Abstract
There is controversy regarding prognosis and treatment of young patients with oral cavity cancer compared to their older counterparts. We conducted a retrospective case-matched analysis of all adult patients younger than 40 years and treated at our institution for a squamous cell carcinoma of the oral cavity. Only non-metastatic adult patients (age >18) with oral tongue cancer were eventually included and matched 1:1 with patients over 40 years of age, at least 20 years older than the cases, with same T and N category and treatment period. Sixty-three patients younger than 40 had an oral cavity squamous cell cancer out of which 57 had an oral tongue primary during the period 1999–2012, and 50 could be matched with an older control. No difference could be seen between younger and older patients with regard to overall, cancer-specific, or progression-free survival. The patterns of failure were similar, although in young patients, almost all failures occurred during the first 2 years following treatment. Although overall survival shows a trend toward lower survival in older patients, cancer-specific survival and analysis of pattern failure suggest that disease prognosis is similar between young and older adults with oral tongue cancer. Further work is needed to identify the younger patients with poorer prognosis who overwhelmingly fail during the first year after treatment and could benefit from treatment intensification. Until then, young adults ought to be treated using standard guidelines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More than 60,000 young adults aged 20–39 are diagnosed with cancer each year in the United States, which represents 4% of all cancers. About 9000 young adults die from cancer each year, making cancer the 4th leading cause of death in this age group, behind accidents, suicide, and homicides [1]. Oral cavity squamous cell carcinoma (OCSCC) predominantly occurs in elderly individuals, following a long history of tobacco and alcohol consumption. Among young adults, the occurrence of OCSCC is rare, representing generally less than 5% of all cases [2]. Oral tongue is the most frequent tumor location in young OCSCC patients. Its incidence in young adults is believed to be increasing, especially in young women with no history of tobacco and alcohol exposure [3, 4]. It has, therefore, been suggested that oral tongue squamous cell carcinoma (SCC) in young adults might be a distinct biological entity, the underlying causes of which remain largely unknown [5, 6].
There are conflicting data regarding the prognosis of OCSCC in young and elderly adults. Some reports suggest a comparable prognosis between the two patients populations [7–10], while others show a poorer prognosis for young adults, which may justify intensified therapy in this subpopulation [11, 12], especially in the absence of the traditional risk factors [6]. Prediction of individual patient prognosis is of the utmost importance for personalizing treatment in balancing the risk of recurrence and the risk of long-term toxicity in these young patients, whose life expectancy once the index cancer is cured is expected to be very long.
We have, therefore, decided to compare the patient population, treatment, and outcome of adults under 40 years of age (defined as young adults) managed at our institution for an oral tongue SCC between 1999 and 2012, with a matched cohort of controls of oral cavity cancer patients 20 years older.
Methods
Patients and matching process
This analysis is a retrospective case-matched study using clinical information collected from electronic patient records. All patients treated for an oral cavity squamous cell cancer between 1999 and 2012 were identified using the institutional head and neck cancer registry. Metastatic patients and patients not followed-up at the institution were excluded. A total of 63 patients younger than 40 years were identified. Additional 1486 patients aged 40–70 years were managed for an oral cavity cancer over the same time period. After excluding tumor sites other than oral tongue, 57 young patients were eligible and included in the analysis. The young adult cases were matched 1:1 with older patient controls. Matching criteria were gender, T (1–2 vs 3–4) stage, N (0–2b vs 2c–3) stage as defined on imaging, period of treatment (±2 years), and age, the controls being at least 40 years of age and at least 20 years older than the cases. The study was conducted with approval from the institutional ethics committee.
Data collected
The electronic medical records of all patients were reviewed retrospectively to determine pretreatment clinical and disease characteristics, management details, and outcomes. Toxicity data were not reliably and uniformly recorded during follow-up, and we, therefore, decided not to collect them as part of this analysis. Human papillomavirus analyses were not conducted on a systematic basis in non-oropharyngeal cancer patients and are not presented here due to a high rate of missing data.
Treatment strategy
All head and neck cancer patients treated at Gustave Roussy are evaluated by a multidisciplinary head and neck tumor board comprising at least a head and neck oncological surgeon, a head and neck radiation oncologist, and a head and neck medical oncologist, and most frequently a dedicated head and neck radiologist. The standard strategy in oral cavity cancer is upfront surgery and adjuvant radiotherapy as indicated by the histopathological staging. Addition of concurrent chemotherapy is discussed according to histopathological findings and risk factors. The use of induction chemotherapy prior to local treatment is not standard, but is occasionally agreed upon owing to the extent of the tumor or its fast growth rate that would render an upfront clear-margin surgery difficult.
Statistical analysis
Proportions were compared using the Fisher exact test. Follow-up was estimated using the reverse Kaplan–Meier method [13]. Overall survival (OS), progression-free survival (PFS), cancer-specific survival (CSS), locoregional control (LC), and distant control (DC) rates were estimated using the Kaplan–Meier method. Survival times were defined as the time between the beginning of treatment, because of varying treatment durations among patients, and the first event. Events were death from any cause for overall survival (OS) and death or tumor progression for PFS. Survival curves were compared using the log-rank test for the univariate analysis and in a multivariate ascending stepwise Cox regression for the multivariate analysis (MVA). Variables associated with disease-free or overall survival with a p value <0.20 were included in the MVA. In the Cox model, continuous variables were dichotomized. All reported p values are two-sided, and p values lower than 0.05 were considered significant. Analyses were conducted using SAS version 9.3.
Results
Description of the population
After matching, only 50 cases were retained with 50 randomly selected controls (no appropriate control was found for the remaining 7 cases). Patient and disease characteristics are described in Table 1. Median age was 32 years (range 19–39) for cases and 53 years (range 42–67) for controls. Regarding matching variables, patients were mostly male (78%) and had T1–T2 disease (70%) and low N stage (N0 in 74%). Regarding other variables, overall stage was not different between cases and controls (p = 0.82), patients had a good performance status (98% for cases and 90% for controls, p = 0.20). The major differences between cases and controls pertained to tumor localization and smoking/drinking habits. While most young patients had tumor limited to the mobile tongue (94%), tumor was extending to floor of mouth invasion in 42% of the older patients (p < 0.0001). Smoking and drinking history increased with advanced patient age (both p < 0.0001).
Treatment strategy
Treatment modalities used are described in Table 2. The use of induction chemotherapy was limited, in keeping with the mostly low observed tumor stages and relevant treatment guidelines, and did not differ between cases and controls (p = 0.55). Surgery was performed in most cases (96%) and controls (92%), but the use of a transoral approach was more frequent in young patients (79 vs. 52%, p = 0.01). Margins were negative in the majority of patients, not differing between cases and controls (p = 0.62). There was no difference in the proportion of primary tumors with lymphovascular invasion (p = 0.16) or perineural invasion (p = 0.12), although data were missing for a large proportion of the control patients (61%). A lymph node dissection was performed in most cases (94%), with no difference between cases and controls (p = 1.00). Among these, positive nodes were found in 16 cases (37%) and 15 controls (33%) (p = 0.66), and around a third of patients in each group had extracapsular nodal spread. Radiotherapy was overall used in less than half of the patients, usually combined with surgery and as a standalone treatment in 2 cases and 4 controls with unresectable disease (p = 0.64).
Disease outcomes and failure patterns
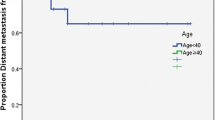
Median follow-up was 86 months (range 12, 186). Eleven deaths occurred among cases and 23 among controls, with actuarial 5 and 8 years survival rates of 81%/81% in cases and 75%/54% in controls (log-rank univariate p value 0.08). Nine of the 11 deaths in cases occurred during the first 2 years, including four deaths in patients staged T2N0. PFS rates at 5 and 8 years were of 72%/72% in cases and 73%/52% in controls. The curves for OS and PFS are displayed in Figs. 1 and 2. The PFS curve shows an interesting qualitative difference between cases and controls, in that the vast majority of PFS events in cases occurred during the first year (10, compared to 3 in the following 6 years), while in controls, the rate was steady (7 in the first years, and then 6 and 4 in years 2 and 3, respectively), as demonstrated on the curve by the plateau after year one for cases and the regular decrease for controls. Nine deaths related to cancers occurred among cases and 12 among controls, with cancer-specific survival (CSS) rates at 5 and 8 years of 82%/82% and 85%/73%. Locoregional recurrences occurred in 12 cases and 10 controls, while distant failures occurred in 4 and 7 patients, respectively. As for PFS, virtually, all cancer deaths occurred within the first 2 years for cases, whereas for older patients, the curves continued to drop after the second-year post-treatment, albeit at a slower rate.
Prognostic analysis
The prognostic analyses are presented in Tables 3 and 4. Female gender, lower tumor stage, and the performance of surgery were statistically associated with improved OS in univariate analysis. Younger patients had a non-significant trend towards improved overall survival, with a hazard ratio (HR) of 0.53 [95% confidence interval (CI) 0.26, 1.10; p = 0.08]. However, in multivariate analysis, only the performance of surgery remained statistically associated with improved survival (HR 0.17; 95% CI 0.06, 0.51; p = 0.001). Regarding CSS and PFS, surgery was the only significant factor in the multivariate analysis (Table 4). Cases and controls had no differences in CSS or PFS, as shown by the multivariate HRs (95% CI, p) of 0.90 (0.36, 2.23; p = 0.81) and 0.84 (0.43, 1.61; p = 0.60), respectively.
Discussion
The present retrospective case-matched study of patients with an oral tongue squamous cell carcinoma failed to identify a difference in cancer-specific survival, locoregional relapse, or distant relapse in patients aged less than 40 years compared to older patients. There was a borderline improved overall survival in younger patients; this trend could be explained by the older age, the more frequent presence of comorbidities, or consumption of alcohol and tobacco in the control group. The only prognostic factor for progression or death was the inability to perform a surgical resection of the tumor, which encompasses patients with the poorest prognosis due to tumor extension or comorbidities.
Given the rarity of OCSCC in young adults, there is a lack of good-quality prospective data in this patient population, which is underrepresented in randomized trials. The rate of patients younger than 40 years of age in the meta-analysis of chemotherapy in carcinoma of the head and neck [14] is inferior to 4% of the approximately 17,000 patients with age data (unpublished data). Our results, together with those published by other teams, help to better define the management of young patients with oral cavity cancer. Given the evident similarity in terms of cancer-related prognosis between young adults and older patients, the treatment of young patients should overall follow the guidelines developed for the general population. However, these guidelines are often insufficient at the individual level. Indeed, our analysis of young patients with OCSCC clearly shows that there is a subset of patients with the early and aggressive relapse although managed following standard guidelines and despite presenting favorable clinical and pathological features. The absence of appropriate prognostic biomarkers and the limited salvage options in case of relapse warrants a particularly close follow-up of such patients after the initial treatment, even in case of favorable features.
Our study complements the most recent and largest comparative studies that evaluated the prognosis of young patients with OCSCC [7, 15–17]. Chang et al. surveyed the Taiwan National Health Insurance Research Database and concluded after performing a multivariate Cox model and a propensity score analysis that young age, defined as 45 years or below, did not confer a worse prognosis for oral cancer patients who underwent wide excision and reconstruction [15]. Lassig et al. matched 87 head and neck SCC patients younger than 45 years to controls aged more than 45 and showed that younger patients had mildly improved overall survival but statistically improved disease-free survival. This was especially true in non-oropharyngeal cancer patients, of which 75% were oral cavity cancer patients [16]. Pytynia and colleagues matched 31 patients with a head and neck malignancy aged 40 years or younger and treated between 1995 and 2001 with 62 controls aged more than 40 years. They concluded that there was no difference between the two age group patients with regard to overall survival, disease-free survival, or time to recurrence [7]. The study by Udeabor et al. showed by retrospectively analyzing their institution’s registry that young adults with oral cavity cancer did not have a worse prognosis than older patients [17]. One conflicting study, by Garavello et al., compared 46 cases and 92 controls with an age limit set at 40 years and found significantly higher recurrence rates in the younger patients [12].
Overall, most of the comparative studies that showed a worse prognosis among younger patients had a small sample size and were not case-matched and, therefore, had a high risk of selection and confusion bias [9]. In contrast, when looking specifically at well-designed case-matched studies, all but one [12] showed similar prognosis in the young and the older patients.
The use of a contemporaneous cohort of older patients for matching and the treatment and follow-up at a tertiary cancer center are the strengths of our study. The main limitations are related to its retrospective nature. Matching was performed on pooled categories and resulted in some differences in overall tumor stage, tumor site, or the use of transoral surgery between cases and controls. Matching was not performed on treatment characteristics, as one of our aims was also to look for different patterns of treatment in younger patients, potentially being more aggressively treated. There was, however, no difference in the use of radiotherapy or chemotherapy between cases and controls in our cohort, and a similar number of non-surgically treated patients, suggesting that the same guidelines have been followed in both age groups. Another limitation is the absence of data on toxicity or patient reported outcomes, which would be important for tailoring treatment to long-term functional or toxicity outcomes. The issue of secondary malignancies in the young population would also be of great interest, although much larger sample size and longer follow-up would probably be needed due to the relative rarity of these events. Finally, no data on potential risk factors specific to the young population could be adequately retrieved in the retrospective setting. As an example, the analysis of human papillomavirus (HPV) status was not routinely performed at the time of the initial treatment. Besides, the relationship between HPV and oral cavity cancers is a matter of debate, even in the young patient subpopulation [6, 18]. Indeed, transcriptionally active HPV is rarely found in oral cavity cancers even in non-smoker and non-drinker young adults. Moreover, no studies to date have demonstrated that the molecular profile or prognosis of these potentially HPV-driven oral cavity malignancies was comparable to those of HPV-induced oropharyngeal cancers [19].
In conclusion, the present case-matched analysis does not indicate a differential prognosis among patients with oral tongue squamous cell carcinoma aged younger or older than 40 years. Treatment guidelines developed for older patients should be used for the young adult population, although the need to adequately stage the patients and minimize and manage long-term side-effects supports the referral of these patients to tertiary centers. Surgery is the mainstay of treatment. Multicenter prospective collaboration is needed to develop biomarkers that could predict tumor aggressiveness to personalize the use of adjuvant modalities following surgery.
References
Ten Leading Causes of Death by Age Group (2010) Office of statistics and programming, national center for injury prevention and control. http://www.cdc.gov/injury/wisqars/pdf/10LCID_All_Deaths_By_Age_Group_2010-a.pdf Accessed 25 April 2016
Müller S, Pan Y, Li R, Chi AC (2008) Changing trends in oral squamous cell carcinoma with particular reference to young patients: 1971–2006. The Emory University experience. Head Neck Pathol 2:60–66. doi:10.1007/s12105-008-0054-5
Patel SC, Carpenter WR, Tyree S, Couch ME, Weissler M, Hackman T et al (2011) Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol Off J Am Soc Clin Oncol 29:1488–1494. doi:10.1200/JCO.2010.31.7883
Shiboski CH, Schmidt BL (2005) Jordan RCK. Tongue and tonsil carcinoma: increasing trends in the US population ages 20–44 years. Cancer 103:1843–1849. doi:10.1002/cncr.20998
Llewellyn CD, Linklater K, Bell J, Johnson NW, Warnakulasuriya S (2004) An analysis of risk factors for oral cancer in young people: a case-control study. Oral Oncol 40:304–313
Bachar G, Hod R, Goldstein DP, Irish JC, Gullane PJ, Brown D et al (2011) Outcome of oral tongue squamous cell carcinoma in patients with and without known risk factors. Oral Oncol 47:45–50. doi:10.1016/j.oraloncology.2010.11.003
Pytynia KB, Grant JR, Etzel CJ, Roberts D, Wei Q, Sturgis EM (2004) Matched analysis of survival in patients with squamous cell carcinoma of the head and neck diagnosed before and after 40 years of age. Arch Otolaryngol Head Neck Surg 130:869–873. doi:10.1001/archotol.130.7.869
Hyam DM, Conway RC, Sathiyaseelan Y, Gebski V, Morgan GJ, Walker DM et al (2003) Tongue cancer: do patients younger than 40 do worse? Aust Dent J 48:50–54
Hilly O, Shkedy Y, Hod R, Soudry E, Mizrachi A, Hamzany Y et al (2013) Carcinoma of the oral tongue in patients younger than 30 years: comparison with patients older than 60 years. Oral Oncol 49:987–990. doi:10.1016/j.oraloncology.2013.07.005
Atula S, Grénman R, Laippala P, Syrjänen S (1996) Cancer of the tongue in patients younger than 40 years. A distinct entity? Arch Otolaryngol Head Neck Surg 122:1313–1319
Sarkaria JN, Harari PM (1994) Oral tongue cancer in young adults less than 40 years of age: rationale for aggressive therapy. Head Neck 16:107–111
Garavello W, Spreafico R, Gaini RM (2007) Oral tongue cancer in young patients: a matched analysis. Oral Oncol 43:894–897. doi:10.1016/j.oraloncology.2006.10.013
Schemper M, Smith TL (1996) A note on quantifying follow-up in studies of failure time. Control Clin Trials 17:343–346
Pignon J-P, le Maître A, Maillard E, Bourhis J, MACH-NC Collaborative Group (2009) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol J Eur Soc Ther Radiol Oncol 92:4–14. doi:10.1016/j.radonc.2009.04.014
Chang T-S, Chang C-M, Ho H-C, Su Y-C, Chen L-F, Chou P et al (2013) Impact of young age on the prognosis for oral cancer: a population-based study in Taiwan. PLoS One 8:e75855. doi:10.1371/journal.pone.0075855
Lassig AAD, Lindgren BR, Fernandes P, Cooper S, Ardeshipour F, Schotzko C et al (2013) The effect of young age on outcomes in head and neck cancer. Laryngoscope 123:1896–1902. doi:10.1002/lary.23932
Udeabor SE, Rana M, Wegener G, Gellrich N-C, Eckardt AM (2012) Squamous cell carcinoma of the oral cavity and the oropharynx in patients less than 40 years of age: a 20-year analysis. Head Neck Oncol 4:28. doi:10.1186/1758-3284-4-28
Majchrzak E, Szybiak B, Wegner A, Pienkowski P, Pazdrowski J, Luczewski L et al (2014) Oral cavity and oropharyngeal squamous cell carcinoma in young adults: a review of the literature. Radiol Oncol 48:1–10. doi:10.2478/raon-2013-0057
Mirghani H, Amen F, Moreau F, St Guily JL (2015) Do high-risk human papillomaviruses cause oral cavity squamous cell carcinoma? Oral Oncol 51:229–236. doi:10.1016/j.oraloncology.2014.11.011
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has a conflict of interest regarding this research.
Funding
No specific funding was used.
Research involving human participants and/or animals
This research is a retrospective assessment of outcomes of patients receiving standard of care treatment, which is not considered as experimental.
Informed consent
Informed consent was waived due to the retrospective nature of the study, which was approved by the institutional review board.
Rights and permissions
About this article
Cite this article
Blanchard, P., Belkhir, F., Temam, S. et al. Outcomes and prognostic factors for squamous cell carcinoma of the oral tongue in young adults: a single-institution case-matched analysis. Eur Arch Otorhinolaryngol 274, 1683–1690 (2017). https://doi.org/10.1007/s00405-016-4419-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-016-4419-1