Abstract
Acute otitis media and otitis media with effusion (OME) are the main causes of hearing impairment in children which require proper treatment, mainly antibiotic therapy. Patients whom were appropriate candidates for adenoidectomy were divided into two groups regarding the presence of middle ear effusion. Adenoid tissue specimens were cultured in both groups and the bacterial flora and anti-microbial resistance pattern were determined. 72 patients were studied, 42 % had OME while 58 % did not. The following bacteria were isolated and cultured from both groups with no meaningful difference in prevalence: Streptococcus viridans (p = 0.265), Staphylococcus aureus (p = 0.72), H. influenza (p = 0.806), Entrococcus. spp (0.391), Streptococcus pneumonia (p = 0.391), nonhemolytic Streptococcus (p = 0.230). Bacterial sensitivity was similar for Amoxicillin–clavulanate (p = 0.935), Amoxicillin (p = 0.935), Cephalexin (p = 0.806), Cefixime (p = 0.391) and Azithromycin in both groups. The two groups showed no meaningful difference considering the bacterial flora of nasopharynx and their sensitivity. Bacteria in both groups were sensitive to Amoxicillin and Amoxicillin–clavulanate and resistant to Azithromycin, Cefixime and Cephalexin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute otitis media (AOM) and otitis media with effusion (OME) are the two most common diseases in young children who are visited by primary care physicians [1]. OME is defined as the presence of effusion (fluid) in the middle ear without any inflammation [2]. Nowadays, this problem is considered as an infectious disease which is commonly seen in 2 to 4-year-old children and is less common after 6 years of age. It occurs more frequently in winter rather than spring or summer [2]. Among the complicated and not fully known etiologies, Eustachian tube dysfunction and bacterial endotoxins are the most widely accepted hypotheses.
Antibiotics were introduced as an inseparable part of OME treatment from the late 1970s to the early 1980s. This was the time interval in which several studies proved the inefficiency of anti-histamines and decongestants in this regard [3]. Although it was initially believed that such effusions are sterile, recent studies revealed that the samples taken from asymptomatic children with MEE contain certain bacteria [4].
Of note, not all patients with adenoid hypertrophy develop OME and not all OME patients have adenoid hypertrophy.
As AOM and OME are now considered as infectious diseases and as the only pathway for the pathogens to reach the middle ear cavity in the presence of an intact tympanic membrane is through the Eustachian tube, we aimed to evaluate and compare the aerobic microbial flora and their resistance to the most common antibiotics used in treating OME in patients with adenoid hypertrophy, with and without OME.
Regarding environmental and demographic factors which affect the frequency of bacterial colonization in the nasopharynx, in particular the changes in the bacterial resistance pattern which is an invaluable guide in empirical therapy, it is essential to identify the nasopharyngeal microbial pattern in each geographical region at certain intervals.
Methods
In this survey, we studied the association between nasopharyngeal microbial flora and their antibiogram and the presence of OME. Seventy-two children who were appropriate candidates for adenoidectomy were selected and divided into two groups, based on the presence or absence of OME. The two groups were matched for age and sex.
All children with the following criteria were excluded from the study: fever or any type of upper respiratory tract infection during surgery or in the past 4 weeks, antibiotic consumption (due to any cause) in the previous 4 weeks, any craniofacial, genetic, hereditary, metabolic or immunologic abnormalities, previous adenoidectomy or otologic surgery and any chronic diseases of the ears except for OME.
Before disinfecting the oral and nasal cavities with antiseptic solutions, a sterile Crow–Davis mouth gag was placed in the mouth and the adenoid tissue was almost totally removed in a single move by a sterile adenoid curette; the specimen was then sent to the microbiology lab under sterile conditions.
The specimens were studied for aerobic bacteria. Two smears which were taken from the superficial and deep parts of the sample underwent microscopic examination after Gram staining.
Then the obtained specimens were cultured on chocolate agar and blood agar media with 5 % sheep blood. After 48 h at 35° of aerobic incubation, the presence of aerobic bacteria was investigated. In case of any growth, proper tests were accomplished to identify the organism. The pattern of resistance in isolated bacteria was assessed according to the CLSI (Clinical and Laboratory Standards Institute) standardized methods [5]. MIC (mean inhibitory concentration) was measured for each antibiotic and the resistance or sensitivity level of the bacteria was determined.
The study protocol was approved by the Ethics Committee of Mashhad University of Medical Sciences and an informed consent was signed by each participant or his/her parent prior to study entrance. Statistical analysis was performed using the SPSS software, version 19.1.
Results
Seventy-two patients were studied; 47 % were male and 53 % female. Thirty (42 %) individuals had OME whereas 42 (58 %) did not. Among the 30 patients with OME, 18 (60 %) were male and 12 (40 %) female.
Based on Chi-Square test with a 95 % confidence interval, no meaningful relationship was found between OME and the patient’s sex (p = 0.194).
Among the 72 studied subject, 46 had the polymicrobial pattern of infection from which 20 (43 %) had OME and 26 (57 %) did not (Table 1).
Using Chi-Square test with a confidence interval of 95 %, no significant correlation was found between the presence of OME and the polymicrobial pattern (p = 0.769).
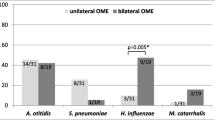
The most common bacteria in the patients with and without OME was streptococcus viridans; yet the statistical analysis showed no meaningful difference between the two groups regarding culture (p = 0.265).
Other bacteria isolated from the cultures in both groups are displayed in Table 2; no meaningful difference was diagnosed between two groups regarding the isolated pathogens.
In the OME group E. coli was isolated from six cultures which were significantly higher than the non-OME group (0.032); however, because it is considered as normal nasopharyngeal flora, it was also regarded as non-pathogen in our study.
The results obtained regarding the resistance and sensitivity of bacteria to the routine antibiotics used in managing OME, are summarized in Table 3.
According to Colmogrov–Smirnov test for MIC, streptococcus viridans did not have a normal distribution in the five antibiotic categories of the two groups; therefore Mann–Whitney test was applied showing no significant correlation between the two groups regarding MIC for this bacteria (p > 0.05 for all five antibiotics).
Discussion
OME is clinically defined as the presence of effusion in the middle ear with no inflammatory signs and symptoms [6]. The pathogenesis of OME is not completely understood. Classic literatures have shown that the effusions of OME result from chronic inflammation in response to residual bacterial degradation components. Nevertheless, the etiopathogenesis of OME appears to be much more complicated [7]. New increasing evidence suggests the major role of biofilms in the pathogenesis of OME [8–10]. Gastroesophageal reflux [11], poor clearance of middle ear fluids [12, 13], allergies [14–17] and finally genetic predisposition [18, 19] may also contribute.
A bacterial organism is identified in approximately one-third of middle ear effusions of children with OME who undergo myringotomy [20–24] and the pathogens are primarily those that are found in the nasopharyngeal biofilms.
Eustachian tube dysfunction seems an important factor in producing middle ear effusion; however, this problem could be completely mechanical or independent from respiratory tract infections [7]. Adenoids have been linked with the etipathogenesis of acute, recurrent, and chronic infectious diseases of the sinonasal and respiratory tract. They may cause mechanical obstruction of the nasopharynx and therefore Eustachian tube, or become a reservoir for resistant bacteria that can cause recurrent infections and permanent inflammation and greatly affect medical treatments [8].
We studied the relationship between OME and nasopharyngeal bacterial flora and the bacterial resistance pattern to antibiotics in 72 adenoidectomy candidates including 34 (47 %) males and 38 (53 %) females under the age of 15, out of which 42 % had OME. To the authors’ knowledge, this is the first study investigating MIC and subsequent bacterial resistance according to CLSI standards.
Evaluating the bacteria in the planktonic but not the biofilm form was the main limitation of the current study. However, the most common bacteria isolated from the specimens of both groups was Streptococcus viridans, followed by Staphylococcus aureus and H. influenza, respectively. No meaningful difference was found between the two groups (with and without OME) regarding the isolated pathogens. Thus, the presence or absence of OME should not affect the antibiotic selection strategy.
In an almost similar study by Ma Clay [25], adenoidectomy candidates whom were divided into two groups based on middle ear involvement were investigated for resistant bacteria in the adenoid tissue culture. They found such pathogens to be significantly more prevalent in patients with middle ear effusion.
Marchisio et al. [26] showed that microbial colonization of the nasopharynx and their resistance patterns are different in patients with OME compared to non-OME cases, which is in contrast to our findings. This difference may be due to geographical varieties or their different antibiotic therapy policy.
In another study by Almac et al. [27] with a larger study population (180 patients), superficial and deep aerobic and anaerobic flora of the adenoid tissue were studied in children with and without middle ear effusion. The authors drew the same conclusion regarding the relationship between OME and nasopharyngeal bacterial flora. This can be attributed to the similar geographical areas and demographical characteristics of the subjects. Linder et al. [28] also reached to the same conclusion.
Saafan et al. [29] conducted a study to determine the role of bacterial biofilm pathogenesis on developing OME. Using both culture and polymerase chain reaction (PCR) techniques, they found that adenoid samples from children with middle ear effusion had higher grade biofilm formation than those without effusion. However, interestingly the adenoid size was significantly lower in the OME group, indicating that bacterial colonization has a greater impact on OME pathogenesis than the size itself.
In general, OME resolves without medical intervention in the majority of patients [30–33]. Regarding the use of antibiotics in OME and based on a systematic review performed in 2012, it was stated that the available evidence does not support the routine use of antibiotics for children with OME [34]. A meta-analyses conducted in 1992 revealed that the clearance rate of OME could be improved during the first month of antibiotic therapy; however, frequent recurrences were observed while no further advantage was reported after the first month of receiving medication [35, 36].
The American Academy of Otolaryngology-Head Neck Surgery (AAOHNS), American Academy of Pediatrics (AAP), and American Academy of Family Physicians (AAFP) clinical practice guideline suggest a single 10 to 14-day course of antibiotics (typically amoxicillin) in certain cases [37]. Hence, the 2008 National Institute for Clinical Excellence (NICE) guidelines does not recommend the administration of antibiotics in the treatment of OME [37].
In the current study we examined five routine first line antibiotics including Amoxicillin, Amoxicillin–clavulanate, Cephalexin, Cefixime and Azithromycin in both groups. The results indicated almost similar sensitivity of pathogens of both groups to the first two antibiotics whereas resistance was observed to the latter three.
Khoramrooz et al. [38] also found Amoxicillin–clavulanate to be effective against the bacterial flora causing OME.
Conclusion
The current study showed that there is no fundamental difference between the bacterial flora of individuals with and without OME. In addition, Amoxicillin and Amoxicillin–clavulanate are still the preferred first line medications in the management of middle ear effusions.
References
Boston M, McCook J, Burke B, Derkay C (2003) Incidence of and risk factors for additional tympanostomy tube insertion in children. Arch Otolaryngol Head Neck Surg 129(3):293–296
Inglis AF, Gates GA (2005) Acute otitis media and otitismedia with effusion. In: Cummings CW, Flint PW, Harlier LA, et al. Cummings Otolaryngology-Head and Neck Surgery. 4th ed. Philadelphia: Mosby. Vol 4, pp 4446–7
Flint PW, Haughey BH, Lund VJ, Niparko JK, Richardson MA, Robbins K, et al (2013) Cummings otolaryngology head and neck surgery. 15th ed. Philadelphia: Mosby
Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, Hayes J et al (2006) Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 296(2):202–211
The European Committee on Anti microbial Susceotibility testing-eucast (2013) Available at: http://mic.eucast.org/Eucast2/SearchController/search.jsp?action=init. Accessed 10 Dec 2013
Seiden AM, Tami TA, Pensak ML, Cotton RT, Glucman JL (2001) Otolaryngology the essentials. 13th ed. USA: Thieme
Kubba H, Pearson JP, Birchall JP (2000) The aetiology of otitis media with effusion: a review. Clin Otolaryngol Allied Sci 25(3):181–194
Ehrlich GD, Veeh R, Wang X et al (2002) Mucosal biofilm formation on middle-ear mucosa in the chinchilla model of otitis media. JAMA 287(13):1710–1715
Hall-Stoodley L, Hu FZ, Gieseke A et al (2006) Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 296(2):202–211
Gok U, Bulut Y, Keles E, Yalcin S, Doymaz MZ (2001) Bacteriological and PCR analysis of clinical material aspirated from otitis media with effusions. Int J Pediatr Otorhinolaryngol 60(1):49–54
Miura MS, Mascaro M, Rosenfeld RM (2012) Association between otitis media and gastroesophageal reflux: a systematic review. Otolaryngol Head Neck Surg 146(3):345–352
Dodson KM, Cohen RS, Rubin BK (2012) Middle ear fluid characteristics in pediatric otitis media with effusion. Int J Pediatr Otorhinolaryngol 76(12):1806–1809
Inagaki M, Sakakura Y, Shimizu T, Majima Y, Ukai K (1988) Ultrastructure of mucous blanket in otitis media with effusion. Ann Otol Rhinol Laryngol 97(3 Pt 1):313–317
Alles R, Parikh A, Hawk L, Darby Y, Romero JN, Scadding G (2001) The prevalence of atopic disorders in children with chronic otitis media with effusion. Pediatr Allergy Immunol 12(2):102–106
Hurst DS, Venge P (2002) The impact of atopy on neutrophil activity in middle ear effusion from children and adults with chronic otitis media. Arch Otolaryngol Head Neck Surg 128(5):561–566
Kreiner-Møller E, Chawes BL, Caye-Thomasen P, Bønnelykke K, Bisgaard H (2012) Allergic rhinitis is associated with otitis media with effusion: a birth cohort study. Clin Exp Allergy 42(11):1615–1620
Martines F, Bentivegna D, Maira E, Sciacca V, Martines E (2011) Risk factors for otitis media with effusion: case-control study in Sicilian school children. Int J Pediatr Otorhinolaryngol 75(6):754–759
Rye MS, Warrington NM, Scaman ES et al (2012) Genome-wide association study to identify the genetic determinants of otitis media susceptibility in childhood. PLoS One 7(10):e48215
Hafrén L, Kentala E, Järvinen TM, Leinonen E, Onkamo P, Kere J, Mattila PS (2012) Genetic background and the risk of otitis media. Int J Pediatr Otorhinolaryngol 76(1):41–44
Bluestone CD, Stephenson JS, Martin LM (1992) Ten-year review of otitis media pathogens. Pediatr Infect Dis J 11(8 Suppl):S7–S11
Poetker DM, Lindstrom DR, Edmiston CE, Krepel CJ, Link TR, Kerschner JE (2005) Microbiology of middle ear effusions from 292 patients undergoing tympanostomy tube placement for middle ear disease. Int J Pediatr Otorhinolaryngol 69(6):799–804
Brook I, Yocum P, Shah K, Feldman B, Epstein S (2001) Microbiology of serous otitis media in children: correlation with age and length of effusion. Ann Otol Rhinol Laryngol 110(1):87–90
Post JC, Preston RA, Aul JJ et al (1995) Molecular analysis of bacterial pathogens in otitis media with effusion. JAMA 273(20):1598–1604
Rayner MG, Zhang Y, Gorry MC, Chen Y, Post JC, Ehrlich GD (1998) Evidence of bacterial metabolic activity in culture-negative otitis media with effusion. JAMA 279(4):296–299
Mc Clay JE (2000) Resistant bacteria in the adenoid. Arch Otolaryngol Head Neck Surg 126(5):625–629
Marchisio P, Claut L, Rognoni A, Esposito S, Passali D, Bellussi L et al (2003) Differences in nasopharyngeal bacterial flora in children with nonsevere recurrent acute otitis media and chronic otitis media with effusion: implications for management. Pediatr Infect Dis J 22(3):262–268
Almac A, Elicora SS, Yumuk Z, Dundar V, Willke A (2009) The relationship between chronic otitis media with effusion and surface and deep flora of hypertrophic adenoids. Int J Pediatr Otorhinolaryngol 73:1438–1440
Linder TE, Marder HP, Munzinger J (1997) Role of adenoids in the pathogenesis of otitis media: a bacteriologic and immunohistochemical analysis. Ann Otol Rhinol Laryngol 106(8):619–623
Saafan ME, Ibrahim WS, Tomoum MO (2013) Role of adenoid biofilm in chronic otitis media with effusion in children. Eur Arch Otorhinolaryngol 270(9):2417–2425
Tos M (1984) Epidemiology and natural history of secretory otitis. Am J Otol 5:459
Williamson IG, Dunleavey J, Bain J, Robinson D (1994) The natural history of otitis media with effusion—a 3-year study of the incidence and prevalence of abnormal tympanograms in four South West Hampshire infant and first schools. J Laryngol Otol 108:930
Rosenfeld RM, Kay D (2003) Natural history of untreated otitis media. Laryngoscope 113:1645
Teele DW, Klein JO, Rosner BA (1980) Epidemiology of otitis media in children. Ann Otol Rhinol Laryngol Suppl 89:5
van Zon A, van der Heijden GJ, van Dongen TM et al (2012) Antibiotics for otitis media with effusion in children. Cochrane Database Syst Rev 9:63
Rosenfeld RM, Post JC (1992) Meta-analysis of antibiotics for the treatment of otitis media with effusion. Otolaryngol Head Neck Surg 106:378
Williams RL, Chalmers TC, Stange KC et al (1993) Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. A meta-analytic attempt to resolve the brouhaha. JAMA 270:1344
American Academy of Family Physicians, American academy of otolaryngology-head and neck surgery, American academy of pediatrics subcommittee on otitis media with effusion (2004) Otitis media with effusion. Pediatrics 113:1412
Khoramrooz SS, Mirsalehian A, Emaneini M, Jabalameli F, Aligholi M, Saedi B et al (2012) Frequency of Alloicoccus otitidis, Streptococcus pneumoniae, Moraxella catarrhalis and Haemophilus influenzae in children with otitis media with effusion (OME) in Iranian patients. Auris Nasus Larynx 39(4):369–373
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nourizadeh, N., Ghazvini, K., Gharavi, V. et al. Evaluation of nasopharyngeal microbial flora and antibiogram and its relation to otitis media with effusion. Eur Arch Otorhinolaryngol 273, 859–863 (2016). https://doi.org/10.1007/s00405-015-3637-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-015-3637-2