Abstract
Purpose
The aim of this study was to compare the effects of Dienogest and medroxyprogesterone acetate (MPA) on the recurrence of endometriosis lesions and clinical symptoms in women undergoing laparoscopic surgery.
Methods
This single center clinical trial was conducted among 106 women with endometriosis undergoing laparoscopic surgery who candidate receiving post-surgery hormone therapy. Participants were allocated to two groups. The first group received Dienogest pills (2 mg) daily for the first three months and then cyclic for three months afterward. The second group received MPA pills twice daily (10 mg) for three months and then cyclic for the next three months. Six months after the intervention, the rate of endometriosis recurrence, the size of endometriosis lesions and pelvic pain were assess and compared between two groups.
Results
Finally, data were evaluated based on 48 and 53 women in the Dienogest and MPA groups, respectively. After 6 months follow-up assessments the pelvic pain score was significantly lower in Dienogest group than MPA group (P < 0.001). There was not statistically difference between two groups in terms of recurrence rate of endometriosis (P = 0.4). Although the size of endometriosis cyst recurrence was smaller in Dienogest group compared to MPA group (P = 0.02).
Conclusions
The findings showed that Dienogest treatment has better effect in reducing pelvic pain and the mean size of the recurrent endometriosis lesions after endometriosis laparoscopic surgery when compared to MPA treatment. Although the recurrent rate of endometriosis was similar between these treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recurrence of endometriosis after laparoscopic surgery is associated with reduced quality of life. After laparoscopic surgery, progesterone medication (mostly medroxyprogesterone acetate (MPA)) is prescribed for these patients. This clinical trial showed that Dienogest treatment has better effect in reducing pelvic pain and the mean size of the recurrent endometriosis lesions after endometriosis laparoscopic surgery when compared to MPA treatment. Although the recurrent rate of endometriosis was similar between these treatments. |
Introduction
Endometriosis is the most common disease observed in 40% of women with chronic pelvic pain [1]. Despite extensive research, the exact pathogenesis of this disease remains to be elucidated. Currently, It is believed that the disease may be a multifactorial phenomenon, meaning that hormonal, environmental, genetic, and lifestyle factors play critical roles in its development. The treatment of endometriosis is personalized and depends on various determinants, such as the type and severity of clinical manifestations (such as pain, infertility, ovarian mass, clinical stage, the patient’s intention for reproduction, age, drugs’ side effects, surgical side effects, and costs. In general, therapeutic methods include pharmaceutical and surgical treatments [2, 3].
Since pharmaceutical treatments alone are often inadequate, surgery is the method of choice to treat endometriosis. The surgical excision of lesions (conservative surgery) not only alleviates pain but also boosts fertility. Because most women with endometriosis are of reproductive age, conservative surgery is preferred over radical surgery in most cases. The nature of endometriosis was believed to be static until the early 1990s, assuming that recurrence after surgery was relatively rare. However, a systematic review estimated the recurrence rate of endometriosis to be 21.5% at 2 years and 40–50% at 5 years, which is higher than what was estimated before. Recurrence and repetitive surgeries can aggravate pain and reduce fertility. Recurrence negatively affects the quality of life and increases costs for the patient and society. Therefore, it is crucial to prevent the recurrence of symptoms and lesions after conservative surgery to maintain pain relief and enhance fertility [4].
The current drugs widely used for the treatment of endometriosis target estrogen or progesterone receptors, because the altered expression of estrogen and progesterone receptors in the endometrial tissue is the major mechanism involved in the pathogenesis of this disease [5]. Few studies have investigated the effect of progesterone on the postoperative recurrence of endometriosis [6].
Progestins reduce the risk of estrogen-related thromboembolic events, which are associated with estrogen-containing contraceptives. Compared to GnRH analogs, high-dose oral progestin therapy, besides being cheaper, is not associated with reduced bone mass. Compared to danazol, progestins are better tolerated, have no androgenic side effects, and have fewer effects on the metabolism of lipids [7]. The progestins commonly used to alleviate endometriosis pain include medroxyprogesterone acetate (MPA), 19-nortestosterone derivatives (norethindrone acetate), and Dienogest [2, 3]. A study reported that MPA reduced the symptoms of pain, pelvic nodularity, and tenderness in more than 80% of patients. However, due to its non-specific binding to androgen and glucocorticoid receptors, MPA negatively affects lipid profile, leading to weight gain and acne formation [8]. Nevertheless, despite its androgenic activity and side effects, MPA is still considered a suitable therapeutic option for endometriosis due to its low cost [7].
Dienogest is fourth-generation progesterone that specifically binds to progesterone receptors. Although this drug presents minor androgenic activity, it locally affects endometriotic lesions, thereby reducing the proliferation of endometrial cells and cytokine production in endometrial stromal cells. Therefore, Dienogest has fewer effects on metabolic parameters [9, 10]. From a therapeutic point of view, Dienogest significantly reduces the size of endometriotic lesions and alleviates the clinical symptoms of the disease. At usual therapeutic doses, Dienogest is associated with considerably lower side effects than other pharmaceutical treatments [11]. So far, limited studies have retrospectively compared the clinical effects of these two drugs [12, 13]. Therefore, this clinical trial aimed to compare the effects of Dienogest and MPA on the recurrence of endometriosis lesions and clinical symptoms in women undergoing laparoscopic surgery.
Methods
Study design
This single-blind controlled randomized clinical trial was conducted in Arash Hospital, Tehran, Iran from December 2020 to June 2022. Written informed consent was obtained from all participants. The study was approved by the Ethics Committee of Tehran University of Medical sciences and IRCT number is IRCT20170917036227N5.
Participants
Women with endometriosis undergoing laparoscopic surgery who candidate receiving post-surgery hormone therapy were enrolled in the study, according to inclusion/exclusion criteria as follows. Those with age between 18 and 45 years old were enrolled. Being pregnant or lactating, willing to become pregnant, history of amenorrhea (no menstruation for ≥ 3 months during the last six months), undetected genital bleeding, recent use of hormonal medications, contraindications for using progesterone, having a risk factor for decreased bone density, undergoing oophorectomy or hysterectomy during the study, and the use of acitretin, anticoagulants were regarded as exclusion criteria.
Intervention
Eligible patients who underwent laparoscopy for endometriosis were included in the study after signing a written consent form. First, a questionnaire was completed to gather demographic information, as well as medical history, history of previous pregnancies, and the current status of endometriosis before surgery (including the disease grade, location, size of endometriosis, etc.). Laboratory tests, including cell blood count (CBC) and biochemical measuring of liver enzymes and lipids, were performed. Vaginal ultrasound was performed to determine the remnants of endometrioma or detect pelvic endometriosis by the same sonographer. The pain severity of dysmenorrhea and dyspareunia, as well as pelvic pain, were measured separately using the visual analog scale (VAS).
Randomization
The participants were divided into two groups using the block randomization table. The first group received Dienogest pills (2 mg) daily for the first three months and then cyclic for three months afterward (from the day 10th of the menstrual cycle for 2 weeks). The second group received medroxyprogesterone acetate pills twice daily (10 mg) for three months and then cyclic for the next three months (from the day 10th of the menstrual cycle for 2 weeks). Six months after the intervention, the same sonographer performed a vaginal ultrasound to detect disease recurrence and the size of endometriosis lesions. Endometriosis recurrence was defined as when trans vaginal ultrasonography indicated the presence of a endometriosis lesion with a more than 2 cm in diameter. The pain severity of dysmenorrhea and dyspareunia and pelvic pain were quantified using the Visual Analogue Scale (VAS) six months after the intervention. Drug side effects, such as headache, alopecia, etc., were recorded using a questionnaire for both groups.
Primary and secondary outcomes
The outcomes included the rate of endometriosis lesions’ recurrence, pain severity of pelvis, dysmenorrhea and dyspareunia six months post-intervention, determination of drug side effects [such as weight gain (Weight gain of 2 kg), headache, depression, sleep disorders at six months post-intervention]. These outcomes were compared between the two groups.
Blinding
For blinding, the order of the treatments was written on cards. Then, the cards were placed inside sealed envelopes, and a random code (i.e., the patient identifier code) was written on each envelope without any order. Only the methodologist of the study was aware of the patients’ corresponding codes. When the physician confirmed that a patient was eligible for participation in the study, a trained nurse would provide the physician with an envelope enclosing the treatment that should have been assigned to the patient. The patients were aware of the type of intervention.
Sample size
The Endometrioma recurrence rate was used to calculate sample size. To be able to detect a 20% difference in endometrioma recurrence rate between two study groups with a 5% recurrence rate [14], alpha 5% and beta 20%, the study required 51 participants in each group.
Data analysis
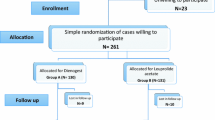
Intention-to-treat analysis was used to maintain the random allocation process. Student's t test, chi square and Mann–Whitney test and linear regression analysis were used to compare outcomes between two groups. P values less than 0.05 were considered significant (Fig. 1).
Results
One hundred six participants were enrolled in this study. Three participant in Dienogest group did not adhere to the assigned treatment regimen (they used traditional medicine) and Two participants got pregnant and were withdrawn from this study. Finally, data were evaluated based on 48 participants in the Dienogest group and 53 women in the MPA group,
The mean ± standard deviation of the age of the participants in the Dinogest group was 35.12 ± 5.81 and in the MPA group was 35.49 ± 4.68. There was not difference between the study groups regarding age, gravidity, BMI, smoking and alcohol consumption and history of surgery. Family history of endometriosis was higher in Dinogest group than the other group. Demographic information of participants was shown in Table 1. The most grade of endometriosis in both groups was grade III (54.2% in Dinogest group and 71.7% in MPA group). In both groups, most participants had endometriosis symptoms for less than a year. As well endometrisis was unilateral in most participants (Table 1).
The assessment of pelvic pain, dysmenorrhea and dyspareunia before and after intervention were shown in Table 2. As shown there was no significant difference in pain scores between two groups before intervention. After 6 month follow-up assessments, the mean pain score of dysmenorrhea, dyspareunia and pelvic pain were significantly decreased in both groups. The mean pelvic pain score in Dinogest group was significantly decreased than MPA group (P value ≤ 0.001). There were not statistically significant between two groups regarding dysmenorrhea and dyspareunia.
Similar results were observed when linear regression analysis was performed and Dinogest consumption was significantly associated with pelvic pain, independent of other variables (Family history, Duration of symptoms and endometriosis laterality) (β for group = 0.85, SE = 0.29, P value = 0.006, 95% CI = 0.25 to 1.44).
There was not any statistically difference between two groups regarding in terms of endometriosis recurrence rate in sonographic findings 6 months after intervention (P value = 0.4).
There were cyst recurrence in 7 patients of Dinogest group and 13 patients in MPA group (14.6% vs. 24.5%). Although the size of endometriosis in Dinogest group was significantly lower than the size of endometriosis in another group. The results are shown in Table 2. 14.3% of relapses in the Dinogest group were unilateral and 85.7% were bilateral. In MPA group, 30.8% of recurrences were unilateral and 69.2% were bilateral. There is no statistically significant difference between the two groups (P value = 0.41).
The drug’s adverse effects were statistically significant difference between two groups (P < 0.001). The most common side effect in Dinogest group was headache and in MPA group was weight gain (Table 3).
Discussion
The results of this study showed that treatment with Dienogest for six months after conservative endometriosis surgery was associated with a lower pelvic pain score compared to post-surgery treatment with MPA. The prevalence of disease recurrence was 14.6% in the Dienogest group and 24.5% in the MPA group; however, this difference between the two groups was not statistically significant. Nevertheless, the 10% difference between the two groups was clinically noticeable. On the other hand, the drug side effects were not significantly different between the two groups. In addition, in the case of disease recurrence, the size of the endometriosis lesions was significantly greater in the MPA group that received Diengest.
Dienogest binds the progesterone receptor, and if it is consumed continually, it inhibits systemic gonadotropin secretion and exerts local anti-proliferative and anti-inflammatory effects on endometriosis lesions [15]. This drug also modulates the production and metabolism of prostaglandin PGE2, PGE2 synthase, cyclooxygenase-2, and microsomal PGE synthase-1. Moreover, Dienogest modulates the production and metabolism of prostaglandins by reducing the activity of the PGE synthase-1 enzyme [16]. According to our literature review, there were limited studies similar to our research, and we found no clinical trials in this area. In line with our study, a 2015 study compared the efficacy of Dienogest and high-dose oral MPA in alleviating endometriosis pain. In the recent prospective study, 98 patients consumed 2-mg Dienogest tablets, and 120 patients received 30–60 mg of MPA for at least six months. The results showed that the extent of endometriosis pain reduction was greater in the Dienogest group than in the MPA group. However, irregular bleeding was significantly more frequent in the Dienogest group than in the MPA group [13].
Another study in 2018 aimed to compare the effectiveness of Dienogest and MPA in the management of endometriosis in terms of menstrual pain, quality of life, tolerability, overall satisfaction, as well as the side effects of the two medications. The recent cross-sectional descriptive study was conducted on sixty 18–55-year-old Chinese women with endometriosis, of whom 30 women were intramuscularly treated with MPA (150 mg) once every three months, and the other 30 women used 2 mg oral Dienogest daily for at least six months. The results of the mentioned study showed that Dienogest was more effective than MPA in the treatment of endometriosis symptoms and alleviating menstrual pain. Besides, tolerability and overall satisfaction were greater in the Dienogest group than in the MPA group [12], which was consistent with our study.
In a study by Yamanaka et al., 126 patients who received neither any medication nor DNG following laparoscopic resection of deep endometriosis were retrospectively examined. The results revealed eight (11.9%) patients in the untreated group, while none in the DNG group experienced disease recurrence [14]. Studies have reported a 12–30% recurrence rate for endometriosis over 2–5 years after conservative surgery [17]. Several studies have suggested that long-term use of Dienogest can be beneficial for preventing endometriosis recurrence [15, 18]. In addition, several studies have pointed out a reduction in the size of recurrent endometrium up to 5 year post-surgery [15].
In a recent systematic review, the patients who received Dienogest maintenance therapy after conservative endometriosis surgery were compared with those who received other treatments, including the levonorgestrel-releasing intrauterine system, and gonadotropin-releasing hormone analogs, or no treatment. This study showed that Dienogest treatment was associated with a lower recurrence rate, suggesting this intervention as a treatment to reduce disease relapse after conservative endometriosis surgery. Nevertheless, this systematic review included only two clinical trials in the field, highlighting the need for conducting more randomized clinical trials in this area [19]. The regrowth of residual lesions and the formation of new lesions are the two main causes of the relapse of endometrial lesions. Vignali et al. found that the recurrence of deep endometriosis after the second surgery often occurred in the same pelvic area that was involved in the first place. There is also a possibility for the development of new lesions in places other than the primary site. Surgery, especially conservative procedures, seems insufficient to remove these lesions completely [4]. The different endometriosis recurrence rates after surgery observed in our study in comparison to other studies may be related to the type of studies (i.e., our study was a clinical trial, while others were cohorts), the populations analyzed, the size and number of lesions, and the type of surgery.
In this study, The most common side effect in Dinogest group was headache and in MPA group was weight gain. The side effects of Dienogest mimic those associated with progesterone (i.e., weight gain, hypertension, breasts pain, and nausea). The pooled analysis of four clinical trials on the safety and tolerability of Dienogest in 332 women with endometriosis receiving 2 mg of the drug daily for 65 weeks showed that the drug was well-tolerated. The most common side effects of the drug included mild to moderate headache, breast discomfort, acne, and depression. Bleeding was the reason for early withdrawal in only 0.6% of the patients [13]. This was a randomized clinical trial, which is a strength of our study. Nonetheless, the patients were followed up only for six months, and we did not assess bone density in our patients, which are among the limitations of this study.
Conclusions
The findings showed that Dienogest treatment has better effect in reducing pelvic pain and the mean size of the recurrent endometriosis lesion when compared to MPA treatment. Although the recurrent rate of endometriosis was similar between these treatment.
Data availability
Data are available upon request, and patient's privacy.
References
Daniels J, Gray R, Hills RK, Latthe P, Buckley L, Gupta J et al (2009) Laparoscopic uterosacral nerve ablation for alleviating chronic pelvic pain: a randomized controlled trial. JAMA 302(9):955–961
Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D’Hooghe T, De Bie B, Heikinheimo O, Horne AW, Kiesel L, Nap A, Prentice A, Saridogan E, Soriano D, Nelen W (2014) ESHRE guideline: management of women with endometriosis. Human Reprod (Oxf, Engl) 29(3):400–412. https://doi.org/10.1093/humrep/det457
Practice Committee of the American Society for Reproductive Medicine (2014) Treatment of pelvic pain associated with endometriosis: a committee opinion. Fertil Steril 101(4):927–935. https://doi.org/10.1016/j.fertnstert.2014.02.012
Koga K, Takamura M, Fujii T, Osuga Y (2015) Prevention of the recurrence of symptom and lesions after conservative surgery for endometriosis. Fertil Steril 104(4):793–801
Tosti C, Pinzauti S, Santulli P, Chapron C, Petraglia F (2015) Pathogenetic mechanisms of deep infiltrating endometriosis. Reprod Sci 22(9):1053–1059
Sagsveen M, Farmer JE, Prentice A, Breeze A (2003) Gonadotrophin-releasing hormone analogues for endometriosis: bone mineral density. Cochrane Database Syst Rev 4:cd001297. https://doi.org/10.1002/14651858.cd001297
Abdul Karim AK, Shafiee MN, Abd Aziz NH, Omar MH, Abdul Ghani NA, Lim PS, Md Zin RR, Mokhtar N (2019) Reviewing the role of progesterone therapy in endometriosis. Gynecol Endocrinol 35(1):10–16
Sönmezer M, Atabekoğlu C, Cengiz B, Dökmeci F, Cengiz S (2005) Depot-medroxyprogesterone acetate in anticoagulated patients with previous hemorrhagic corpus luteum. Eur J Contracept Reprod Health Care 10(1):9–14
Yamanaka K, Xu B, Suganuma I, Kusuki I, Mita S, Shimizu Y, Mizuguchi K, Kitawaki J (2012) Dienogest inhibits aromatase and cyclooxygenase-2 expression and prostaglandin E2 production in human endometriotic stromal cells in spheroid culture. Fertil Steril 97(2):477–482
Sasagawa S, Shimizu Y, Kami H, Takeuchi T, Mita S, Imada K, Kato S, Mizuguchi K (2008) Dienogest is a selective progesterone receptor agonist in transactivation analysis with potent oral endometrial activity due to its efficient pharmacokinetic profile. Steroids 73(2):222–231
Köhler G, Faustmann TA, Gerlinger C, Seitz C, Mueck AO (2010) A dose-ranging study to determine the efficacy and safety of 1, 2, and 4 mg of Dienogest daily for endometriosis. Int J Gynecol Obstet 108(1):21–25
Wong YYI, Lee MHM (2018) Dienogest versus medroxyprogesterone acetate for control of menstrual pain in Chinese women with endometriosis. Hong Kong J Gynaecol Obstet Midwifery 18(2):91–97
Oh S (2015) The comparison between 2 mg Dienogest and high-dose medroxyprogesterone acetate on oral treatment of endometriosis. J Minim Invasive Gynecol 22(6):S170
Yamanaka A, Hada T, Matsumoto T, Kanno K, Shirane A, Yanai S, Nakajima S, Ebisawa K, Ota Y, Andou M (2017) Effect of Dienogest on pain and ovarian endometrioma occurrence after laparoscopic resection of uterosacral ligaments with deep infiltrating endometriosis. Eur J Obstet Gynecol Reprod Biol 216:51–55
Murji A, Biberoğlu K, Leng J, Mueller MD, Römer T, Vignali M, Yarmolinskaya M (2020) Use of Dienogest in endometriosis: a narrative literature review and expert commentary. Curr Med Res Opin 36(5):895–907
Bedaiwy MA, Allaire C, Alfaraj S (2017) Long-term medical management of endometriosis with Dienogest and with a gonadotropin-releasing hormone agonist and add-back hormone therapy. Fertil Steril 107(3):537–548
Ota Y, Andou M, Yanai S, Nakajima S, Fukuda M, Takano M, Kurotsuchi S, Ebisawa K, Hada T, Ota I (2015) Long-term administration of Dienogest reduces recurrence after excision of endometrioma. J Endometr Pelvic Pain Disord 7(2):63–67
Chandra A, Rho AM, Jeong K, Yu T, Jeon JH, Park SY, Lee SR, Moon H-S, Chung HW (2018) Clinical experience of long-term use of Dienogest after surgery for ovarian endometrioma. Obstet Gynecol Sci 61(1):111–117
Liu Y, Gong H, Gou J, Liu X, Li Z (2021) Dienogest as a maintenance treatment for endometriosis following surgery: a systematic review and meta-analysis. Front Med 8:652505
Funding
The study was not founded. This study was support by Tehran University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
MVD, HR, RH contributed to design. HR, RH conducted the study, LH and HR prepared the manuscript, HR analyzed the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Ethical approval
The study was approved by the Ethics Committee of Tehran University of Medical sciences and IRCT number is IRCT20170917036227N5. All patients who agreed to participate in the study provided signed informed consent.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vahid-Dastjerdi, M., Hosseini, R., Rodi, H. et al. Comparison of the effectiveness of Dienogest with medroxyprogesterone acetate in the treatment of pelvic pain and recurrence of endometriosis after laparoscopic surgery. Arch Gynecol Obstet 308, 149–155 (2023). https://doi.org/10.1007/s00404-022-06898-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06898-2