Abstract
A randomized placebo-controlled study has demonstrated no effect of prednisolone in customary dosage on idiopathic sudden sensorineural hearing loss (ISSNHL). The aim of the present paper is to analyse a larger patient group by meta-analysis of data from the RCT together with a corresponding material drawn from the Swedish national database for ISSNHL. Data from 192 patients, 18–80 years with ISSNHL, were available. All had an acute hearing loss of at least 30 dB measured as PTA in the three most affected contiguous frequencies. All patients had been enrolled within one week after onset and evaluated by audiograms after 3 months. 45/99 (RCT) and 54/99 (the database) had been treated with prednisolone in tapering doses from 60 mg daily and 42/93 with placebo (RCT) or 51/93 with no treatment (the database). Primary outcome was the mean hearing improvement on day 90 for the different groups. A mean difference of >10 dB improvement was required to demonstrate a treatment effect for prednisolone compared to placebo/no treatment. No significant difference was seen between the prednisolone group and placebo/no treatment (p = 0.06). Total recovery was 38 % in prednisolone group, 40 % in the placebo and 14 % in the no treatment group. Vertigo at the onset of hearing loss and age at onset had an equal negative prognostic value in all groups and signs of inflammation had a positive effect. Prednisolone in customary dosage does not influence recovery after ISSNHL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last 20 years, corticosteroids have become, both nationally and internationally, the most common treatment for idiopathic sudden sensorineural hearing loss (ISSNHL); the oral dosage has successively been increased and i.v. treatment introduced, without scientific evidence of any better results. A recent Cochrane report 2013 still declares that the effect of corticosteroids on ISSNHL is yet to be proven [1].
Despite the fact that ISSNHL is still idiopathic, different treatment options have been introduced continuously for the past 80 years. All attempts to treat are based on one or another theory on the pathogenesis of the disease: the vascular theory, probably the oldest, is based on knowledge of the cochlear end-arterial vascular supply as described by Siebenmann [2] and Naybena [3]. Early on, treating physicians often prescribed bed rest, reasoning that vaso-constriction resulted in the hearing loss and might be caused by stress/sympathetic stimulation. Alternative treatments were blockage of the cervical sympathetic through surgery or by local application of anaesthetics [4]. In the 1970s, rheological treatment was introduced. For example Dextran® 40 was used as a hemodilutive and became the treatment of choice [5], often together with other vasoactive drugs. However, in the 1990s, the rheological theory was contradicted by a sufficiently large randomized, three-armed study in Switzerland by Probst et al. [6], comparing dextran therapy with pentoxyphyllin (vasodilator) and placebo (physiological saline), where no significant difference could be seen between the three arms. Although there was evidence that dextran was not better than placebo, this type of treatment is still part of the “shot gun therapy” in many countries.
During development of the antibiotic era after 1950, different antibiotics were tried since a few cases of sudden hearing loss were shown to have been caused by lues [7] and later on signs of borreliosis were found in other cases.
In the USA, a treatment policy based on using corticosteroids developed during the 1970s on the hypothesis that most ISSNHL cases had a viral/inflammatory cause [8]. Antiviral therapy has been tried after hearing loss was noted in connection both with herpes and other viruses. Thus far, no study has shown any effect of antiviral treatment [9, 10].
The important placebo-controlled study by Wilson et al. [11], where a positive effect seemed to be demonstrated by corticosteroids, has later been criticized in Cochrane reports as being of low methodological quality [1, 12]. Despite this, the Wilson study is still always cited as the grounds for using corticosteroid therapy. Several later investigations with corticosteroids have not been able to demonstrate any specific effect—only tendencies [13]. A sufficient number of patients are always required to show evidence of effect when studying a disease that, without treatment, has about a 30 % chance of total, and another 30 % chance of partial recovery [14–17].
In 2012, the authors published an RCT which demonstrated no significant effect by prednisolone on ISSNHL [18]. That RCT included a relatively low number of analysed patients. By adding data from those patients in the Swedish national database for ISSNHL, using the same inclusion criteria and the same treatment as in the RCT, our aim is to perform a small meta-analysis to strengthen the results. The ENT clinics in Sweden have had different treatment policies with respect to ISSNHL; at some large clinics corticosteroids have never been given and at other corticosteroids have for years been the rule.
A second aim is to evaluate whether medical treatment per se has an effect on outcome on ISSNHL by comparison of the placebo group from the RCT and the patients from the database with no medication.
Methods
Material
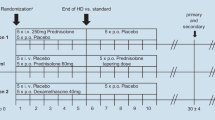
Between 2003 and 2008, 23 ENT centres in all parts of Sweden contributed to a database (the Swedish national database for ISSNHL) including data from 400 patients. Fourteen of those clinics also separately participated in a randomized triple-blind placebo-controlled trial on ISSNHL, during 2006–2010. For the present study, analyses of data from both the database and the RCT were used for all patients that fulfilled following criteria.
Age should be from 18 to 80 years with a sudden unilateral onset of sensorineural hearing loss developing within 24 h without any known aetiology (no earlier or present ear diseases). The average decrease in hearing threshold for the three most affected contiguous frequencies of the affected ear should be ≥30 dB HL. Enrollment and treatment had been started within seven days from onset. All patients had given informed consent to participate.
Exclusion criteria were the common medical reasons for not using corticosteroids: pregnancy, diabetes, chronic infections, peptic ulcer, uncompensated heart disease, recent surgery, or psychiatric disease. The patients’ ordinary medication for concomitant disease had been permitted. Vascular, antiviral or corticosteroid treatment with hypothetic effect on the hearing loss was not permitted.
Clinical examination
A case report form (CRF) had been collected for each participating patient. The patient CRF consisted of a questionnaire, audiograms, information on radiological investigations (MRI or CT), laboratory work-ups, brainstem response audiometry (BRA) and vestibular work-up (if such diagnostic examinations had been done) and information on adverse events and or serious adverse events. The questionnaire covered the time course of the onset of the hearing loss and associated symptoms such as tinnitus and vertigo, potential precipitating events preceding the SSNHL, the patient’s past medical history, medication for concomitant disease, hearing loss and associated symptoms before the SSNHL and family history of different diseases especially hearing loss. A pure tone audiogram, at the first visit and after 3 months, had been analysed.
Intervention
All the patients included in the analysis had either gotten treatment with prednisolone or placebo or no medication. The patients drawn from the database were only selected if they had received the same or equivalent treatment as in the RCT, which was prednisolone as 10 mg capsules given as a single dose of 60 mg daily for 3 days, thereafter reduced by 10 mg per day, with a total treatment time of at least eight days.
The control group from the RCT consisted of all patients who had been treated exactly as was the prednisolone-treated group, but with placebo and had audiograms both at the start and after 3 months. The control group from the database was those patients who had received no medication, neither prednisolone nor any other specific medication, but had been examined with audiograms both at the initial visit and after 3 months and fulfilled the inclusion criteria. These patients came mainly from clinics where no corticosteroid treatment was the rule.
Primary outcome measures
The effect of corticosteroid treatment is the primary endpoint and was to be evaluated by measuring and characterizing of the hearing loss before and after 3 months. The evaluation of the audiograms was based on changes of the hearing thresholds at the frequencies 125, 250, 500, 1k, 1.5k, 2k, 3k, 4k, and 6k Hz. The differences of the mean of the hearing thresholds at the three most affected contiguous frequencies characterized the hearing loss for four different frequency regions. The hearing loss was estimated in comparison with either an earlier audiogram or the unaffected ear. For details see Ref. [16].
The audiogram taken at enrolment and the audiogram taken 3 months later were compared to determine the degree of hearing improvement and hearing recovery (Table 1).
A mean difference in improvement of >10 dB between the whole prednisolone-treated group and the entire placebo/no medication group would be in favour of corticosteroid therapy when calculated from the hearing loss at the onset (the average decrease for the three most affected contiguous frequencies).
Secondary outcome measures
The prognostic value on the hearing loss of all items in the CRF (regardless of treatment) was regarded as “secondary outcome”.
Statistical analysis
To substantiate a treatment effect of >10 dB for a prednisolone group compared to placebo group, roughly 200 patients had been estimated to be included to give 99.6 % power in the previous RCT. Since that number was not possible to attain, meta-analysis was now used in an attempt to validate the previous results [18]. The analyses of primary and secondary endpoints were performed by comparisons of the initial audiogram and the audiogram taken at the follow-up after 90 days.
As had been planned from the beginning, multiple regression analysis was used with selected variables (age, heredity for hearing loss, vertigo, tinnitus, time from onset of SSNHL to first ENT visit, prescribed rest or sick leave, affected frequency regions) which were forced into the model together with prednisolone and controls. In the first step, interactions between treatment and some selected variables were studied. These were between treatment and age, treatment and time from onset of SSNHL to first ENT visit, treatment and affected frequency regions, and treatment and baseline pure tone average for the affected frequencies. This was to see if any of the selected interactions had a significant covariance with recovery. Interactions which were not significant were removed from further analyses.
The second step in the multiple regression analyses was to include variables using the stepwise forward method if p < 0.05, to see if any single variable had a significant covariance with recovery. Three dummy variables were created out of the four categories of frequency regions and used to indicate the absence or presence of some categorical effect that might be expected to shift the outcome. These three dummy variables were not tested individually in the stepwise forward process; the choice was between including none or all (partial F test).
The effect of prednisolone and placebo was tested with single-sided null hypothesis since we did not expect a deterioration of hearing due to the corticosteroids: H0 = the efficacy of prednisolone on recovery ≤10 dB, H1 = the efficacy of prednisolone on recovery >10 dB.
For all other variables, a double-sided null hypothesis was tested that the variable had no effect on recovery.
A value of p < 0.05 was used for all tests for statistical significance.
Results
The present meta-analysis consists of data from a total of 192 patients who all had been treated according to inclusion, 87/103 from the RCT and 105/400 patients out of the Swedish national database. 99 had received prednisolone and 93 placebo or no medication. Baseline characteristics data for the four groups are described in Table 2.
The hearing of each patient had been evaluated at the first visit at the ENT clinics, at the final follow-up, audiograms were missing for two patients in prednisolone group (RCT), one patient in the corticosteroid group from the Swedish database and two patients in the no-medication group from the Swedish national database. These five patients were excluded from further analyses.
Hearing improvement and recovery are shown in Table 3.
Total hearing recovery, meaning a difference of <10 dB between the audiogram taken before the disease and the audiogram taken after 3 months, was observed in 32 % of all patients (60/187). In the prednisolone group 38 % recovered completely, in the placebo group 40 %, and in the group without any treatment 14 % (Table 3).
Primary endpoint: effect of treatment
The null hypothesis was that the efficacy of prednisolone on recovery should be ≤10 dB. No significant difference in hearing recovery due to effect of treatment was observed between the prednisolone group and the placebo group plus the no-medication group, the estimated treatment efficacy was 6.10 dB (p = 0.886).
The recovery with respect to which part of the frequency region that was affected by the hearing loss is presented in Table 4.
Secondary endpoints: prognostic factors
Three variables were significantly related to outcome regardless of treatment or not, namely age at onset of hearing loss, presence of vertigo and abnormal findings of laboratory work-ups.
The patients’ age at baseline influenced the degree of hearing improvement: the effect was −0.35 dB, which means that the hearing improvement was 0.35 dB less per year of the patient’s age at the onset of disease (p = 0.003).
Presence of vertigo at the onset of ISSNHL was associated, regardless of treatment, with less hearing improvement. The effect of vertigo was −15.2 dB (p < 0.001).
115 of 187 patients had laboratory testing on blood samples. Of these, 37 had abnormal findings. These abnormal laboratory findings at the onset of the disease were associated with a better prognosis for hearing improvement irrespective of treatment or not. This effect was +11.0 dB (p = 0.009).
The majority of those with abnormal laboratory findings had signs of inflammation/infection (CRP >10 mg/L, erythrocyte sedimentation rate >20 mm, leukocyte count >10 × 109 mL/L, haemoglobin count (Hb) <120 g/L, thrombocyte count >150 × 109 mL/L, and/or positive Borrelia tests (IgG antibodies and IgM antibodies) with or without ongoing clinical infection) [17].
Comparison between the placebo and the no-medication group
No significant difference in hearing recovery was observed between the placebo group and no-medication group. The estimated placebo efficacy was 5.90 dB (p = 0.208).
The only two factors associated with less hearing improvement regardless of placebo treatment or no medication, was age at the initial contact (−0.33 dB, p = 0.041) and presence of vertigo (−23.5 dB, p = 0.000).
Discussion
The present analysis has been performed to clarify, with a sufficient power, whether corticosteroids do affect the clinical course of unilateral ISSNHL. The included number of patient data (187) was estimated to give a high precision in the results.
Almost 3,000 papers on ISSNHL have been published in the English literature during the last 100 years. Attempts to treat the hearing loss with a multitude of different drugs with either vascular/rheological or anti-inflammatory properties are described [5, 6, 9, 11]. Most authors are convinced that several different diseases can cause the symptom of hearing loss and might be included in the diagnosis ISSNHL. That can be a reason why no single treatment has given clear evidence of effect [19]. In Germany, an expert group has suggested that different shapes of the audiogram tells about where in the cochlea the damage may be situated and thereby explain the pathogenesis [20]: a low-frequency loss might regarded as a sign of hydrops, a mid-frequency loss could be a sign of vascular interference, a high-frequency hearing loss of up to 40 dB should be possible dysfunction of outer hair cells, and a hearing loss of more than 40 dB could be due to dysfunction/damage also to the inner hair cells. A flat loss can be considered as an interference with the vascular stria giving an acute hearing loss due to proceeding acute ionic balance disturbance. It would be of utmost interest to see if different treatment policies will develop according to this hypothesis, since so far only corticosteroids, also i.v. and in higher dosage, and/or rheological treatment are suggested [20]. This theoretical approach has its weakness that it does not fit with either experience from clinical praxis or investigations including the present study, which show that all hearing losses with different audiogram configuration seem to have a chance to spontaneous recovery. The recovery can either occur as quickly as the hearing loss came or more slowly within a couple of months [18].
The present data show that irrespective of treatment, a third of the patients recover completely, a half partially and only 16 % have no regression (Table 3). These data seem to be in accordance with Byl [15], who also stated that the long-term results seem to be independent of the given treatment.
The strength of the present attempt at a meta-analysis is that there are two different sets of patient material identically treated with corticosteroids, both with almost the same results showing no significant effect of corticosteroids. The weakness is the question of possible bias in the choice between using corticosteroids or not for the patients from the National database. Analysis of the non-treated patients showed that they came mostly from clinics where the treatment tradition was no treatment with corticosteroid, which should minimize the bias. However, looking at the database material for those who had received corticosteroid showed that some patients had, in spite of this, been given steroids at those non-treatment clinics. One can assume that patient pressure had been stronger than the clinics treatment policy. The question of bias is primarily restricted to these patients: are they different in any way from those who just follow the doctors’ recommendations? Even so, there were too few of these to have any influence on the final result.
Looking at “total recovery”, the no-medication group from the Swedish National Database seemed to recover completely more seldom than the other three groups. This may speak for an unspecific beneficial effect of treatment per se, the so-called placebo effect, and underlines the importance of investigating and giving care to all patients who develop an ISSNHL.
Corticosteroids have, in most countries, successively become the base for all treatment of ISSNHL since the report by Wilson [11] and the dosage has increased. Over the last ten years, i.v. administration of corticosteroids has become common, without scientific ground and local administration into the affected ear has more and more been the focus for research [21]. However, the theoretical basis is rather weak as to why and how the corticosteroids work other than that receptors have been found in the inner ear [22]. The local treatment has mostly been rescue medication after an initial oral corticosteroid treatment has failed [13]. Probably more basic research has to be done, with more studies of local administration of drugs directly to the inner ear before clinical trials of sufficient power can be a reality.
A positive prognostic sign for hearing recovery for both groups of patients in the present analysis, regardless of treatment or not, was to have abnormal laboratory data. These signs were within the category of inflammation such as a CRP >10, SR >20 or a high count of leucocytes. Since information about whether laboratory tests were taken was not part of either in the RCT nor requested for the Swedish National Database, these findings, although significant, could not be followed up on an individual basis. However, inflammation in relation to ISSNHL would be very interesting as a focus for new investigations.
Conclusion
With a sufficient number of patients, it has been possible to demonstrate that corticosteroids orally, in a high tapering dosage, do not have a significant effect in the treatment of ISSNHL. Is it time to change the focus of research to find new ways to treat ISSNHL?
References
Wei BPC, Stathopoulos D, O’Leary S (2013) Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst Rev (7):CD003998. doi:10.1002/14651858.CD003998.pub3
Siebenmann F (1894) Die Blutgejiisse im Lgbyrinthe des menschlichen Ohres. Wiesbaden
Naybena D (1923) A study in the comparative anatomy of the blood vascular system of the internal ear in mammalia and homo. Kioto Acta Scholae Med 6:1–13
Singleton GT (1971) Cervical sympathetic chain block in sudden deafness. Laryngoscope 81:734–736
Kellerhals B, Hippert F, Pfaltz CR (1971) Treatment of acute acoustic trauma with low molecular weight dextran. Pract Otorhinolaryngol (Basel) 33:260–264
Probst R, Tschopp K, Ludin E et al (1992) A randomized, double-blind, placebo-controlled study of dextran/pentoxifylline medication in acute acoustic trauma and sudden hearing loss. Acta Otolaryngol 112:435–443
Nadol JB Jr (1975) Hearing loss of acquired syphilis: diagnosis confirmed by incudectomy. Laryngoscope 85:1888–1897
Wilson WR, Veltri RW, Laird N, Sprinkle PM (1983) Viral and epidemiologic studies of idiopathic sudden hearing loss. Otolaryngol Head Neck Surg 91:653–658
Stokroos RJ, Albers FWJ, Tenvergert EM (1998) Antiviral treatment of Idiopathic Sudden Sensorineural Hearing Loss: a prospective, randomized, double-blind clinical trial. Acta Otolaryngol 118:488–495
Westerlaken BO, Stokroos RJ, Wit HP et al (2003) Treatment of idiopathic sudden sensorineural hearing loss with antiviral therapy: a prospective, randomized, double-blind clinical trial. Ann Otol Rhinol Laryngol 112:993–1000
Wilson WR, Byl FM, Laird N (1980) The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Arch Otolaryngol 106:772–776
Wei BPC, Mubiru S, O’Leary S (2009) Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst Rev
Lautermann J, Sudhoff H, Junker R (2005) Transtympanic corticoid therapy for acute profound hearing loss. Eur Arch Otorhinolaryngol 262:587–591
Mattox DE, Simmons FB (1977) Natural history of sudden sensorineural hearing loss. Ann Otol Rhinol Laryngol 86:463–480
Byl FM Jr (1984) Sudden hearing loss: eight years’ experience and suggested prognostic table. Laryngoscope 94:647–661
Nosrati-Zarenoe R, Arlinger S, Hultcrantz E (2007) Idiopathic sudden sensorineural hearing loss: results drawn from the Swedish national database. Acta Otolaryngol 127:1168–1175
Nosrati-Zarenoe R, Hansson M, Hultcrantz E (2010) Assessment of diagnostic approaches to idiopathic sudden sensorineural hearing loss and their influence on treatment and outcome. Acta Otolaryngol 130:384–391
Nosrati-Zarenoe R, Hultcrantz E (2012) Corticosteroid treatment of idiopathic sudden sensorineural hearing loss: randomized triple-blind placebo-controlled trial. Otol Neurotol 33:523–531
Conlin AE, Parnes LS (2007) Treatment of sudden sensorineural hearing loss II. A meta-analysis. Arch Otolaryngol Head Neck Surg 133:582–586
Arnold W, Brusis T, Canis M, Hesse G, Arolsen B, Klemm E, Löhler J et al (2014) Leitlinie der Dt. Ges. f. Hals-Nasen-Ohren-Heilkunde, Kopf- und Hals-Chirurgie. http://www.awmf.org/uploads/tx_szleitlinien/017-010l_S1_Hoersturz_2014-02.pdf
Seggas I, Koltsidopoulos P, Bibas A et al (2011) Intratympanic steroid therapy for sudden hearing loss: a review of the literature. Otol Neurotol 32:29–35
Meltser I, Tahera Y, Canlon B (2009) Glucocorticoid receptor and mitogen-activated protein kinase activity after restraint stress and acoustic trauma. J Neurotrauma 26:1835–1845
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hultcrantz, E., Nosrati-Zarenoe, R. Corticosteroid treatment of idiopathic sudden sensorineural hearing loss: analysis of an RCT and material drawn from the Swedish national database. Eur Arch Otorhinolaryngol 272, 3169–3175 (2015). https://doi.org/10.1007/s00405-014-3360-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-014-3360-4