Abstract
Efinaconazole 10 % solution is a new triazole antifungal agent developed for the topical treatment of fungal infections of the nails. The current study examined the effect of intratympanic application of efinaconazole 10 % solution in the guinea pig ear. Sixteen male Hartley guinea pigs (weight 501–620 g) were divided into 3 groups to be treated with efinaconazole 10 % solution, gentamicin (50 mg/mL), or saline solution. Topical solutions of 0.2 mL were applied through a small hole made at the tympanic bulla once daily for 7 consecutive days. Post-intervention auditory brainstem responses were obtained 7 days after the last treatment. The extent of middle ear damage and hair cell loss was investigated. The efinaconazole- and gentamicin-treated groups showed severe deterioration in auditory brainstem response threshold. Middle ear examination revealed extensive changes in the efinaconazole-treated group and medium changes in the gentamicin-treated group. Hair cells were preserved in the efinaconazole- and saline-treated groups, but severe damage was seen in the gentamicin group. In conclusion, efinaconazole 10 % solution applied intratympanically to the guinea pig middle ear caused significant middle ear inflammation and hearing impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Otomycosis is defined as fungal infection of the external auditory canal and the middle ear [1]. Otomycosis is not a rare disease, because unnecessary use of antibiotic eardrops for the treatment of otitis media and otitis externa has increased the prevalence of otomycosis [2]. The most common fungal species isolated from temperate climates are Candida and Aspergillus. Treatment of otomycosis is not simple. First-line treatment involves cleaning of the external ear canal and application of topical antifungal agents. In cases where fungal infection persists, other topical medicines such as gentian violet and acetic acid/propylene glycol drops are used [2]. Propylene glycol and gentian violet are potent antifungal agents, but both have been shown to be ototoxic [3, 4].
Tympanic membrane perforation is a common condition. In such circumstances, topically applied eardrops can enter the middle ear and may reach the cochlea by diffusion through the round window. Oral antifungals are useful, but systemic adverse events may arise. Given the risk of ototoxicity from topically applied eardrops when treating ear infections concurrent with tympanic membrane perforation, potent but non-ototoxic topical antifungal eardrops have been long awaited.
Efinaconazole 10 % topical solution is a new topical antifungal agent with unique physicochemical properties, potent antifungal activity, and a low surface tension formulation, all of which are considered instrumental in enhancing penetration and achieving clinical success in the treatment of onychomycosis, a fungal infection of the nails [5]. Efinaconazole 10 % solution has been on the market since late 2013 as Jublia® (Valeant Pharmaceuticals, Quebec, Canada) or Clenafin® (Kaken Pharmaceutical, Tokyo, Japan). Efinaconazole 10 % solution is prepared specifically for topical application on the nail, which is thick and hard. Strong permeability and potency of efinaconazole solution might be harmful to the middle and inner ear, which are sensitive and delicate. This study was designed to examine the effects of intratympanic efinaconazole 10 % solution in the guinea pig.
Materials and methods
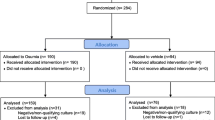
A total of 16 male Hartley guinea pigs, weighing between 501 and 620 g, were anesthetized by intraperitoneal injection of 40 mg/kg ketamine and 10 mg/kg xylazine for auditory brainstem response (ABR) audiometry measurement. The animal care and ethics committee of university approved all procedures.
Auditory brainstem response (ABR) audiometry
All auditory tests were conducted with the anesthetized animal placed in an electromagnetically shielded room. ABRs were measured with a real-time signal processing system (Tucker-Davis Technologies, Alachua, FL) following the previous reports [6, 7]. In short, calibrated tone bursts (duration, 1 ms; rise-fall time, 0.1 ms; phase change, 0°) were synthesized with SigGenRP software using a TDT RP2.1 real-time processor (Tucker-Davis Technologies) and presented at 10 bursts/s at frequencies of 4, 8, 16, and 32 kHz. Total of 1000 tone bursts at each tested frequency/amplitude combination was averaged. The threshold was established as the response at 5 dB above this final level [8].
Intratympanic application of drugs
After ABRs were obtained, the mastoid bulla was exposed with a retroauricular incision. A small hole was made in the mastoid bulla with a 1-mm diamond burr [7]. A 23-gauge needle was used to inject 200 μL of 50 mg/mL gentamicin solution (n = 4), 10 % efinaconazole (n = 7), or 0.9 % saline (n = 5) into the mastoid bulla. We chose 10 % as the concentration of efinaconazole because the commercially available efinaconazole solution is 10 % only, and it is used without dilution for the nail. The gentamicin solution (G1397, Sigma, St. Louis, MI) and efinaconazole 10 % solution (Clenafin®, Kaken Pharmaceutical, Tokyo, Japan) was directly used without dilution. Intratympanic injections were performed once a day for 7 days without anesthesia. This small hole kept patent for the 7-day injections in all animals. Post-intervention ABRs were obtained 7 days after the last treatment.
Middle ear scoring
After post-intervention ABRs were obtained, animals were deeply anesthetized and killed by transcardial perfusion with phosphate buffered saline (PBS) (137 mM NaCl, 10.1 mM Na2HPO4, 1.8 mM KH2PO4, 2.7 mM KCl, pH 7.4) followed by PBS with 4 % paraformaldehyde. The temporal bones were obtained and examined as in previous experiment [7]. The extent of damage to the middle ear was scored following the system proposed by Pawlowski et al. [9]. To determine the severity of damage, the state of the middle ear was analyzed by two examiners (K. N. and Y. H.) by blinded manner. Scores were reported from 1 (no damage) to 5 (severe and profound damage) for mucosal thickening, hyperemia, hematoma, and mucosal adhesion.
Tissue processing and hair cell counting
After middle ear scoring, the cochleae were perfused and fixed in 4 % paraformaldehyde in PBS at 4 °C for 7 days. Cochleae were decalcified in 10 % ethylenediaminetetraacetic acid for 14 days. The basal turn of the organ of Corti was isolated by microdissection and washed with PBS-TX (137 mM NaCl, 10.1 mM Na2HPO4, 1.8 mM KH2PO4, 2.7 mM KCl, pH 7.4, 0.3 % Triton X-100) for 10 min. The tissue was then incubated for 20 min with rhodamine phalloidin (1:40, #R415; Molecular Probes, Eugene, OR) in PBS-TX, washed again in PBS-TX, and mounted on coverslips. Tissues were observed under a fluorescence microscope (BZ-9000; Keyence, Osaka, Japan), and hair cells of the basal turn of the organ of Corti were counted [6].
Statistical analysis
Values are shown as mean ± standard deviation (SD). Differences in ABR thresholds, middle ear damage score, and hair cell counts among the 3 groups were tested using the Kruskal–Wallis rank test and the Scheffé post hoc test. Values of P < .05 were considered statistically significant. All statistical analyses were performed using the Stata/MP version 11.1 statistical software (StataCorp LP, College Station, TX).
Results
A total of 16 animals completed the study. Figure 1 shows ABR thresholds before intervention. No significant difference in hearing level was found among the three groups. Figure 2 shows thresholds in each group 7 days after the last treatment. Post-intervention ABR threshold showed significant differences at all frequencies with Kruskal–Wallis test. Both the gentamicin and efinaconazole groups showed significant differences from the saline group at 4, 16, and 32 kHz. No significant differences between the gentamicin and efinaconazole groups were seen at any frequency.
Pathological analysis performed 7 days after the last treatment revealed mild middle ear changes in the gentamicin group and severe middle ear changes in the efinaconazole group (Fig. 3). The middle ear was unaffected in the saline group. Mucosal thickening was seen in the gentamicin and efinaconazole groups (Fig. 4). Hematoma was only seen in the gentamicin group. Quantification of changes found that mean (SD) cumulative scores for middle ear damage were 5.0 (1.0) for the saline group, 8.8 (2.8) for the gentamicin group, and 142.1 (2.1) for the efinaconazole group. Cumulative scores showed significant differences (Kruskal–Wallis test; P = .005 and χ 2 test = 10.794). Scores were significantly worse for the efinaconazole group than for the saline (P < .001).
Loss of outer hair cells was minimal in the saline group and mild in the efinaconazole group (Fig. 5a, b). With gentamicin treatment, profound hair cell loss was observed (Fig. 5c). Although hair cell loss was more frequent in the efinaconazole group than in the saline group, no significant difference was seen (Fig. 6).
Discussion
ABR thresholds showed significant deteriorations after efinaconazole and gentamicin treatments. ABR thresholds were intact in the saline group. The result shows that efinaconazole and gentamicin applied intratympanically at the original concentration caused significant hearing impairment. Examination of middle ear mucosa found severe middle ear inflammation in the efinaconazole group (Fig. 3). Total damage score was worse in the efinaconazole group than in the gentamicin group. In particular, mucosal thickening was prominently severe in the efinaconazole group (Fig. 4). Hair cell loss was observed in the efinaconazole group, but was not statistically significant (Figs. 5, 6).
Otomycosis is a relatively common fungal infection of the external ear canal, with a reported prevalence of 9–30.4 % in patients presenting with symptoms of otitis or inflammatory conditions of the ear [1, 10, 11]. Otomycosis is most commonly associated with fungi of the genus Candida (60 %), followed by Aspergillus (40 %) [10]. Potent and safe ototopical antifungal agents are needed, because the incidence of otomycosis is increasing with the overuse of ototopical antibiotics [2]. Other predisposing factors are humid climate, impaired immune system, open mastoid cavities, hearing aids, and autoinoculation of the ear canal by patients suffering from fungal skin infections [12]. Many topical antifungal agents are commercially available and used for otomycosis. The most commonly used drugs are clotrimazole and ketoconazole, offering efficacy rates over 80 % [1]. Not all topical antifungal drugs are safe when applied to the middle ear. Clotrimazole, miconazole, nystatin, tolnaftate, fluconazole, and ketoconazole are non-ototoxic, but acetic acid, cresylate, Locacorten®-Vioform®, and gentian violet are all ototoxic [4, 12].
Onychomycosis, infection of the nail unit, has not been curable using topical antifungal agents, but new topical antifungal agents effective against onychomycosis have recently become commercially available. Topical efinaconazole 10 % solution has shown relatively comparable efficacy to rates reported for oral itraconazole against mild-to-moderate onychomycosis [13]. Physicochemical properties, antifungal activity, and the nature of the vehicle formulation are all believed to contribute to the therapeutic outcome in onychomycosis patients. Efinaconazole 10 % solution is a clear solution containing ethanol, lipophilic esters, and cyclomethicone. These ingredients help create a low surface tension optimal for application to the dry nail plate surface, lateral and proximal nail folds, hyponychium, and any undersurface of the nail plate accessible due to distal onycholysis [13]. Efinaconazole 10 % solution did not cause contact sensitization and induced only minimal skin irritation [14]. We thought use of such a potent topical antifungal agent in the ear might be greatly beneficial to many patients suffering from intractable otomycosis.
The antifungal activity of efinaconazole is mediated through inhibition of ergosterol biosynthesis, resulting in depletion of ergosterol and accumulation of lanosterol, which in turn compromises the structure and function of fungal cell membranes [15]. Efinaconazole is active against a broad spectrum of pathogenic fungi, including Candida and Aspergillus, the most common pathogens causing otomycosis [16]. Topical application of efinaconazole 10 % solution showed very low concentration of efinaconazole and its metabolite H3 in blood [17].
Efinaconazole, fluconazole, itraconazole, and voriconazole are triazoles. Triazoles are insoluble in water, so organic solvent is necessary to dissolve efinaconazole. The formulations for the marketed drugs Jublia® and Clenafin® contain ethanol, butylated hydroxytoluene, C12–15 alkyl lactate, citric acid, cyclomethicone, diisopropyl adipate, disodium edetate, and purified water in addition to efinaconazole [15]. Ethanol shows ototoxicity against both the middle and inner ear. Injection of 70 % ethanol through the ventilation tube for 5 days showed middle ear, vestibular, and cochlear ototoxicity [18]. We showed ototoxicity of 70 % ethanol-based antifog solution [7]. Various studies showed that higher concentrations of ethanol cause more pronounced effects [19, 20].
Propylene glycol is one of the major ingredients of antifungal drugs and shown to be ototoxic [3]. Efinaconazole 10 % solution does not contain propylene glycol. Ototoxicity of butylated hydroxytoluene, C12–15 alkyl lactate, citric acid, cyclomethicone, diisopropyl adipate, and disodium edetate has not been studied. Butylated hydroxytoluene is an antioxidant and common food preservative. C12–15 alkyl lactate, cyclomethicone, and diisopropyl adipate are emollients and, in certain circumstances, useful solvents. Citric acid is a weak organic acid and natural preservative. Disodium edetate is a chelating agent. Polyethylene glycol 400 is used in medications as a solvent and known to be non-ototoxic [3]. If efinaconazole is soluble and stable in polyethylene glycol 400, it is worthwhile to test its ototoxicity in animal models.
Hair cell damage was investigated with the surface preparations. Sporadic hair cell loss was observed at the basal turn in the efinaconazole group (Fig. 5b). No significant difference in hair cell loss was evident between the efinaconazole and saline groups (Fig. 6). Although we could not obtain enough samples from mid and apical turn of the cochlea for statistical analysis, similar pattern was observed. The mechanism of hearing impairment caused by topical efinaconazole 10 % solution seems more likely to depend on conductive hearing impairment due to middle ear inflammation than sensorineural hearing loss related to hair cell damage. There is a possibility that efinaconazole could not pass through the round window membrane into the inner ear because of severe middle ear inflammation. To examine permeability of efinaconazole into the inner ear, concentration of efinaconazole in perilymph should be measured in the future. Since most of the damage was secondary to middle ear issues, the effect might be transient and reversible over time.
Efinaconazole 10 % solution is prepared specifically for topical application on the nail. Efinaconazole infiltrates the dry nail plate surface, lateral and proximal nail folds, hyponychium, and any undersurface of the nail plate accessible due to distal onycholysis [14]. Compared to nail, the middle ear mucosa is much thinner and more delicate. Use of efinaconazole as a topical eardrop would require development of an efinaconazole preparation with optimal concentration and non-ototoxic solvent.
In conclusion, we found that efinaconazole 10 % solution is harmful in a guinea pig animal model, resulting in damage mainly to the middle ear.
References
Munguia R, Daniel SJ (2008) Ototopical antifungals and otomycosis: a review. Int J Pediatr Otorhinolaryngol 72:453–459. doi:10.1016/j.ijporl.2007.12.005
Jackman A, Ward R, April M, Bent J (2005) Topical antibiotic induced otomycosis. Int J Pediatr Otorhinolaryngol 69:857–860. doi:10.1016/j.ijporl.2005.01.022
Marsh RR, Tom LW (1989) Ototoxicity of antimycotics. Otolaryngol Head Neck Surg 100:134–136
Tom LW (2000) Ototoxicity of common topical antimycotic preparations. Laryngoscope 110:509–516. doi:10.1097/00005537-200004000-00003
Zeichner JA, Stein Gold L, Korotzer A (2014) Penetration of ((14)C)-efinaconazole topical solution, 10 %, does not appear to be influenced by nail polish. J Clin Aesthet Dermatol 7:34–36
Oshima H, Nomura K, Yamazaki M et al (2014) Ototoxic effect of daptomycin applied to the guinea pig middle ear. Acta Otolaryngol 134:679–683. doi:10.3109/00016489.2014.898186
Nomura K, Oshima H, Yamauchi D et al (2014) Ototoxic effect of ultrastop antifog solution applied to the guinea pig middle ear. Otolaryngol Head Neck Surg 151:840–844. doi:10.1177/0194599814545749
Zettel ML, Zhu X, O’Neill WE, Frisina RD (2007) Age-related decline in Kv3.1b expression in the mouse auditory brainstem correlates with functional deficits in the medial olivocochlear efferent system. J Assoc Res Otolaryngol 8:280–293. doi:10.1007/s10162-007-0075-x
Pawlowski KS, Si E, Wright CG et al (2010) Ototoxicity of topical azithromycin solutions in the guinea pig. Arch Otolaryngol Head Neck Surg 136:481–487. doi:10.1001/archoto.2010.54
Kurnatowski P, Filipiak A (2001) Otomycosis: prevalence, clinical symptoms, therapeutic procedure. Mycoses 44:472–479
Ho T, Vrabec JT, Yoo D, Coker NJ (2006) Otomycosis: clinical features and treatment implications. Otolaryngol Head Neck Surg 135:787–791. doi:10.1016/j.otohns.2006.07.008
Daniel SJ (2012) Topical treatment of chronic suppurative otitis media. Curr Infect Dis Rep 14:121–127. doi:10.1007/s11908-012-0246-8
Del Rosso JQ (2014) The role of topical antifungal therapy for onychomycosis and the emergence of newer agents. J Clin Aesthet Dermatol 7:10–18
Del Rosso JQ, Reece B, Smith K, Miller T (2013) Efinaconazole 10 % solution: a new topical treatment for onychomycosis: contact sensitization and skin irritation potential. J Clin Aesthet Dermatol 6:20–24
Jo W, Glynn M, Nejishima H et al (2014) Nonclinical safety assessment of efinaconazole solution (10 %) for onychomycosis treatment. Regul Toxicol Pharmacol 70:242–253. doi:10.1016/j.yrtph.2014.07.012
Jo Siu WJ, Tatsumi Y, Senda H et al (2013) Comparison of in vitro antifungal activities of efinaconazole and currently available antifungal agents against a variety of pathogenic fungi associated with onychomycosis. Antimicrob Agents Chemother 57:1610–1616. doi:10.1128/AAC.02056-12
Jarratt M, Siu WJ, Yamakawa E et al (2013) Safety and pharmacokinetics of efinaconazole 10 % solution in healthy volunteers and patients with severe onychomycosis. J Drugs Dermatol 12:1010–1016
Perez R, Freeman S, Sohmer H, Sichel JY (2000) Vestibular and cochlear ototoxicity of topical antiseptics assessed by evoked potentials. Laryngoscope 110:1522–1527. doi:10.1097/00005537-200009000-00021
Aktaş S, Basoglu MS, Aslan H et al (2013) Hearing loss effects of administering boric alcohol solution prepared with alcohol in various degrees on guinea pigs (an experimental study). Int J Pediatr Otorhinolaryngol 77:1465–1468. doi:10.1016/j.ijporl.2013.06.010
Morizono T, Sikora MA (1981) Ototoxicity of ethanol in the tympanic cleft in animals. Acta Otolaryngol 92:33–40
Conflict of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arakawa, K., Nomura, K., Oshima, H. et al. Effect of intratympanic application of efinaconazole 10 % solution in the guinea pig. Eur Arch Otorhinolaryngol 273, 1137–1142 (2016). https://doi.org/10.1007/s00405-015-3669-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-015-3669-7