Abstract
The role of IL-25 and IL-33 in the aetiology and pathogenesis of nasal polyps has been controversial in the literature. The objective of the study is to detect serum and tissue levels of IL-25 and IL-33 in patients with (CRSwNP) or without (CRSsNP) nasal polyps using enzyme-linked immunosorbent assay (ELISA). Study group consisted of 20 CRSwNP and 20 CRSsNP patients. Control group comprised of 20 volunteers who had been operated with septum deviation without any additional sinonasal pathology, allergy, systemic disease, or medication use. All groups preoperatively underwent paranasal CT examinations and sinonasal pathologies were recorded based on Lund–Mackay radiological staging system. IL-25 and IL-33 levels in serum and tissue samples were analyzed using the ELISA method. Serum IL-25 and IL-33 levels in CRSsNP, CRSwNP, and control groups did not differ statistically significantly (p = 0.345 and p = 0.338). Any statistically significant difference was not detected in mean tissue IL-25 levels among CRSsNP, CRSwNP, and control groups (p = 0.698). Mean tissue IL-33 level in the CRSwNP group was statistically significantly lower when compared with those of CRSsNP and control groups (p < 0.001 and p < 0.001, respectively). A statistically significant negative correlation was detected between tissue IL-33 levels and Lund–Mackay CT scores (r = −0.436 and p = 0.005). In the present study, we conceivably contributed to scarce number of studies conducted on this issue and we think that further studies will better clarify the role of IL-25 and IL-33 in the development of nasal polyps.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic rhinosinusitis (CRS) is a widespread inflammation of paranasal sinus and nasal mucosa and it can be defined as CRS coursing with (CRSwNP) or without polyps (CRSsNP). CRSwNP is currently one of the most important health problems with a worldwide prevalence ranging between 2 and 5 percent [1]. In cases with CRS, the presence of polyps has been suggested to be a significant factor in the development of treatment-resistant cases and postoperative recurrences [2, 3]. The most dominant inflammatory cell type is eosinophils [4]. In histological studies, increased eosinophilic activation is seen when compared with the normal nasal mucosa [5]. From activated eosinophils, T-helper 2 cytokines, such as IL-5, and lipid mediators, such as RANTES and eotaxins, are released. All these substances exert proinflammatory activities and cause increase in adhesion molecules, activation and regulation of vascular permeability, and mucus secretion [6–8].
Interleukin-25 was first defined in 2001 by Hurst et al. [9]. This protein consists of 177 amino acids, which are produced in Th2 T cells and mast cells in in vitro media and epithelial cells [10]. Basophils and neutrophils have been demonstrated as a source of IL-25 [11]. As has been indicated in many reports, scarce amounts of these substances are produced in brain, kidney, prostate, testicles, adrenal gland, spinal cord, and trachea. However, increased amounts of IL-25 have been detected in gastrointestinal system and uterus. Intraperitoneal injection of IL-25 has been demonstrated to induce eosinophilia, splenomegaly, and increase in splenic plasma cells. Besides, it causes increases in the amounts of IgE and IgG1 [10]. IL-25 also induces Th2-dependent cytokines and release of IL-13 in all tissues. However, IL-25 pioneers gene expression in other tissues and pathological changes in mucosal tissues, which have also attracted great deal of attention [10]. Some data support critical role of IL-25 in the pathogenesis of asthma. In sensitized murine lungs, increased levels of IL-25 have been observed following inhalation of antigenic agents [12]. Another recent study suggested that sinonasal epithelia and infiltrating mast cells might be involved in the pathogenesis of CRS with NPs by producing IL-25 [13].
Another Th2 cytokine is Interleukin-33, which was first identified in cerebral cells of dogs by Onda et al. in 1999 [14]. In the year 2003, Baekkevold et al. cited IL-33 as a nuclear factor secreted from venules coated with tall columnar epithelium [15]. It is a member of the IL-1 cytokine family and has been demonstrated as potential inducer of Th2-type responses via IL-IR-dependent protein ST2 receptors. Its molecular weight is 30kD and it belongs to the group β-folded proteins [15]. When IL-33 receptor is bound to ST2, Th2 type is included in the inflammatory process and it starts to be expressed in mast cells, dendritic cells, and Th2 cells [16].
IL-33 is thought to be the most potential triggering factor of TH2-type inflammation in mucosal tissues in relationship with thymic stromal lymphopoietin (TSLP) [17]. Basophils, mast cells, eosinophils, natural killer cells, and Th2 lymphocytes are target cells, and in the presence of IL-33, Th2 cells synthesize increased amounts of IL-5 and IL-13 [18, 19]. Mast cells increase levels of proinflammatory cytokines as IL-1-β, IL-6, IL-13, and TNF-α Basophils and eosinophils respond to IL-33 via increased integrin synthesis [20].
Taken together, studies summarized above demonstrate that IL-25 and IL-33 play important roles in Th2-type inflammation. Because both cytokines are produced by the immune cells and tissue cells, we aimed to study both serum and tissue levels of IL-25 and IL-33 in CRSwNP or CRSsNP polyps using enzyme-linked immunosorbent assay (ELISA) method so as to investigate their involvement in ethiopathogenesis of CRSwNP and CRSsNP. This is the first study to analyse these two cytokines simultaneously in serum and tissue of in chronic sinusitis.
Materials and methods
Study design
The study has been conducted in accordance with the principles of the Helsinki Declaration and approved by the local Institutional Review Board (19.09.2013/25.2). Written informed consent was obtained from all subjects.
Among patients aged over 18 who consulted to outpatient clinic of the Otorhinolaryngology Department of Antalya Training and Research Hospital with complaints of nasal stuffiness, nasal and postnasal discharge and facial pain at least for the last 12 weeks and those with CRSwNP and/or CRSsNP as detected during endoscopic and paranasal sinus tomographic examinations were included in the study [21]. Study group consisted of 20 CRSwNP and 20 CRSsNP rhinosinusitis patients. Smokers, patients with a systemic disease, those who had been previously operated for nasal polyposis and/or chronic sinusitis, craniofacial malformations, immune deficiencies, malignant disease and patients who had been using any immunomodulator treatment for the last 4 weeks, were not included in the study. Control group of the study comprised of 20 volunteers who had been operated with the indication of septum deviation without any additional sinonasal pathology, allergy, systemic disease, or medication use.
All patients in the study and the control groups preoperatively underwent paranasal CT examinations and sinonasal pathologies of the patients were recorded based on Lund–Mackay radiological staging system [22].
Blood serum samples were drawn from all patients in the study and the control groups before the operation. During functional endoscopic sinus surgery, polyp tissue material from the CRSwNP patients and uncinate process tissue samples from cases with CRSsNP were harvested. Tissue samples were retrieved from the right lower concha of the control group of patients who would be operated for the correction of nasal septum deviation.
Measurement of IL-25 and IL-33
IL-25 and IL-33 levels in serum and tissue samples retrieved from the patient and the control groups were analyzed using the ELISA method in the Akdeniz University Department of Health Sciences Research and Application Center (SBAUM). Blood samples were centrifuged at 4000 rpm for 15 min, and their sera were separated. Sera was kept at −20 °C in polypropylene tubes Tissue samples were cut in pieces, of 100 mg, and weighed on sensitive scales. Tissue samples were washed with phosphate buffer solution and homogenized in 1 ml phosphate buffer solution and kept overnight at −20 °C. They were thawed under room temperature and then frozen two more times to fragment cell membranes. Aliquots of tissue homogenisates was centrifuged at 5000 g and at 2–8 °C for 5 min. Supernatants were taken out and preserved in polypropylene tubes at −20 °C to be used for measurements. IL-25 and IL-33 levels in conserved serum and tissue samples were measured using Human Interleukin 25 (IL-25) ELISA (Cusabio, USA) and Human IL-33 ELISA (Boster, USA) kits, respectively. The minimal detection limits for these kits are 62.5 and 15.6 pg/mL for IL-25 and IL-33, respectively.
Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences 11.5 for Windows (SPSS Inc., Chicago, IL, USA). A normal distribution of the quantitative data was checked using Kolmogorov–Smirnov and Homogeneity Levene’s tests. The differences were assessed by two-tailed Student’s t test for two groups and by One-Way ANOVA test for more than two groups. The differences between groups were assessed by the Kruskal–Wallis analysis of variance followed by Conover’s multiple t test to identify the significant values. Nominal variables were assessed by likelihood ratio test. Correlation was examined using the Spearman correlation coefficient. Continuous data were presented as mean ± standard deviation or median [minimum–maximum], as appropriate. All differences associated with a chance probability of 0.05 or less were considered statistically significant.
Results
Study group comprised total of 40 patients (20 CRSwNP and 20 CRSsNP patients) and control group consisted of 20 patients. Radiological staging of the patients in the study group was evaluated based on Lund–Mackay staging system, and mean values for the patients in groups of CRSwNP and CRSsNP were calculated as 17.7 and 7.8, respectively. Demographic and clinical findings of the patients are summarized in Table 1.
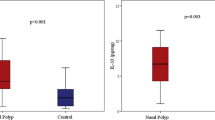
Serum IL-25 and IL-33 levels were measured both in the study and the control groups (Fig. 1a, b). Mean serum IL-25 and IL-33 levels in CRSwNP, CRSsNP, and control groups did not differ statistically significantly. (p = 0.345 and p = 0.338, respectively) Besides tissue IL-25 and IL-33 levels in the study and control groups were analyzed (Fig. 2a, b). Any statistically significant difference was not detected in mean tissue IL-25 levels among CRSwNP, CRSsNP, and control groups (p = 0.698). A statistically significant difference was detected among groups as for tissue IL-33 levels (p < 0.001). Mean tissue IL-33 level in the CRSwNP group was statistically significantly lower when compared with those of CRSsNP and control groups (p < 0.001 and p < 0.001, respectively). However, any statistically significant difference was not observed between CRSsNP and control groups (p = 0.194).
Tissue IL-25 (a) and Tissue IL-33 (b) levels determined by ELISA of CRSsNP (n = 20), CRSwNP (n = 20), and control (n = 20) groups. The Kruskal–Wallis test was used. No statistical differences in tissue IL-25 levels among the three groups were determined. Tissue IL-33 levels were lower in CRSwNP than other two groups (p < 0.001)
In patients with CRSwNP and CRSsNP, a statistically significant correlation was not detected between serum IL-25 levels and Lund–Mackay CT scores (r = −0.044 and p = 0.788). In the entire patient population, a statistically significant correlation was not detected between tissue IL-25 levels and Lund–Mackay CT scores (r = −0.077 and p = 0.637). Similarly, a statistically significant difference was not detected between serum IL-33 levels, Lund–Mackay CT scores, and outcomes obtained (r = 0.006 and p = 0.969). However, a statistically significant negative correlation was detected between tissue IL-33 levels and Lund–Mackay CT scores (r = −0.436 and p = 0.005) (Fig. 3).
Discussion
Chronic rhinosinusitis is a widespread disease characterized by the inflammation of nasal and paranasal sinus mucosa [1]. Currently, it is classified as CRS with or without nasal polyps. Even though the ethiology and pathogenesis of the CRS are not clear, there have been reports that certain bacteria, viruses, and fungi might be involved in the disease process [23]. It is also suggested that the inflammation might develop as a result of impaired cytokine and chemokine balance in nasal mucosa [24]. Immunological mechanism in CRS has not been fully clarified yet. Although many theories have been suggested for the ethiopathogenesis of nasal polyposis, currently, all these theories have acknowledged mucosal oedema as the basic pathology leading to the formation of polyps. It has also been emphasized that mucosal oedema is caused by inflammatory mediators and cytokine and adhesion molecules play a role in its ethiopathogenesis [25].
In our study, serum and tissue IL-25 levels were not significantly different between groups. In contrast with two recent studies by Shin et al., and Lam et al., who demonstrated that tissue levels of IL-25, were higher in CRSwNP patients compared with CRSsNP patients and healthy controls [13, 26]. Although our study, in which considerably more patients than both the above studies, also revealed an increase of IL-25 levels in the nasal polyp group, this increase did not reach to statistical significance. One disadvantage of our study compared to these studies was that we did not group patients according to eosinophilia status.
In our study, serum IL-33 levels in the CRSwNP group were higher when compared with the other groups, while, generally, mean values were not statistically significant among groups. However, tissue IL-33 levels were statistically significantly different among CRSsNP, CRSwNP, and control groups, while mean tissue IL-33 level in the CRSwNP group was statistically significant lower relative to CRSsNP and control groups. Similarly, Baba et al. recently investigated IL-33/ST2 levels in the groups with eosinophilic CRS and non-eosinophilic CRS patients using immunuhistochemical methods, such as ELISA and PCR, and detected higher percentages of ST2-positive cells in the eosinophilic CRS group [27]. In addition, in line with our results, IL-33 levels were determined to lower in the presence of polyp.
In our study, we also investigated the relationship between CT scores, serum, and tissue IL-25 and 33 levels in patient groups. We detected a statistically significant negative correlation between tissue IL-33 levels and CT scores. Correlations between other groups and CT scores were not found to be statistically significant. Previously, other studies have investigated the relationship between clinical signs and interleukin expression in patients with CRS. Hu et al. detected a statistically significant positive correlation between CT scores of the patients with CRS and their IL-17A expressions [28]. Shen et al. found a positive correlation between IL-17A levels and CT scores in patients with nasal polyps [29]. Lam et al. investigated epithelial endotypes and clinical severity of the CRS and detected a statistically significant negative correlation between IL-25 levels and CT scores, but no correlation between CT scores and IL-33 levels [30]. The reason underlying the negative correlation may be caused by different immune cells infiltrating the tissue. Since the infiltration of the immune cells to tissues requires adhesion molecules, we speculate that the adhesion molecules on the polyp tissue might be different than that of the chronic sinusitis tissue without polyp formation.
Differences between literature data support the assertion that cytokine profiles in CRS with and without polyps have not been precisely clarified yet. As suggested previously, the comprehension of the pathophysiological role of both IL-25 and IL-33 in CRSwNP will constitute a basis for the development of IL-25 and 33 antagonists in the treatment of this widespread health problem [13, 31].
Conclusion
This is the first study that simultaneously analyse the levels of IL-25 and IL-33 from both sera and tissues of CRS patients. Even though we have not detected significantly higher levels of these cytokines in our patients, we conceivably contributed to scarce number of studies conducted on this issue. Further studies will better clarify the role of IL-25 and IL-33 in the pathogenesis of nasal polyps.
References
Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, Bachert C, Baraniuk J, Baroody FM, Benninger MS, Brook I, Chowdhury BA, Druce HM, Durham S, Ferguson B, Gwaltney JM, Kaliner M, Kennedy DW, Lund V, Naclerio R, Piccirillo JF, Rohane P, Simon R, Slavin RG, Togias A, Wald ER, Zinreich SJ (2004) American Academy of Allergy, Asthma and Immunology (AAAAI); American Academy of Otolaryngic Allergy (AAOA); American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS); American College of Allergy, Asthma and Immunology (ACAAI); American Rhinologic Society (ARS). Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol 114:S155–S212
Litvack JR, Griest S, James KE, Smith TL (2007) Endoscopic and quality-of-life outcomes after revision endoscopic sinus surgery. Laryngoscope 117:2233–2238
Bhattacharyya N (2007). Influence of polyps on outcomes after endoscopic sinus surgery. Laryngoscope 117:1834–1838
Jankowski R (1996) Eosinophils in the pathophysiology of nasal polyposis. Acta Otolaryngol 116:160–163
Stoop AE, van der Heijden HA, Biewenga J, van der Baan S (1993) Eosinophils in nasal polyps and nasal mucosa: an immunohistochemical study. J Allergy Clin Immunol 91:616–622
Hogan SP, Rosenberg HF, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME (2008) Eosinophils: biological properties and role in health and disease. Clin Exp Allergy 38:709–750
Bachert C, Gevaert P, Holtappels G, Cuvelier C, van Cauwenberge P (2000) Nasal polyposis: from cytokines to growth. Am J Rhinol 14:279–290
Kato A (2015) Immunopathoogy of chronic rhinosinusitis. Allergol Int 64:121–130
Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, Brieland JK, Zurawski SM, Chapman RW, Zurawski G, Coffman RL (2002) New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol 169:443–453
Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM (2001) IL-25 induces IL-4, IL-5 and IL-13 and Th2-associated pathologies in vivo. Immunity 15:985–995
Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ (2007) IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med 204:1837–1847
Tamachi T, Maezawa Y, Ikeda K, Kagami S, Hatano M, Seto Y, Suto A, Suzuki K, Watanabe N, Saito Y, Tokuhisa T, Iwamoto I, Nakajima H (2006) IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J Allergy Clin Immunol 118:606–614
Shin HW, Kim DK, Park MH, Eun KM, Lee M, So D, Kong IG, Mo JH, Yang MS, Jin HR, Park JW, Kim DW (2015) IL-25 as a novel therapeutic target in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol 135:1476–1485
Onda H, Kasuya H, Takakura K, Hori T, Imaizumi T, Takeuchi T, Inoue I, Takeda J (1999) Identification of genes differentially expressed in canine vasospastic cerebral arteries after subarachnoid hemorrhage. J Cereb Blood Flow Metab 19:1279–1288
Baekkevold ES, Roussigné M, Yamanaka T, Johansen FE, Jahnsen FL, Amalric F, Brandtzaeg P, Erard M, Haraldsen G, Girard JP (2003) Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol 163:69–79
Coyle AJ, Lloyd C, Tian J, Nguyen T, Erikkson C, Wang L, Ottoson P, Persson P, Delaney T, Lehar S, Lin S, Poisson L, Meisel C, Kamradt T, Bjerke T, Levinson D, Gutierrez-Ramos JC (1999) Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med 190:895–902
Paul WE, Zhu J (2010) How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol 10:225–235
Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA (2005) IL-33, an interleukin-1 like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23:479–490
Lam EPS, Kariyawasam HH, Rana BMJ, Durham SR, McKenzie ANJ, Powell N, Orban N, Lennartz-Walker M, Hopkins C, Ying S, Rimmer J, Lund VJ, Cousins DJ, Till SJ (2016) IL-25/IL-33–responsive TH2 cells characterize nasal polyps with a default TH17 signature in nasal mucosa. J Allergy Clin Immunol 137:1514–1524
Suzukawa M, Iikura M, Koketsu R, Nagase H, Tamura C, Komiya A, Nakae S, Matsushima K, Ohta K, Yamamoto K, Yamaguchi M (2008) An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J Immunol 181:5981–5989
Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, Georgalas C, Goossens H, Harvey R, Hellings P, Hopkins C, Jones N, Joos G, Kalogjera L, Kern B, Kowalski M, Price D, Riechelmann H, Schlosser R, Senior B, Thomas M, Toskala E, Voegels R, de Wang Y, Wormald PJ (2012) EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 50(1):1–12
Lund VJ, Mackay IS (1993) Staging in rhinosinusitis. Rhinology 31:183–184
Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge C, Bachert C (2006) Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 61:1280–1289
Kern Robert C, Conley David B, Walsh William, Chandra Rakesh, Kato Atsushi, Tripathi-Peters Anju, Grammer Leslie C, Schleimer Robert P (2008) Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol 22:549–559
Sachse F, Becker K, von Eiff C, Metze D, Rudack C (2010) Staphylococcus aureus invades the epithelium in nasal polyposi and induces IL-6 in nasal epithelial cells in vitro. Allergy 65:1430–1437
Lam M, Hull L, Imrie A, Snidvongs K, Chin D, Pratt E, Kalish L, Sacks R, Earls P, Sewell W, Harvey RJ (2015) Interleukin-25 and interleukin-33 as mediators of eosinophilic inflammation in chronic rhinosinusitis. Am J Rhinol Allergy 29(3):175–181
Baba S, Kondo K, Kanaya K, Suzukawa K, Ushio M, Urata S, Asakage T, Kakigi A, Suzukawa M, Ohta K, Yamasoba T (2014) Expression of IL-33 and its Receptor ST2 in Chronic Rhinosinusitis With Nasal Polyps. Laryngoscope 124:115–122
Hu XD, Bao YY, Zhou SH, Yao HT, Mao JY, Ji XX, Wu XH (2013) Interleukin-17A expression in patients with chronic rhinosinusitis and its relationship with clinical features. J Int Med Res 41:777–784
Shen Y, Pan CK, Tang XY, Yang YC, Ke X, Kou W, Wang XQ, Hong SL (2011) Significance of interleukin-17A in patients with nasal polyposis. Asian Pac J Allergy Immunol 29:169–175
Lam M, Hull L, McLachlan R, Snidvongs K, Chin D, Pratt E, Kalish L, Sacks R, Earls P, Sewell W, Harvey RJ (2013) Clinical severity and epithelial endotypes in chronic rhinosinusitis. Int Forum Allergy Rhinol 3:121–128
Ohno T, Morita H, Arae K, Matsumoto K, Nakae S (2012) Interleukin-33 in allergy. Allergy 67:1203–1214
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any conflict of interest, financial, or otherwise.
Rights and permissions
About this article
Cite this article
Ozturan, A., Eyigor, H., Eyigor, M. et al. The role of IL-25 and IL-33 in chronic rhinosinusitis with or without nasal polyps. Eur Arch Otorhinolaryngol 274, 283–288 (2017). https://doi.org/10.1007/s00405-016-4260-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-016-4260-6