Abstract
Otolith function in subjects with vitamin D deficiency/insufficiency is investigated through vestibular-evoked myogenic potentials (VEMP) and subjective visual vertical (SVV) testing. The study group included 62 patients with vitamin D deficiency/insufficiency (30 females, 32 males), with age range 24–56 years (40.6 ± 9.1). The control group included 44 healthy volunteers of similar age and gender distribution. The entire study group had: (1) serum level of 25-hydroxyvitamin D <30 ng/ml; (2) normal bone mineral density as indicated by dual-energy X-ray absorptiometry with T-score >−1; (3) normal middle ear function; (4) Age is ≤60 years. All subjects enrolled in the current study underwent audiovestibular evaluation consisting of pure-tone audiometry, immittancemetry, cervical VEMP (cVEMP), ocular VEMP (oVEMP), and SSV. The entire control group had normal cVEMP, two subjects had abnormal oVEMP. Thirty-three subjects (53 %) in the study group had abnormal oVEMP and 31 subjects (50 %) had abnormal cVEMP. Forty-one (66 %) had abnormal VEMP when abnormal VEMP was considered as either abnormal oVEMP or cVEMP. The entire control and study groups had normal SSV test results. Vitamin D deficiency may be associated with development of otolith dysfunction affecting both the utricle and saccule. This was suggested by the high prevalence of abnormal ocular vestibular-evoked myogenic potentials (oVEMP) and cervical vestibular-evoked myogenic potentials (cVEMP) in the study group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction and rationale
The last decade has witnessed a dramatic increase in both clinical and public awareness of the health implications associated with vitamin D status. Sun exposure in moderation is the major source of vitamin D for most humans; very few foods naturally contain vitamin D, and foods that are fortified with vitamin D are often inadequate to satisfy vitamin D requirements [1]. Middle East, including Kuwait where this study is performed, receives abundant sunlight all year round, yet vitamin D deficiency is endemic in the Middle East due to inadequate exposure to sunlight, either because of the dressing style or avoidance of the extremely hot sun. Moreover, vitamin D deficiency affects upwards a billion people all over the world [2].
According to serum level of 25-hydroxyvitamin D, vitamin D is considered sufficient (≥30 ng/ml), insufficient (20–29 ng/ml) or deficient (<20 ng/ml). Vitamin D deficiency/insufficiency precipitates and exacerbates osteopenia/osteoporosis in adults. It has also been associated with increased risk of common cancers, autoimmune diseases, hypertension, and infectious diseases [1]. Several studies emphasized the importance of vitamin D for normal audiovestibular function. Vitamin D receptor abnormalities in the mice were associated with hearing loss [3], and vestibular disorders [4]. Moreover, aural symptoms and fullness during pregnancy are thought to be due to vitamin D insufficiency [3]. Brookes [5] reported that vitamin D supplement therapy improved the hearing in two patients out of four patients with sensorineural hearing loss due to vitamin D deficiency. Recently, it was reported that low vitamin D levels were associated with development and recurrence of benign paroxysmal positional vertigo (BPPV); this was attributed to the possible disturbance in vestibular endolymph Ca2+ level resulting in formation of abnormal otoconia [6].
The authors of the present study hypothesized that if vitamin D deficiency would result in otoconial abnormalities in the utricle, and possibly the saccule that causes BPPV, then otolith dysfunction would be expected. Vestibular-evoked myogenic potentials (VEMP); both ocular VEMP (oVEMP) and cervical VEMP (cVEMP), and subjective visual vertical (SVV) are clinical tools that are widely used for otolith function assessment [7, 8]. The aim of this work is to study the VEMP and SVV test results in patients with vitamin D deficiency/insufficiency. This is the first study that clinically investigates otolith function in patients with vitamin D deficiency/insufficiency.
Materials and methods
The current study is a prospective cohort study at a tertiary medical center.
Subjects
This study was conducted between May 2012 and September 2013 in Hadiclinic hospital, Kuwait. The study group included 62 patients with vitamin D deficiency/insufficiency (30 females, 32 males), with age range 24–56 years (40.6 ± 9.1). The control group included 44 healthy volunteers (21 females, 23 males), with no neurotologic symptoms and age range of 23–57 years (38.6 ± 8.9). Informed consent was obtained from all subjects, and the study was approved by the Ethical Committee at Hadiclinic Hospital.
Methods
One hundred six subjects were recruited in the present study. They were selected among those performing serum level assessment of 25-hydroxy vitamin D either for routine checkup, which is very common in Kuwait due to the high prevalence of vitamin D deficiency, or due to non-otologic complaints, e.g., lethargy, bone aches. No subjects complaining of dizziness or auditory symptoms were included in the present study. The inclusion criteria of the present study were: (1) serum level of 25-hydroxy vitamin D <30 ng/ml for the study group and ≥30 for the controls; (2) normal bone mineral density (BMD) as indicated by dual-energy X-ray absorptiometry (DXA) with T-score >−1; (3) immittance test results indicating normal middle ear functions; bilateral type (A) tympanograms and intact acoustic reflex; (4) age is ≤60 years.
All subjects enrolled in the current study underwent complete clinical history and audiovestibular evaluation consisting of pure-tone audiometry using Madsen audiometer Orbiter 922, acoustic immittance testing using Madsen immittancemeter Zodiac 901, cVEMP and oVEMP testing using a Bio-Logic Navigator Pro system, ver 4.2, and subjective visual vertical test. None of the control or the study groups had supplementary therapy of vitamin D during the last year before inclusion in the study. The study and control groups were tested in overlapping manner by the same examiner and using same equipment.
VEMP testing technique
The order of testing, whether to start with oVEMP/cVEMP or right/left ear, was randomly determined for each subject. Stimuli used were 95 dBnHL; 500 Hz tone bursts of alternating polarity. They were presented through earphones at 5 Hz repetition rate with 1 ms rise/fall time, 2 ms plateau. The analysis window was 100 ms wide and responses to 200 stimuli were averaged. The amplitude of the first positive–negative peak, P13–N23 for cVEMP, and the first negative–positive peak, N1–P1 for oVEMP were recorded to detect abnormal VEMP response
Cervical VEMP
The cVEMP test was performed in the sitting position with the head rotated away from the stimulated side. Surface electromyographic activity was recorded with surface electrodes. The active electrode was placed on the middle third of the sternocleidomastoid muscles ipsilateral to the tested ear, with the reference electrode placed on the upper third of the sternum and the ground electrode on the forehead. A feedback method was used to enforce sternocleidomastoid muscle contraction, in which subjects pushed their jaw against a handheld inflated cuff to generate a specified cuff pressure; the electromyogram (EMG) signal was amplified and band pass filtered (30–1,500 Hz).
Ocular VEMP
The oVEMP test was performed in the sitting position, and the subject was instructed to look superomedially at a small fixed target one meter from the eyes. The visual angle was approximately 30° upward. Each subject’s eyes remained fixed on the target throughout the test. A five-minute rest period was given before testing the second ear to avoid the effect of extraocular muscle strain. The active electrodes were placed on the face, oriented vertically and approximately one cm below the center of the lower eyelid just inferior to the contralateral eye for sound stimulation. The reference electrode was placed about one cm below the active electrode on the cheek, and the ground electrode was placed on the forehead, and the EMG signal was amplified and band pass filtered (10–300 Hz).
Abnormal VEMP was defined as: (1) VEMP pattern of ‘no response’; which meant the absence of a meaningful waveform (2) VEMP asymmetry; interaural difference of VEMP amplitude exceeding the mean +2 SD of the control group. (3) Delayed peak latency exceeding the mean +2 SD of the control group.
Subjective visual vertical (SSV) testing
SVV was measured using a small rotatable luminous line in the upright body position in a completely darkened room. The patient was seated in a chair and the head and chin were fixed on a forehead-chin rest in an upright position 50 cm away from the SVV device. The length of the luminous straight line on a computer screen was 20 cm. After the luminous line was randomly tilted automatically, the patient was asked to rotate the bar to the position they perceived as vertical using a hand controller. By convention, the angular deviations of the virtual line were defined as positive if tilted clockwise and negative if tilted counterclockwise in relation to the real vertical. To minimize the learning effect, each subject performed five static SVV measures previous to the real assessment, which were not included in the results of this study, then six measures were performed and the final result was determined by the mean value of these measurements [9]. Based on the results with normal subjects in our center, the upper limit of the normal range of the SVV tilt was set as ±2.5°. SVV tilts outside the angel of −2.5° to +2.5° were determined to be pathological.
Statistical analysis
For statistical analysis, the values for control and the study groups were compared using Student’s t test, ANOVA and post hoc Bonferroni test to test the equality of different means. Chi-square test or Fisher’s exact test was used to compare the distribution of qualitative data. Pearson product-moment correlation coefficient was used to test the correlation between serum level of 25-hydroxyvitamin D and different VEMP parameters. Pearson correlation coefficient for two binary variables was used to test the correlation between coincidence of oVEMP and cVEMP in the study group. A “p” value of ≤0.05 indicated statistical significance. The computer program used was SPSS, release 18.0.
Results
Demographics and clinical features of the study group
There were no significant differences between the control and the study groups regarding both age and gender distribution; the study group had significant lower 25-hydroxyvitamin D level than the controls (P < 0.001, Table 1). Nine subjects (14 %) of the study group reported a past history of infrequent mild attacks of dizziness related to head movement and position during the last year before evaluation; one of them had a canalith repositioning maneuver 4 month before evaluation for treatment of BPPV. The other eight subjects did not seek medical examination as their dizziness was modest and had no impact on their work or leisure activities. The vestibular symptoms had abated in all of the nine subjects by the time of the study. No vestibular symptoms were reported in the control group. Eighteen subjects of the study group (29 %) were referred for vitamin D assessment because they complained of bone aches and six subjects (10 %) because they complained of lethargy, the rest of the subjects were doing routine checkup for their 25-hydroxyvitamin D level. Audiometric evaluation revealed that all subjects participating in this study either had normal hearing or hearing loss that coincides with the subject’s age. The pure-tone averages for the 0.5, 1, and 2 kHz of the study and control groups were 17.4 ± 8.8 and 20.2 ± 8.2, respectively. Student’s t test revealed this difference to be insignificant (p > 0.05).
VEMP testing
The entire control group had normal cVEMP, one subject had prolonged oVEMP in one ear and other subject had absent oVEMP in one ear. Twenty-three subjects (37 %) in the study group disclosed abnormal cVEMP and oVEMP. Ten subjects (16 %) had abnormal oVEMP and eight subjects (13 %) had abnormal cVEMP. Pearson correlation coefficient detected a strong relation between incidence of oVEMP and cVEMP abnormalities in the study group (r = 0.42). The correlation coefficient revealed moderate correlation between the latency of cVEMP and oVEMP (r = 3,215). Forty-one subjects (66 %) had abnormal VEMP when abnormal VEMP was considered as either abnormal oVEMP or cVEMP. Thirty-one (50 %) subjects in the study group had abnormal VEMP in both ears, while 10 subjects (16 %) had abnormal VEMP in one ear only. Table 2 shows the VEMP wave peak latencies, amplitudes, and amplitude asymmetries in the controls, vitamin D insufficiency and deficiency subgroups. The N10 and P13 peak latencies were significantly delayed in the study group compared to the controls (p < 0.05). No significant differences in VEMP parameters were detected between vitamin D deficiency and insufficiency subgroups.
-
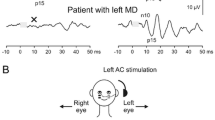
Out of the 248 VEMP traces registered in the study group (four VEMP were registered for each subject multiplied by 62 subjects), 105 tests (42 %) were abnormal; 53 abnormal oVEMP (13 absent response and 40 delayed wave peaks), and 52 abnormal cVEMP (nine absent response and 43 delayed wave peaks). Figure 1 shows the distribution of VEMP results in the ears of the control and the study groups. Fisher’s exact test disclosed significant higher prevalence of VEMP abnormalities in the study group compared to the control group (p < 0.001). Figure 2 shows an example for cVEMP registered in a subject of the study group. Figure 3 shows scatter plot of 25-hydroxy vitamin D level versus VEMP latencies (10/16).
Fig. 1 Distribution of VEMP results in the ears of the control and the study groups. The number in each box represents the number of ears showing the specified VEMP condition. The total number of the control column is 88 ears, and the study column is 124 ears. Fisher’s exact test disclosed significant higher prevalence of oVEMP and cVEMP abnormalities in the study group compared to the control group (p < 0.001), non-significant differences between the distribution of oVEMP and cVEMP were revealed using Chi-square test in the study group, and Fisher’s exact test in the control group (p > 0.05)
Fig. 2 -
No correlation was detected between the serum level of 25-hydroxyvitamin D and the VEMP peak latencies, amplitude or number of abnormal tests or the type of abnormal response (absent/delayed) in the study group.
C-SSV testing
The entire control and study groups had normal SSV test results. The mean ± SD was 0.4 ± 1.2° for the study group and 0.6 ± 1.4° for the control group (p > 0.05).
Discussion
The present study revealed that 66 % of subjects with vitamin D deficiency/insufficiency may have otolith function as suggested by their abnormal VEMP results. The otolith dysfunction is mostly induced through disturbance of calcium homeostasis in the inner ear. The calcium concentration of the endolymph (cochlea 23 μmol L−1, and vestibule 280 μmol L−1) is much lower than that of the perilymph (1,100 μmol L−1). The low Ca2+ level is essential for the normal transduction of sound, formation of otoconia and normal balance (4, 10). A Ca2+ absorption system in inner ear epithelial cells is present to maintain the low Ca2+ of vestibular endolymph. The Ca2+ absorptive system is comprised of an apical membrane entry pathway (the Ca2+ selective TRPV5/6 channel), a cytosolic Ca2+ buffer binding protein (calbindin-D9 K/D28 K), and basolateral Ca2+ exit pathways; Na–Ca exchangers (NCX1/2/3) and plasma membrane Ca2+-ATPases (PMCA1/2/3/4) [4]. Yamauchi et al. [4, 11] reported that 1,25-dihydroxyvitamin D3 plays an important role in the homeostasis of endolymph Ca2+; 1,25-dihydroxyvitamin D3 up-regulates the expression of TRVP5, calbindin-D9 K, calbindin-D28 K and NCX2 in the semicircular canal duct epithelium, while it shows small but significant downregulation of PMCA4. These findings suggest that vitamin D deficiency would result in elevation of Ca2+ concentration in the vestibular endolymph. Joeng et al. [6] reported that normal serum level of vitamin D is essential for development of normal otoconia through keeping the Ca2+ concentration in the vestibular endolymph at the normal critical level, as either low or high Ca2+ would result in abnormal otoconia. Two studies described pathological morphological changes of the otoconia in response to disorders causing abnormal Ca2+ metabolism with subsequent increase in vestibular endolymph Ca2+. Nakaya et al. [10] studied the endolymphatic Ca2+ level in knockout mice that were genetically engineered to show absent “pendrin” protein. Absence of pendrin resulted in acidification of the endolymph due to loss of secretion of HCO3−, which in turn resulted in consequent inhibition of TRPV5 and TRPV6 mediated Ca2+ absorption and increased endolymphatic Ca2+ level (2,600 μmol L−1 instead of the normal 280 μmol L−1). Interestingly, the otoconial crystals lost their relatively fine stony appearance and formed giant crystals in the utricles of knockout mice. Again, large otoconial crystals were reported by Vibert et al. in the utricles of osteopenic/osteoporotic mice. They explained the increased size of the otoconia by that: abnormal calcium metabolism may be associated with abnormal crystallization of the otoconia; this would activate a mechanism of control at the molecular level of the organic core of the otoconia by increasing the protein core to compensate the abnormal shell. Such morphometric changes of the utricular ultrastructure, by generating altered otoconia, might induce otolith dysfunction [12]. Though these studies detected morphological changes in the utricular otoconia, yet the similarities in utricular and saccular composition suggest that similar changes in the saccular otoconia may be present.
Vitamin D deficiency is considered an important predisposing factor of osteoporosis/osteopenia [13]. Osteoporosis/osteopenia is associated with morphological changes of the utricular otoconia, otolith dysfunction and repeated BPPV [12, 14]; the authors restricted inclusion in the present study to those with normal BMD, as evidenced by T-score higher than −1, to avoid any confounding effect of osteoporosis/osteopenia on VEMP or SVV test results.
VEMP and SVV are complementary used for assessing otolith function [7, 8]. On using Air conduction loud sound stimuli, the oVEMP is mostly reflecting utricular activity and the cVEMP would mostly reflect the saccular activity [15]. VEMP abnormalities may signify the degree of neuronal degeneration in the utricle and the saccule; mild lesions may be associated with delayed peak latencies, while more significant lesions would be associated with absent VEMP [16, 17]. The present study indicates that vitamin D deficiency may be associated with development of otolith dysfunction affecting both the utricle and saccule. This was evidenced by the high prevalence of abnormal oVEMP and cVEMP in the study group. Detailed analysis of the correlation between 25-hydroxyvitamin D levels and delayed VEMP peaks latencies detected ‘qualitative’ rather than ‘quantitative’ relation, i.e., there was an association between coincidence of both vitamin D insufficiency/deficiency and delayed VEMP peaks, but the degree of vitamin D deficiency was not necessarily related to the degree of VEMP peak delay. Vitamin D level and VEMP peak latency might have sigmoidal relationship; the vitamin D level versus VEMP peak latency scatter plot (Fig. 3) revealed a relation that is close to S-shaped curve; the well-known dose/response curve. The sigmoid curve suggests that there is a critical level of 25-hydroxyvitamin D level that is required for development of normal VEMP peak latencies, below such level substantial increase in VEMP latency occurs. 25-hydroxyvitamin D level may not be the only factor affecting the extent of VEMP abnormalities. Other contributing factors may include individual variation in otolith sensitivity to vitamin D deficiency among the population, rendering some subjects amenable for severe otolith dysfunction in spite of mild vitamin D deficiency and vice versa. Other suggested factors may be the duration and chronicity of the vitamin deficiency. The hazardous effect of severe vitamin D deficiency may be ameliorated by intermittent periods of sufficient vitamin supplementation either by oral intake or adequate sun exposure in vacations. However, establishment of the causal link between abnormal VEMP and vitamin D deficiency would require animal experiments studying the otolith composition in lab animal with vitamin D deficiency, and clinical trials that look for recovery of abnormal VEMP by vitamin D supplementation therapy.
SVV is effective in detecting unilateral and acute otolith dysfunction, but it gives normal results in chronic or bilateral lesions [14]. Cnyrim et al. [18] studied the recovery of abnormal SVV in patients with vestibular neuritis and Wallenberg’s syndrome, to study the development of central vestibular compensation in acute peripheral and acute central vestibular lesions. The study revealed a significant decay of SVV displacement in the course of time for the entire sample of patients. The daily decay rate was 5.4 % (95 % CI 3.6–7.1 %) for both peripheral and central vestibular lesions. SVV returned back to normal level in less than 30 days [18]. The normal SVV results in the present study, even in those with unilateral VEMP abnormalities, may indicate that the otolith dysfunction was not acute.
References
Holick MF, Chen TC (2008) Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 87(4):1080S–1086S
Al-Mohaimeed A, Khan N, Naeem Z, Al-Mogbel E (2012) Vitamin D status among women in middle east. J Health Sci 2(6):49–56
Zou J, Minasyan A, Keisala T, Zhang Y, Wang JH, Lou YR et al (2008) Progressive hearing loss in mice with a mutated vitamin D receptor gene. Audiol Neurootol 13(4):219–230
Yamauchi D, Nakaya K, Raveendran NN, Harbidge D, Singh R, Wangemann P et al (2010) Expression of epithelial calcium transport system in rat cochlea and vestibular labyrinth. BMC Physiol 10:1–12
Brookes GB (1983) Vitamin D deficiency: a new cause of cochlear deafness. J Laryngol Otol 97(5):405–420
Jeong SH, Kim JS, Shin JW, Kim S, Lee H, Lee AY, Kim JM, Jo H, Song J, Ghim Y (2013) Decreased serum vitamin D in idiopathic benign paroxysmal positional vertigo. J Neurol 260(3):832–838
Nagai N, Ogawa Y, Hagiwara A, Otsuka K, Inagaki T, Shimizu S et al (2014) Ocular vestibular evoked myogenic potentials induced by bone-conducted vibration in patients with unilateral inner ear disease. Acta Otolaryngol 134:151–158
Nechel C, Toupet M, Bodsona I (2001) The subjective visual vertical in otolith functions and disorders. In: Tran Ba Huy P, Toupet M (eds). Adv Otorhinolaryngol. Basel, Karger. (58): 77–87
Pavan TZ, Funabashi M, Carneiro JA, Pontelli TE, Tedeschi W, Colafêmina JF et al (2012) Software for subjective visual vertical assessment: an observational cross-sectional study. Braz J Otorhinolaryngol 78(5):51–58
Nakaya K, Harbidge DG, Wangemann P, Schultz BD, Green ED, Wall SM et al (2007) Lack of pendrin HCO3- transport elevates vestibular endolymphatic [Ca2+] by inhibition of acid-sensitive TRPV5 and TRPV6 channels. Am J Physiol Renal Physiol 292(5):F1314–F1321
Yamauchi D, Raveendran NN, Pondugula SR, Kampalli SB, Sanneman JD, Harbidge DG et al (2005) Vitamin D upregulates expression of ECaC1 mRNA in semicircular canal. Biochem Biophys Res Commun 331(4):1353–1357
Vibert D, Sans A, Kompis M, Travo C, Muhlbauer RC, Tschudi I, Boukhaddaoui H, Häusler R (2008) Ultrastructural changes in otoconia of osteoporotic rats. Audiol Neurootol 13(5):293–301
Francis J, Bonner J, Grabois M, Shipp K, Lindsay J, Gold D (2003) Health professional’s guide to rehabilitation of the patient with osteoporosis. Osteoporos Int 14(Suppl 2):S1–S22
Jang YS, Kang MK (2009) Relationship between bone mineral density and clinical features in women with idiopathic benign paroxysmal positional vertigo. Otol Neurotol 30:95–100
Curthoys IS, Iwasaki S, Chihara Y, Ushio M, McGarvie LA, Burgess AM (2011) The ocular vestibular-evoked myogenic potential to air-conducted sound; probable superior vestibular nerve origin. Clin Neurophysiol 122:611–616
Eryaman E, Oz I, Ozker B, Erbek S, Erbek S (2012) Evaluation of vestibular evoked myogenic potentials during benign paroxysmal positional vertigo attacks; neuroepithelial degeneration? B-ENT 8(4):247–250
Talaat HS, Metwaly MA, Abdelrouf H, Khafagy A, Isak H (2013) Vestibular evoked myogenic potentials in idiopathic posterior canal benign paroxysmal positional vertigo. Hear Balance Commun 11(4):176–181
Cnyrim C, Rettinger N, Mansmann U, Brandt T, Strupp M (2007) Central compensation of deviated subjective visual vertical in Wallenberg’s syndrome. J Neurol Neurosurg Psychiatry 78:527–528
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanyelbhaa, H., Sanyelbhaa, A. Vestibular-evoked myogenic potentials and subjective visual vertical testing in patients with vitamin D deficiency/insufficiency. Eur Arch Otorhinolaryngol 272, 3233–3239 (2015). https://doi.org/10.1007/s00405-014-3395-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-014-3395-6