Abstract
Purpose
The aim of this study was to examine whether there is a correlation between different types of ventricular septal defects (VSD) and chromosomal abnormalities in the low-risk setting of non-invasive prenatal testing (NIPT) and to evaluate the prognosis of fetuses with varying types of VSD.
Methods
Cases of pregnant women who underwent amniocentesis due to fetal VSD were collected by Tianjin Central Hospital of Obstetrics and Gynecology from May 2017 to May 2022. Exclusions were made for those without NIPT, with high-risk NIPT results, genetic disorders, and those lost to follow-up. Data collected included ultrasound classification of VSD, prenatal NIPT results, copy-number variations (CNVs) results, and neonatal outcomes.
Results
The prevalence of pathogenic CNVs was investigated in 74 cases of VSDs. Of these cases, 45 were isolated VSDs (9 muscular and 36 non-muscular) and 29 were non-isolated VSDs (10 with intracardiac and 19 with extra-cardiac structural anomalies). The results revealed that the incidence of pathogenic CNVs was lower in isolated VSDs compared to non-isolated VSDs in a low-risk NIPT condition (χ2 = 9.344, P = 0.002). There was no significant difference in the prevalence of pathogenic CNVs between VSDs with intracardiac and extra-cardiac structural anomalies (P = 0.541). Moreover, VSDs associated with intracardiac structural anomalies had the highest rate of surgical intervention.
Conclusion
When NIPT is low-risk and VSD is isolated, the likelihood of fetal chromosomal defects is not increased. However, if there are intra- or extra-cardiac structural abnormalities present alongside VSD, the possibility of pathogenic CNV is considerably greater, necessitating invasive prenatal diagnosis. Isolated muscular VSDs usually do not require surgery, which can be used as a basis for prenatal counseling regarding fetal VSD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
This study indicated that when NIPT is low-risk, an isolated VSD does not increase the probability of chromosomal abnormalities in the fetus; however, if the VSD is non-isolated and associated with either intra- or extracardiac structural issues or FGR, the risk of pathogenic CNV is significantly higher, thus recommending invasive prenatal diagnosis. Furthermore, it was observed that isolated muscular septal defects usually do not require surgical treatment, which provides a useful basis for prenatal counseling regarding fetal VSD. |
Introduction
Congenital heart disease (CHD) is a common birth defect that affects between 3 and 11 out of every 1000 live births globally. Among the various types of CHD, ventricular septal defect (VSD) is the most frequent, accounting for approximately 35% of all neonatal CHD cases. Unfortunately, those with VSD usually experience a lower survival rate than the general population [1]. VSD can occur as an individual anomaly or as part of more complex heart conditions such as tetralogy of Fallot, univentricular atrioventricular junction, transposition of the great arteries, and aortic constriction/interruption [2]. It has been reported that 20–40% of VSDs are associated with chromosomal abnormalities. Isolated VSDs may not significantly increase the risk of fetal chromosomal abnormalities in cases of low-risk prenatal screening. In contrast, the rate of chromosomal abnormalities tends to be higher in non-isolated VSDs, suggesting that invasive prenatal diagnosis should be offered in cases of non-isolated VSDs [2, 3]. The correlation between different types of VSD and chromosomal abnormalities in the Chinese population remains unclear. Non-invasive prenatal testing (NIPT) has advanced screening capabilities, improving the detection of trisomy 13, 18, and 21, as well as specific chromosomal microdeletion/microduplication syndromes [4], but it may not identify all chromosomal abnormalities in fetuses with VSD. This study seeks to explore the relationship between different subtypes of VSD and fetal chromosomal abnormalities in the context of low-risk NIPT, to reduce the potential for missed or false diagnoses and reduce the risk of fetal loss from invasive prenatal diagnosis in low-risk groups.
Materials and methods
Clinical data
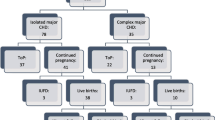
This study was conducted at Tianjin Central Hospital of Obstetrics and Gynecology focusing on pregnant women who underwent amniocentesis due to fetal VSD between May 2017 and May 2022. The study included women with either isolated or non-isolated VSDs diagnosed through echocardiography, while excluding those without NIPT, with high-risk NIPT, genetic disorders, and those lost to follow-up. High-risk NIPT often encompass chromosomal number abnormalities, fragment deletions or duplications, and polymorphisms. Pregnancy outcomes were monitored through phone calls and follow-up of newborns for at least 6 months after delivery. Data collected and analyzed included prenatal and postpartum diagnoses and prognoses, NIPT and CNV results need for surgical intervention, and natural healing of VSDs either in utero or postnatally. The process of data collection and analysis is illustrated in Fig. 1.
The ultrasonic diagnosis criteria and classification of fetal VSD
A transabdominal ultrasound was performed using a color Doppler ultrasound machine (Voluson E10, GE, USA), with a C1-5-D probe that operates at a frequency of 3.5–5.5 MHz to detect fetal VSD. The continuous disruption of the ventricular septum in the parasternal four-chamber view, along with enhanced echo at the septal end, was observed using color Doppler imaging to confirm the diagnosis. The diagnosis was further validated by our hospital’s cardiac ultrasound experts and prenatal diagnosis specialists, each with over 5 years of experience. Following a VSD diagnosis, genetic counseling is provided, and amniocentesis is recommended for genetic testing.
The classification of VSD into isolated and non-isolated types was based on the presence of other cardiac and extra-cardiac abnormalities on ultrasound examination. Isolated VSD (IVSD) was further divided into muscle VSD (MSD) and non-muscle VSD (non-MSD), with the latter including perimembranous and mixed-type defects. Non-isolated VSD (NIVSD) included intracardiac and extra-cardiac abnormalities. Intracardiac abnormalities included atrioventricular septal defect, coarctation of the aorta, and tetralogy of Fallot. Additionally, extra-cardiac abnormalities included enlarged lateral ventricles, enlarged double renal pelvis, digestive tract obstruction, hypospadias, short long bones, and other abnormalities, such as fetal growth restriction (FGR).
Chromosomal examination
NIPT screening was performed using non-invasive prenatal subchromosomal copy-number variation detection (NIPSCCD) method, which is based on the shallow‐depth whole genome sequencing [5]. Samples were sequenced on the Nextseq550AR platform. Subchromosomal segments with absolute values of the final Z‐scores above 1.28 were considered indicative of CNVs, while Z‐scores of a whole chromosome above 3 were considered as trisomy 21/18/13. These were classified as high-risk for NIPT.
Chromosome G-banding karyotype analysis and CNV-seq were performed at 18–24 weeks of gestation based on high-throughput sequencing. Fluorescence in situ hybridization (FISH) and CNV-seq were also conducted after 24 weeks. The ACMG 2019 guidelines were used to classify the results of CNV-seq. After a thorough evaluation of CNV-seq, G-banding karyotype analysis, or FISH, the cases in this study were categorized as either pathogenic CNVs (P) or non-pathogenic CNVs. The non-pathogenic CNVs consisted of CNVs of uncertain significance (VUS) and benign CNVs (B). This allowed women and their families to make an informed decision on whether to continue or terminate the pregnancy, based on the presence of pathogenic CNVs, gestational age, and the severity of fetal structural abnormalities.
Statistical analysis
SPSS 24.0 was utilized in data processing. For numerical (continuous) variables, we used medians and 25th and 75th percentiles (P25-P75) for variables with non-normal distribution. To compare the incidence of pathogenic CNV in various kinds of VSD and the rate of surgical intervention between the groups, χ2 test (with continuous correction or Fisher’s test) was employed. A P value below 0.05 showed that the difference was statistically significant.
Results
Analysis of the composition ratio of each type of VSD and the incidence of pathogenic CNV
The basic information of the 74 VSD patients is shown in Table 1. Table 2 showed that out of the 74 fetuses with VSD, 45 (60.8%) had IVSD and 29 (39.2%) had NIVSD. Of the isolated cases, 9 (12.2%) had MSD and 36 (48.6%) had non-MSD. Among the NIVSD cases, 10 (13.5%) had VSD combined with intracardiac structural anomalies (ISA) and 19 (25.7%) had VSD combined with extra-cardiac structural anomalies (ESA). Regarding the incidence of pathogenic CNV in different types of VSD, no pathogenic CNV was found in any of the isolated MSD or non-MSD cases (0/9, 0%; 0/36, 0%). However, 20% (2/10) of patients with VSD combined with ISA and 26.3% (5/19) of patients with VSD combined with ESA had pathogenic CNV (Table 2). Additionally, non-pathogenic CNV included clinically significant unknown and normal CNV.
Comparison of pathogenic CNV rates among groups
There was a significant difference in the occurrence of pathogenic CNVs between isolated and non-isolated VSDs, with none of the IVSDs exhibiting the condition, while 24.1% (7/29) of the NIVSD cases did, as indicated by a Chi-squared test (χ2 = 9.344, P = 0.002). An analysis of the NIVSD subgroups revealed that CNVs were present in 20% (2/10) of cases with ISA and 26.3% (5/19) of cases with ESA, although no significant difference was detected between the 2 groups (Table 3). In contrast, none of the IVSD subgroups had pathogenic CNVs.
Clinical characteristics and pregnancy outcome of pathogenic CNV and CNV of unknown clinical significance
Out of the 74 cases, 7 were identified as pathogenic CNVs (Nos. 1–7), including a VSD combined with an aberrant left subclavian artery and aortic stenosis (No. 1), VSD with aneurysm of membranous ventricular septum (AMVS) and left heart enlargement (No. 2), and 3 VSDs with FGR with or without polyhydramnios and heart enlargement (Nos. 3, 4, 6). All pregnant women in the previously mentioned 7 cases opted for termination of pregnancy (TOP). Another seven cases had CNVs of unknown clinical significance (Nos. 8–14). These included one VSD with hypospadias (No. 13), one tetralogy of Fallot (No. 14), three isolated non-MSDs (Nos. 8–10), and two isolated MSDs (Nos. 11–12). Except for No. 13, who chose TOP, the remaining pregnant women chose to continue their pregnancies, with good outcomes. The CNV results and VSD phenotypes are detailed in Tables 4 and 5.
The rate of surgical interventions in neonates with various forms of VSD
Among the 67 cases of non-pathogenic CNV, 7 opted to TOP for personal reasons, 1 infant passed away due to metabolic disease after delivery, and the remaining 59 neonates were born healthy and survived. Details of these cases are presented in Table 6. Excluding TOP and deaths, the rate of surgical intervention for newborns or infants with isolated MSD was 0% (0/9), while for those with isolated non-MSD, it was 20% (7/35), with no significant difference between the 2 groups. The surgery rate for newborns or infants with VSD combined with ISA was significantly higher than for those with isolated MSD (83.3% vs 0%, P = 0.002). However, there was no significant difference in surgery rates between the isolated MSD group and the NIVSD combined with ESA (P = 0.5) (Table 6).
Discussion
Recent research has revealed a strong connection between VSD and chromosomal abnormalities, particularly trisomies of 21, 18, and 13, as well as sex chromosome abnormalities [6]. However, the prenatal chromosomal serological screening or NIPT results of these pregnant women were not mentioned. In this research, all seven cases of pathogenic CNV had chromosomal deletions or duplications with fragment sizes ranging from 1.25 to 14.2 Mb, but NIPT screening failed to identify them. This could be due to the limitations of NIPT which may not cover all chromosomal fragments. The sensitivity of NIPT in detecting CNVs smaller than 3 Mb was only 78.57% [7]. Factors, such as the location of the chromosomal deletions or repetitive fragments, false-positive rates, fetal free DNA concentration, and sequencing depth, could also impact the results of the detection [8]. Despite the fact that some pregnant women with invasive prenatal testing for fetal VSD may belong to a high-risk screening population, many studies have not taken into account the prenatal chromosome serological screening or NIPT results of these women. This study aimed to explore the relationship between different types of VSD in fetuses, chromosomal abnormalities, and prognosis under low-risk NIPT conditions.
Studies have revealed that out of 568 fetuses with IVSD, 8 had a pathogenic CNV. This rate was similar to the rate of the general pregnant population of 1.6–1.7% [9]. Investigations conducted on pregnancies with low comprehensive risk assessment and IVSD before childbirth indicated that the incidence of chromosomal abnormalities was 0.7% lower than the rate in the general pregnant population [6]. Additionally, isolated MSD may be a benign variation [6]. In contrast, NIVSD had a chromosomal abnormality rate of 14.6% [9]. Our research found that in the context of low-risk NIPT, 45 fetuses with IVSD had no pathogenic CNVs, indicating that the defect may be a benign variation. The incidence of NIVSD-associated CNV was significantly higher than that of IVSD. This was particularly evident when combined with extra-cardiac structural abnormalities (26.3%), which was lower than the 40% chromosomal abnormalities of VSD with extra-cardiac abnormalities reported by Alan et al. [10]. The case data omit high-risk VSD of NIPT, implying that for fetuses with IVSD, invasive prenatal diagnosis was not necessary when NIPT was low risk. Conversely, for those with NIVSD, even if NIPT was low risk, it is strongly advised to perform invasive prenatal diagnosis, particularly if combined with ESA, to avoid any missed diagnosis and potential detrimental pregnancy outcomes.
Analysis of pathogenic CNV malformations revealed that five out of seven cases were VSD with extra-cardiac structural issues, and three of them had FGR. It is uncertain whether the association between VSD and FGR is caused by hemodynamic alterations or changes in the placenta-heart axis during the early stages of embryonic development [11]. Further research is needed to determine if VSD is more likely to combine with FGR [12]. Consequently, it is recommended that pregnant women with NIVSD, particularly when associated with FGR, should undergo invasive prenatal diagnosis and CNV examination.
Reports suggest that the rate of spontaneous closure for isolated MSD and isolated perimembranous VSD in fetuses was 31/64 and 3/11, respectively. At 2 years of age, the closure rates were 92.2% and 45.5%, respectively [13]. Generally, MSD close in utero or during the initial 2 years of life, while isolated perimembranous VSD may require intervention postnatally [3]. This study also found that the closure rate for MSD was 55.6%, compared to 5.6% for non-MSD. This is consistent with our study’s findings, which indicate that MSD have the best chance of closing naturally and may not need surgical treatment, whereas isolated non-MSD should be evaluated carefully. It has been reported that a defect size of ≥ 4 mm is a predictor of non-spontaneous closure for perimembranous VSD [14]. Another study revealed that those with defects larger than 3 mm did not close spontaneously [15]. In this study, the average size of isolated MSD was 2.18 mm, with a maximum size of 3.5 mm, and no surgical intervention was performed. On the other hand, the average size of non-MSD was 3.07 mm, with 8 cases larger than 4 mm and a 20% rate of surgical intervention (data not shown). Therefore, we speculated that the size of the VSD played a role in the disparity of natural healing rates between non-MSD and MSD. The average size of VSD in the operative and non-operative groups was 3.89 mm and 2.87 mm, respectively (data not shown). Although no significant difference was observed, the size of the defect should still be taken into account for better patient care. Research suggests that the prognosis of VSD patients with chromosomal abnormalities combined with ESA may be more favorable than those with isolated non-MSD and VSD combined with ISA. We found that the surgical intervention rate for VSD combined with ISA was 83.3%, while for VSD combined with ESA, the rate was 11.1% after eliminating chromosomal issues. Despite the small sample size, further studies are needed to confirm this.
This study is limited by the small sample size and being conducted at a single center. Furthermore, the follow-up data for newborns were collected via telephone, which could be inaccurate, and the decision to perform surgical interventions is based on certain human factors. Finally, it is possible that advancements in technology or specific genetic factors may lead to additional finding in newborns with prenatally detected VSDs that were not identified during prenatal screening. For example, one case of IVSD passed away due to a genetic metabolic disease after birth. In conclusion, this article explored the connection between various types of VSD and chromosomal abnormalities in the context of low-risk NIPT. The results indicated that IVSDs do not increase the risk of chromosomal irregularities when undergoing low-risk NIPT screening, eliminating the need for invasive prenatal diagnosis. However, for NIVSD cases, it is recommended to undergo invasive prenatal diagnosis, especially if combined with ESA, to avoid missed diagnoses and unsatisfactory pregnancy outcomes. It is important to note that when VSD is accompanied by FGR, invasive prenatal diagnosis is suggested. IVSDs, however, generally heal without needing surgery. A thorough examination should be conducted for IVSDs with ESA. Surgery is usually necessary for VSDs combined with ISA. Going forward, more attention should be given to the size and type of VSD when conducting research and offering clinical advice, to better determine if spontaneous closure or surgery is needed. This study provides a theoretical foundation for the connection between various types of VSDs and chromosomes under the condition of low-risk NIPT, thus supporting prenatal consultation for VSD patients.
Data availability
The datasets generated and/or analysed during the current study are available in the [Science Data Bank] repository, [https://www.scidb.cn/en], the data named “Correlation between types of Ventricular Septal Defect and chromosomal abnormalities in Low-Risk Non-Invasive Prenatal Testing”.
References
Eckerström F, Nyboe C, Maagaard M et al (2023) Survival of patients with congenital ventricular septal defect. Eur Heart J 44:54–61. https://doi.org/10.1093/eurheartj/ehac618
Cheng K, Zhou H, Fu F et al (2022) Should prenatal chromosomal microarray analysis be offered for isolated ventricular septal defect? A single-center retrospective study from China. Front Cardiovasc Med 9:988438. https://doi.org/10.3389/fcvm.2022.988438
RaucherSternfeld A, Sheffy A, Tamir A et al (2022) Isolated ventricular septal defects demonstrated by fetal echocardiography: prenatal course and postnatal outcome. J Matern Fetal Neonatal 35:129–133. https://doi.org/10.1080/14767058.2020.1712710
Chen Y, Lu L, Zhang Y et al (2022) Clinical application of expanded noninvasive prenatal testing for fetal chromosome abnormalities in a cohort of 39,580 pregnancies. Am J Med Genet A 188:1426–1434. https://doi.org/10.1002/ajmg.a.62657
Yu D, Zhang K, Han M et al (2019) Noninvasive prenatal testing for fetal subchromosomal copy number variations and chromosomal aneuploidy by low-pass whole-genome sequencing. Mol Genet Genomic Med 7:e674. https://doi.org/10.1002/mgg3.674
Vedel C, Rode L, Jørgensen FS et al (2021) Prenatally detected isolated ventricular septum defects and the association with chromosomal aberrations-a nationwide register-based study from Denmark. Prenatal Diagn 41:347–353. https://doi.org/10.1002/pd.5853
Hyblova M, Harsanyova M, Nikulenkov-Grochova D et al (2020) Validation of copy number variants detection from pregnant plasma using low-pass whole-genome sequencing in noninvasive prenatal testing-like settings. Diagnostics (Basel) 10:569. https://doi.org/10.3390/diagnostics10080569
Liu W, Yang J, Zhang J et al (2021) Consensus on technological standards for non-invasive prenatal screening of pathogenic copy number variations by high-throughput sequencing of maternal plasma cell-free DNA. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 38:613–619. https://doi.org/10.3760/cma.j.cn511374-20201208-00855
Maya I, Singer A, Yonath H et al (2020) What have we learned from 691 prenatal chromosomal microarrays for ventricular septal defects? Acta Obstet. Gynecol Scand 99:757–764. https://doi.org/10.1111/aogs.13708
Allan LD, Sharland GK, Milburn A et al (1994) Prospective diagnosis of 1006 consecutive cases of congenital heart disease in the fetus. J Am Coll Cardiol 23:1452–1458. https://doi.org/10.1016/0735-1097(94)90391-3
Smith M, Nicoll A (2018) Perinatal outcomes following mid trimester detection of isolated short foetal femur length. J Obstet Gynaecol 38:727. https://doi.org/10.1080/01443615.2018.1444408
Ghanchi A, Derridj N, Bonnet D et al (2020) Children born with congenital heart defects and growth restriction at birth: a systematic review and meta-analysis. Int J Environ Res Public Health 17:3056. https://doi.org/10.3390/ijerph17093056
Raucher A, Sternfeld, Sheffy A, Tamir A et al (2022) Isolated ventricular septal defects demonstrated by fetal echocardiography: prenatal course and postnatal outcome. J Matern Fetal Neonatal Med 35:129–133. https://doi.org/10.1080/14767058.2020.1712710
Zhao Q-M, Niu C, Liu F et al (2019) Spontaneous closure rates of ventricular septal defects (6750 consecutive neonates). Am J Cardiol 124:613–617. https://doi.org/10.1016/j.amjcard.2019.05.022
GordinKopylov L, Dekel N, Maymon R et al (2022) Prenatally diagnosed isolated perimembranous ventricular septal defect: genetic and clinical implications. Prenatal Diagn 42:461–468. https://doi.org/10.1002/pd.6128
Acknowledgements
The authors gratefully acknowledge the Prenatal Diagnosis Center and Ultrasound Center of Tianjin Central Hospital of Obstetrics and Gynecology in supporting this collaborative research.
Funding
This work was funded by National Key Specialties and Diseases Cohort Project (GJZDZKZBDL2022-04); Open Fund of Tianjin Central Hospital of Gynecology Obstetrics/Tianjin Key Laboratory of human development and reproductive regulation (2022XH10 and 2022XHY03); Natural Science Foundation of Tianjin (22JCYBJC01110).
Author information
Authors and Affiliations
Contributions
Xiaomin Zhao: the acquisition, analysis, or interpretation of data for the work; Yongmei Shen: drafting the work or revising it critically for important intellectual content; Dexuan Kong and Liying Yao: ultrasonography; Shanshan Li and Wen Li: writing—review and editing; Ying Chang: substantial contributions to the conception or design of the work and final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval and consent to participate
The studies involving human participants were reviewed and approved by Human Research Ethics Committee of Tianjin Central Hospital of Obstetrics and Gynecology. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All participants signed a written informed consent form prior to their participation in this study.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, X., Shen, Y., Kong, D. et al. Correlation between types of ventricular septal defect and chromosomal abnormalities in low-risk non-invasive prenatal testing. Arch Gynecol Obstet 310, 1517–1523 (2024). https://doi.org/10.1007/s00404-024-07566-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-024-07566-3