillectomy is poorly defined in this age group and children <2 years of age are commonly excluded from practice guidelines and recommendations for the management of pediatric OSAS [1]. Consequently, surgeons treating these young children have little guidance upon which to base their management.
The aim of the present paper is to analyze (1) the pattern of UA obstruction in ≤2-year-old children during DISE; (2) whether findings during DISE may be helpful in clinical decision making for this young age group; (3) the outcome of DISE-directed upper airway surgery in young children with OSAS.
Methods
Since 2011, it is our policy that all children diagnosed with OSAS and considered for UA surgery undergo an endoscopic examination of the UA during drug-induced sleep [10] at the time of the planned surgical procedure. Data obtained during DISE, polysomnographic data, demographics, and treatment outcomes are registered in a database. We performed a retrospective analysis of these data limited to children ≤2 years old at the time of PSG.
Diagnosis of OSAS was based upon a combination of clinical signs and symptoms of UA obstruction and full-night polysomnography (PSG). All children undergo a clinical examination by a pediatric ENT surgeon, and tonsil size is scored according to Brodsky [11]. BMI-z scores were calculated according to Flemish growth curves for boys and girls.
All children underwent nocturnal PSG for at least 6 h at the Pediatric Sleep Disorders Center of the Antwerp University Hospital, Belgium as previously reported [12]. Polysomnography was manually scored by certified technicians according to international guidelines [13]. The obstructive apnea–hypopnea index (oAHI) was defined as the number of obstructive apneas and hypopneas per hour of sleep. OSAS was defined as an oAHI ≥2/hr [1]. OSAS was classified as mild (oAHI between 2 and 5/hr), moderate (oAHI ≥5 and up to 10/hr), or severe (oAHI ≥10/hr) [14]. A respiratory event was classified as central apnea when it meets the apnea definition (≥90% drop in airflow compared to pre-event baseline respiration), is associated with the absence of respiratory effort throughout the entire duration of the event and at least one of the following criteria is met: the event lasts 20 s or longer; the event lasts at least 2 breaths during baseline breathing and is associated with an arousal and/or ≥3% drop in oxygen desaturation. The central apnea index (CAI) is defined as the number of central apneas/hour of sleep. Sigh and movement-related central apneas were not included in the CAI.
DISE was performed in the operation theatre by a single pediatric ENT surgeon (AB). The procedure and scoring system have been described in detail elsewhere [12]. Obstruction of >50% at a given airway level is considered clinically significant.
Multilevel obstruction was defined as the presence of 1 or more UA abnormalities outside the adenotonsillar region. When DISE showed a clinically relevant obstruction at the level of adenoids and/or tonsils, the child was intubated and ventilated at the end of the endoscopic examination and an adenotomy or tonsillectomy ± adenotomy was performed. Surgery was performed with cold instruments, and in case of tonsillectomy, the anterior and posterior tonsillar pillars were sutured with Vicryl 3.0 sutures. All children were monitored at the pediatric ward with continuous oximetry on the first postoperative night.
The study was approved by the local Ethics Committee (B30020107827). The parents are informed that surgical decision making will be based upon DISE findings and that there are 3 different therapeutic options that may ensue: no surgery because obstruction is located outside the adenotonsillar region, adenoidectomy, or adenotonsillectomy. Written informed consent about these options and DISE is obtained.
Statistical analysis was performed with IBM SPSS statistics version 22. Data are reported as median value with lower and upper quartile. Pre–postoperative findings are compared by Wilcoxon Signed Rank test; correlations between variables were calculated using Pearson’s Correlation coefficient. Statistical significance is accepted at p < 0.05.
Results
During the study period, DISE was performed in 41 <2-year-old children. Non-surgical treatment was proposed in 5 of them. Thirty-six underwent a surgical intervention, pre–postoperative data are available for 28 patients, and these constitute the study group. The study group included 17 boys, 11 girls; age 1.5 years (1.3–1.8), BMI-z score 0.5 (−0.7 to 1.3) and severe OSAS, obstructive apnoea/hypopnoea index (oAHI) 13.8/hr (7.5–28.3). Four had a history of prior adenoidectomy. One child had achondroplasia and another had a history of prematurity; the other children had no comorbid conditions.
All but 3 had significant (>50%) obstruction at the level of the adenoids, and all but 5 had significant (>50%) obstruction at the level of the tonsils.
None of the children had UA obstruction at the level of the palate or tongue base during DISE. A collapse of the epiglottis was observed in 6 and late-onset laryngomalacia in 4 patients. Five children had a circumferential narrowing/collapse at the oropharyngeal or hypoharyngeal level scored as hypotonia. Half of the children included had multilevel collapse (additional site of UA collapse outside the adenotonsillar region).
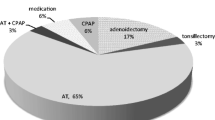
DISE-directed treatment consisted of adenoidectomy (n = 4), tonsillectomy (n = 1), and AT (n = 23). One child received pre-operative CPAP treatment and 1 child with limited tonsillar obstruction underwent AT because of severe pre-operative OSAS and oAHI of 39.9/hr and oxygen desaturations up to 69%.
Time between surgery and postoperative PSG was 3.4 months (3.1–3.7). Pre-operative oAHI did not correlate to age or Brodsky tonsil score. There was a significant improvement in the following parameters: oAHI 13.8/hr (7.5–28.3) vs. 0.9 (0.4–2.4) p < 0.001; CAI 3.1/hr (1.6–4.7) vs 1.4 (0.8–2.7) p = 0.002; MeanSat 96.6% (95.5–97.3) vs 97.3 (97.0–98.0) p = 0.005; and MinSat 85.5% (82.0–87.0) p < 0.001. Pre–postoperative oAHI for each individual patient is displayed in Fig. 1.
Twenty children (71.4%) had a postoperative oAHI <2/hr. Eigth had persistent disease that was mild (n=4), moderate (n=1), and severe in 3. Among the 3 children with persistent severe OSAS (oAHI ≥ 10/hr), one was found to have a hypotonic upper airway DISE and he had a cold during PSG. In the remaining 2, persistent OSAS could not be predicted by DISE or comorbidity. The presence of laryngomalacia or collapse of the epiglottis during DISE did not affect treatment outcome; these children had a postopreative oAHI <2/hr. A summary of DISE findings and pre–postoperative oAHI for each individual patient is presented in Table 1.
A 3rd PSG after 16 weeks treatment with montelukast in 2/3 children with persistent severe OSAS showed an oAHI <2/hr, resulting in an overall cure rate of 78.5%.
Regarding treatment outcome as a function of surgery, 16 out of 23 (69.5%) of children treated by AT obtained a cure. Adenoidectomy cured only 2 out of 4 patients.
There were no major complications. The mean hospital stay was 2 days in 19 children. Reasons for prolonged hospital stay were poor oral intake (n = 3), desaturations requiring supplemental oxygen (n = 4), bleeding without need for reintervention (n = 1), and fever requiring antibiotics (n = 2).
Discussion
To the best of our knowledge, this is the first study reporting on the systematic use of DISE and outcome of DISE-directed surgery documented by PSG in infants and young children with OSAS and the largest series on UA surgery in young children documented by pre–postoperative PSG.
Upper airway evaluation by means of DISE revealed some interesting similarities and differences compared to older (>2 year) children.
Adenotonsillar hypertrophy was the major cause of UA obstruction, but 50% of the children had multilevel obstruction (additional collapse outside the adenotonsillar region). Few previous studies describe DISE findings in young children. In the pilot paper by Croft et al., 3 children were 1 year old and 3 were 2 years old in a total sample of 15 [5]. Four children in the study by Croft et al. underwent adenotonsillectomy based upon UA evaluation and had a normal postoperative sleep study. In one Down syndrome child with a large tongue, an expectant treatment was proposed and another 2 years old child with Down syndrome was tracheotomized because of a collapse of the entire UA with small adenoids and tonsils. Chan et al. in a study aimed to develop a new scoring system for DISE, reported on 14 ≤ 2-year-old children [15]. However, their data are difficult to compare to the present study as 8/14 children in the paper by Chan et al. had a neurological comorbidity or a chromosomal abnormalitiy. Among the 6 patients without comorbidity, 3 had UA obstruction encompassing the upper and lower airway complex. We also observed multilevel collapse in 50% of the patients. Goldberg et al. performed endoscopic examinations in 39 patients, mean age 15 months, but nearly half of them were found to be hypotonic most often associated with a systemic disorder or a genetic syndrome. In children with a normal muscle tone, the prevalence of dynamic abnormalities (pharyngeal wall collapse and laryngomalacia) was 100% in those <1 year old, compared to 66% in patients between 1 and 2 year old and 17% in those >2 year old. This decline in dynamic abnormalities was only observed in children with normal muscle tone. In contrast, the prevalence of fixed abnormalities narrow nasal passages, adenotonsillar hypertrophy, and tongue enlargement increased with age both in children with normal tone and those with hypotonia.
Although half of the patients in our study had multilevel collapse, UA obstruction identified outside of the adenotonsillar region did not have a major impact on treatment outcome. All children with adenotonsillar hypertrophy and a collapse of the epiglottis or late-onset laryngomalacia were cured by AT.
Late-onset laryngomalacia as described by Richter et al. should be distinguished from congenital laryngomalacia [16]. Whereas the latter is characterized by a congenital onset and the presence of stridor, the former is a type 1 laryngomalacia (redundant mucosa prolapsing over the arythenoids upon inspiration) associated with OSAS symptoms. In our study, none of the children with laryngomalacia presented with stridor. It is likely that adenotonsillar hypertrophy was the predominant obstructing pathology with mild laryngomalacia as a secondary cause for UA obstruction. This could explain our observation that the presence of laryngomalacia did not affect treatment outcome after AT (all children were cured). A finding that is in line with data reported by Revell et al. [17] Resolution of OSAS symptoms and normalization of PSG may be obtained after AT in a subgroup of children with adenotonsillar hypertrophy and mild laryngomalacia.
In these children, a fixed airway obstruction such as adenotonsillar hypertrophy is associated with a secondary dynamic UA obstruction resulting from the increased inspiratory negative pressure generated by to overcome the fixed obstruction as described by Goldberg et al. [4] In such a case, the relief of UA obstruction in the adenotonsillar region would lower the negative intraluminal pressure more downstream in the upper airway, thus preventing floppy structures such as the epiglottis or supra-arythenoidal mucosa to collapse with inspiration. [4, 17] However, some children may require both AT and supraglottoplasty and careful follow-up, and repeat PSG is recommended to assess the effect of AT on late-onset laryngomalacia.
None of our patients was found to have tongue base obstruction. There are different possible explanations for this finding. Infants and young children have a small and shorter upper airway with a higher positioned larynx. In case of obstructing tonsils, there may not be enough room for the tongue to fall backwards and cause UA obstruction. Alternatively, lingual tonsillar hypertrophy has been identified as a cause of tongue base obstruction in children with a history of previous adenotonsillectomy. Since none of our study patients had a history of AT and only 4 had a history of prior adenoidectomy with a short time interval (usually a few months) between adenoidectomy and DISE, they may not have had enough time to develop lingual tonsillar hypertrophy.
The systematic use of DISE in children undergoing UA surgery for OSAS (especially in those without comorbidity or previous surgery) has been questioned [18]. However, we and others have shown that intra-operative decision making as to the relative contribution of adenotonsillar hypertrophy and obstruction at other UA levels based upon DISE findings is clinically useful and may allow for a more individually tailored treatment plan [4, 5, 12, 17]. This may be even more important in young children in whom the airway is small, prone to collapse due to pharyngeal airway dysfunction.
There still remains discussion whether adenoidectomy alone is effective for treatment of OSAS in a child who presents with adenoid hypertrophy [19] and Brodsky tonsil score is a poor predictor of OSAS severity and has limited value in clinical decision making [20] In young children, adenoidectomy is often preferred by the surgeon because it is associated with less morbidity and faster recovery than tonsillectomy. In the study by Robison et al., the age at tonsillectomy was greater than that of any other surgical intervention and all but one patient underwent tonsillectomy because earlier adenoidectomy failed to resolve OSAS [7]. In the paper by Robison et al., 27.5% of the patients who underwent primary adenoidectomy required a tonsillectomy or revision adenoidectomy with tonsillectomy. Similarly, Kay et al. reported that 28.7% of children <2 years at the time of adenoidectomy required a tonsillectomy within 5 years [21]. In addition, if adenoids were removed for upper airway obstruction, the patients were about 2 times more likely to require a subsequent tonsillectomy.
In our present study, a limited number of children were cured by adenoidectomy or tonsillectomy alone suggesting that DISE may be helpful to select the most appropriate intervention and avoid unneccesary morbidity in this young patient group. Without a carefully performed UA evaluation, surgical management is based upon trial and error and this should be avoided in a population that is especially at risk for postoperative complications. Given the small number of children treated with adenoidectomy alone versus tonsillectomy, no meaningful comparisons between both techniques can be made in terms of efficacy or complication ratio. Nevertheless, the high success rate of AT suggests that this type of surgery may be justified when both adenoids and tonsils are found to obstruct the UA during DISE.
In our view, the role of DISE in children ≤2 years of age is that it may guide surgical decision making in cases which may require AT, even at young age, to resolve OSAS and thus preventing the need for subsequent tonsillectomy at older age.
For the children with persistent severe OSAS, adjuvant medical treatment with montelukast was proposed for 16 weeks and this resulted in an objective improvement with cure of OSAS documented in 2. In the 3rd patient, CPAP was recommended.
Young age is a risk factor for postoperative complications and according to international guidelines, all our patients remained at least 1 night in the pediatric ward for continuous monitoring of pulse oximetry [22, 23]. The majority could be discharged the day after surgery, and there were no major complications encountered. These figures are similar to those reported by Chen et al., where the average length of stay was 2.7 days and postoperative complications occurred in 25% of the otherwise healthy patients (poor oral intake or persistent hypoxia with oxygen requirement).
We acknowledge that a lack of a control group of children where no DISE is used is a limitation of our study. We do not have data for comparison from our own center, and comparison with outcome data reported in literature is hampered because most previous studies included infants and young children with comorbidities [9, 24].
Conclusions
Drug-induced sedation endoscopy was found to be a useful tool for UA evaluation in young children with OSAS and considered candidate for surgical treatment. Based upon findings during DISE, the majority underwent AT without major complications and with favorable treatment outcomes. DISE may be helpful to identify children that are less likely to respond, those in whom AT should be performed at the first surgery, or those that may benefit from limited surgery by adenoidectomy or tonsillectomy alone. It also provides more insight into UA dynamics in these young patients and illustrated differences in UA behavior compared to older children and adults. However, it is possible that our findings are not applicable to a broader category of children including those with comorbidity and further studies including syndromic children or those with craniofacial abnormalities may reveal other UA findings and different treatment outcomes.
References
Kaditis AG, Alonso Alvarez ML, Boudewyns A, Alexopoulos EI, Ersu R, Joosten K, Larramona H, Miano S, Narang I, Trang H, Tsaoussoglou M, Vandenbussche N, Villa MP, Van Waardenburg D, Weber S, Verhulst S (2016) Obstructive sleep disordered breathing in 2- to 18-year-old children: diagnosis and management. Eur Respir J 47(1):69–94. doi:10.1183/13993003.00385-2015
Katz ES, Mitchell RB, D’Ambrosio CM (2012) Obstructive sleep apnea in infants. Am J Respir Crit Care Med 185(8):805–816. doi:10.1164/rccm.201108-1455CI
DeHaan KL, Seton C, Fitzgerald DA, Waters KA, MacLean JE (2015) Polysomnography for the diagnosis of sleep disordered breathing in children under 2 years of age. Pediatr Pulmonol. doi:10.1002/ppul.23169
Goldberg S, Shatz A, Picard E, Wexler I, Schwartz S, Swed E, Zilber L, Kerem E (2005) Endoscopic findings in children with obstructive sleep apnea: effects of age and hypotonia. Pediatr Pulmonol 40(3):205–210. doi:10.1002/ppul.20230
Croft CB, Thomson HG, Samuels MP, Southall DP (1990) Endoscopic evaluation and treatment of sleep-associated upper airway obstruction in infants and young children. Clin Otolaryngol Allied Sci 15(3):209–216
Chan DK, Liming BJ, Horn DL, Parikh SR (2014) A new scoring system for upper airway pediatric sleep endoscopy. JAMA Otolaryngol Head Neck Surg 140 (7):595–602. doi:10.1001/jamaoto.2014.612
Robison JG, Wilson C, Otteson TD, Chakravorty SS, Mehta DK (2013) Analysis of outcomes in treatment of obstructive sleep apnea in infants. Laryngoscope 123(9):2306–2314. doi:10.1002/lary.23685
Cheng J, Elden L (2013) Outcomes in children under 12 months of age undergoing adenotonsillectomy for sleep-disordered breathing. Laryngoscope 123(9):2281–2284. doi:10.1002/lary.23796
Brigance JS, Miyamoto RC, Schilt P, Houston D, Wiebke JL, Givan D, Matt BH (2009) Surgical management of obstructive sleep apnea in infants and young toddlers. Otolaryngol Head Neck Surg 140(6):912–916. doi:10.1016/j.otohns.2009.01.034
Paradise JL, Smith CG, Bluestone CD (1976) Tympanometric detection of middle ear effusion in infants and young children. Pediatrics 58(2):198–210
Brodsky L (1989) Modern assessment of tonsils and adenoids. Pediatr Clin North Am 36 (6):1551–1569
Boudewyns A, Verhulst S, Maris M, Saldien V, Van de Heyning P (2014) Drug-induced sedation endoscopy in pediatric obstructive sleep apnea syndrome. Sleep Med 15(12):1526–1531. doi:10.1016/j.sleep.2014.06.016
Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM, American Academy of Sleep M (2012) Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J Clin Sleep Med 8(5):597–619. doi:10.5664/jcsm.2172
Marcus CL, Moore RH, Rosen CL, Giordani B, Garetz SL, Taylor HG, Mitchell RB, Amin R, Katz ES, Arens R, Paruthi S, Muzumdar H, Gozal D, Thomas NH, Ware J, Beebe D, Snyder K, Elden L, Sprecher RC, Willging P, Jones D, Bent JP, Hoban T, Chervin RD, Ellenberg SS, Redline S, Childhood Adenotonsillectomy T (2013) A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 368(25):2366–2376. doi:10.1056/NEJMoa1215881
Chan DK, Liming BJ, Horn DL, Parikh SR (2014) A New Scoring System for Upper Airway Pediatric Sleep Endoscopy. JAMA Otolaryngol Head Neck Surg. doi:10.1001/jamaoto.2014.612
Richter GT, Rutter MJ, deAlarcon A, Orvidas LJ, Thompson DM (2008) Late-onset laryngomalacia: a variant of disease. Arch Otolaryngol Head Neck Surg 134(1):75–80. doi:10.1001/archoto.2007.17
Revell SM, Clark WD (2011) Late-onset laryngomalacia: a cause of pediatric obstructive sleep apnea. Int J Pediatr Otorhinolaryngol 75(2):231–238. doi:10.1016/j.ijporl.2010.11.007
Galluzzi F, Pignataro L, Gaini RM, Garavello W (2015) Drug induced sleep endoscopy in the decision-making process of children with obstructive sleep apnea. Sleep Med 16(3):331–335. doi:10.1016/j.sleep.2014.10.017
Caldwell P, Hensley R, Machaalani R, Cheng A, Waters K (2011) How effective is adenoidectomy alone for treatment of obstructive sleep apnoea in a child who presents with adenoid hypertrophy? J Paediatr Child Health 47(8):568–571. doi:10.1111/j.1440-1754.2011.02154.x
Nolan J, Brietzke SE (2011) Systematic review of pediatric tonsil size and polysomnogram-measured obstructive sleep apnea severity. Otolaryngol Head Neck Surg 144(6):844–850. doi:10.1177/0194599811400683
Kay DJ, Bryson PC, Casselbrant M (2005) Rates and risk factors for subsequent tonsillectomy after prior adenoidectomy: a regression analysis. Arch Otolaryngol Head Neck Surg 131(3):252–255. doi:10.1001/archotol.131.3.252
Baugh RF, Archer SM, Mitchell RB, Rosenfeld RM, Amin R, Burns JJ, Darrow DH, Giordano T, Litman RS, Li KK, Mannix ME, Schwartz RH, Setzen G, Wald ER, Wall E, Sandberg G, Patel MM, American Academy of O-H, Neck Surgery F (2011) Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg 144(1 Suppl):S1–S30. doi:10.1177/0194599810389949
Gross JB, Bachenberg KL, Benumof JL, Caplan RA, Connis RT, Cote CJ, Nickinovich DG, Prachand V, Ward DS, Weaver EM, Ydens L, Yu S, American Society of Anesthesiologists Task Force on Perioperative M (2006) Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology 104 (5):1081–1093 (quiz 1117–1088)
Mitchell RB, Kelly J (2005) Outcome of adenotonsillectomy for obstructive sleep apnea in children under 3 years. Otolaryngol Head Neck Surg 132(5):681–684. doi:10.1016/j.otohns.2004.12.010
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boudewyns, A., Van de Heyning, P. & Verhulst, S. Drug-induced sedation endoscopy in children <2 years with obstructive sleep apnea syndrome: upper airway findings and treatment outcomes. Eur Arch Otorhinolaryngol 274, 2319–2325 (2017). https://doi.org/10.1007/s00405-017-4481-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-017-4481-3