Abstract
Purpose
Bacterial vaginosis (BV) is a dysbiosis of the vaginal microbiome and a condition found in 20–30% of all women. Literature describing the possible link between BV and subfertility is increasing. Newer techniques such as quantitative polymerase chain reactions (qPCR) detect BV more accurately than traditional methods but come with high costs. The association between pH and BV as diagnosed using traditional methods is well-established in a symptomatic population. This study is the first to investigate the association between pH and BV diagnosed by qPCR in an asymptomatic subfertile population and to examine the usefulness of pH as a means of cost reduction.
Methods
Data of 170 pH–qPCR combinations were used from a prospective cohort study examining bacterial vaginosis in a subfertile population. 102 women received a vaginal swab and pH measurement at baseline and subsequent advanced reproductive technology (ART) treatments. The swabs are analysed using the AmpliSens®Florocenosis/Bacterial vaginosis-FRT qPCR kit.
Results
pH is strongly associated with BV as diagnosed by qPCR (OR 3.06, p = 0.000, CI 1.65–5.68). The cut-off point for pH ≥ 4.7 maximised diagnostic performance [AUC 0.74 (CI 0.66–0.83), sensitivity 76%] and reduced costs by 60%.
Conclusion
This study shows that the vaginal pH for a multi-ethnic, asymptomatic population of women attending fertility clinics is strongly associated with BV qPCR outcome. Using the cut-off of pH of 4.7 has a high sensitivity for diagnosis of BV by qPCR and can be achieved at a cost reduction of 60%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
It is cost reductive to only diagnose bacterial vaginosis by qPCR in an asymptomatic subfertile patient when the vaginal pH-value is ≥ 4.7. |
Introduction
Subfertility is a relatively common and increasing problem over the last decades [1]. In the Netherlands, subfertile couples are eligible for advanced reproductive technologies (ART) such as in-vitro fertilisation (IVF) or intra-uterine insemination (IUI) when the 1-year prognosis for spontaneous pregnancy is under 30% [2]. For many people, having children constitutes a major part of their existence and an inability to do so can be a traumatic experience [3]. In recent years, dysbiosis of the vaginal microbiome or bacterial vaginosis (BV) has been associated with preterm birth, endometritis, subfertility and the success rates of fertility treatments [4,5,6,7]. The mechanism behind the effect on subfertility is still unclear. Some studies report that BV has no effect on conception rates but is significantly associated with early pregnancy loss (RR 1.68, 95% CI 1.24–2.27), indicative of a problem related to implantation [8, 9].
BV is characterised by a shift in the vaginal microbiome from a Lactobacillus dominated profile to a more variable profile with anaerobic and facultative anaerobes. The overgrowth of these anaerobes causes symptoms of abnormal discharge, fishy odour and itching. However, half of the BV cases are asymptomatic. Typical treatment consists of a 7-day course of either oral or vaginal clindamycin or metronidazole and is known to have high recurrence over 50% of cases within 1 year [10]. The exact aetiology of BV is unclear but influencing factors include: vaginal douching, ethnicity, obesity, smoking, sexually transmissible diseases or unprotected sexual behaviour [11,12,13].
The gold standard for the diagnosis of BV is the Nugent score utilising gram-stained smears. The technique requires a skilled microbiologist and is time-consuming. The Amsel criteria are an alternative due to their simplicity and comparable performance but still require a microscope. Both methods are quite cumbersome and the Amsel criteria, by virtue of using clinical criteria, favours symptomatic BV cases. The use of the newer quantitative polymerase chain reactions (qPCR) methods allows for a more objective and accurate diagnosis of BV [4, 14]. A recent study compared the Nugent score and a BV qPCR assay to a full microbiome analysis using 16s ribosomal DNA-sequencing. The Nugent score attained a sensitivity for BV of 63.9%, while the BV qPCR assay achieved 80.6%, both methods had a specificity of ≥ 92.4%. This indicates a substantial performance gap between traditional methods and qPCR [14].
A disadvantage of using qPCR-tests is that they are expensive. Measuring a vaginal pH (as used in the Amsel criteria) is a simple and cheap procedure. pH is a good predictor for BV diagnosed by the Nugent score, but it is unknown whether this holds true for asymptomatic cases detected by qPCR [15, 16].
In some fertility clinics, commercial qPCR or microbiome testing is already available for subfertile couples. Searching for the cause of their subfertility, couples are willing to pay a high price for extra examinations, even when evidence is still insufficient [17]. The goal of this study is to examine the association of pH value with asymptomatic BV as diagnosed using qPCR, in a bid to potentially drive down costs related to using qPCR or microbiome testing in fertility clinics.
Materials and methods
Data were extracted from the database of an ongoing prospective cohort study examining the impact of BV in fertility patients (approved by medical ethics committee Leiden-Delft-Den Haag, reference Z21.031). Inclusions for this prospective study were ongoing at the time of writing. Patients visited the fertility outpatient clinic of The Haaglanden Medical Centre (The Hague, The Netherlands). Eligible women undergoing an initial fertility assessment (IFA) were included and followed for up to five ART treatments.
Participants
Women of 18 years and older were eligible for inclusion. After the initial assessment, women were either managed expectantly or treated using IVF, IUI or ovulation induction depending on the cause and duration of subfertility. Eligible women were measured at baseline and those treated with IVF or IUI received further swabs and pH measurements. The exclusion criteria were a history of three or more miscarriages, an inability to speak neither Dutch nor English, the use of antibiotics in the previous month or the use of prophylactic antibiotics in general. Incomplete combinations of pH measurements and vaginal swabs were excluded from the analysis.
Data collection
An e-swab (Copan Italia SpA, Brescia, Italy) was taken from the posterior fornix after inserting a speculum while wearing gloves. The pH measurements were performed using a pH-Fix 4.0–7.0 (ref 92137, Macherey–Nagel, Düren, Germany). The pH strip measured in the following increments: 4, 4.4, 4.7, 5, 5.3, 5.8, 6.1, 6.5, and 7.0. The measurements were not performed if the woman was menstruating or post-coital. For the subsequent ART procedures, the combination of swab and pH measurement was performed prior to the ovum pick up or insemination.
The swabs were analysed by an external laboratory (NMDL & DDL laboratory, Rijswijk, the Netherlands), using a CE-IVD marked multiplex quantitative PCR assay, the AmpliSens® Florocenosis/Bacterial vaginosis-FRT PCR kit (InterLabService, Moscow, Russia). Based on the presence of Lactobacillus species, Gardnerella vaginalis, Atopobium vaginae (recently reclassified as Fannyhessea vaginae [18]) and total amount of bacteria, swabs were categorised as BV positive (amount of G. vaginalis and/or A. vaginae is almost equal or exceeds the amount of Lactobacillus spp.), BV negative (G. vaginalis and/or A. vaginae are absent or its amount is substantially less than the Lactobacillus spp. amount), unspecified dysbiosis (amount of Lactobacillus spp. is reduced relative to the total amount of bacteria, whereas G. vaginalis and/or A. vaginae are absent or its amount is substantially less than total amount of bacteria) or suspected dysbiosis (amount of G. vaginalis and/or A. vaginae is similar to the amount of Lactobacillus spp. but does not exceed the limit value) using the software tool provided by the kit manufacturer. All swab results other than BV negative were classified as a BV positive qPCR result for this study [14]. Relevant information about patient characteristics and treatments (such as age, duration of subfertility, antibiotic use) was extracted from the electronic patient dossiers. This information was managed using Castor EDC (electronic data capture, 2021), a cloud-based clinical data management service.
Outcome
The main outcome was the association between a positive qPCR result and the vaginal pH value. The secondary outcomes of interest were the test characteristics of each pH value for BV namely, sensitivity, specificity, area under the curve (AUC) and diagnostic odds ratio (OR).
Statistical analysis
IBM SPSS statistics for Macintosh, Version 27, released 2020, was used for all analysis. Continuous parametric variables were analysed using an unpaired t test. Continuous non-parametric variables were analysed using the Mann–Whitney U test. Categorical variables were analysed using the Chi2 or Fisher’s exact test. To account for the repeated measurements per case a general estimating equations analysis (GEE) was used. ROC curves were made to find the cut-off that maximises sensitivity and specificity. Contingency tables were used to assess the performance of each pH value for predicting BV.
Results
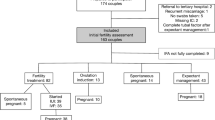
A total of 102 eligible women were included. In total 170 pH and qPCR swab combinations were available for analysis. Figure 1 shows the included number of combinations for each timepoint (IFA and ART treatments). One woman contributed five combinations, and four women contributed four combinations. All other women contributed three or less combinations (data not shown).
The baseline characteristics of women with at least one set of pH and BV measurement are shown in Table 1. The total number of women is 102 instead of 99 to account for three women who had an incomplete measurement set at baseline but contributed valid pH swab combinations at following measurements. Only eight patients mentioned mild discharge complaints, with no need to treat with antibiotics. Most of the population were of Caucasian decent (45% non-Caucasian) and had a high social economic status (62% of total). Most women were expectantly managed or conceived spontaneously. Of the 29 women who were expectantly managed, seven switched to IUI after several months. Of the nine women receiving ovulation induction, one switched to IUI. One woman changed after one IUI procedure to IVF. One woman had an escape IUI because of poor response during IVF treatment.
In some women, their qPCR results differed over time. Seven women who received IVF, and two women who received IUI showed a negative qPCR result at baseline but became positive during their treatment. In one of them, her qPCR result changed to positive at her second IUI attempt, after using antibiotics for a urinary tract infection. Two women (one IUI, one IVF treatment) changed from positive, to negative, to positive again.
The main outcomes are shown in Tables 2, 3. Of all 170 swabs, 125 (74%) were classified as BV negative, 37 as BV positive, five as ‘unspecified dysbiotic’ and three as ‘suspected dysbiosis’. Therefore, 45 (26%) were considered a positive qPCR result. A significant relationship between pH and qPCR outcome was found [p = 0.000, OR 3.06; 95% confidence interval (CI) 1.65–5.68] (Table 2).
A pH value ≥ 4.7 was the best predictor for a positive qPCR result (OR 7.8; 3.35–18.20, p value < 0.001). The cut-off for pH of 4.7 has the highest AUC (AUC 0.74, 95% CI 0.66–0.83) compared to other cut-off values (pH > 4.4, > 5, > 5.3 and higher). The cut-off for pH of 4.7 has a sensitivity of 76% and specificity of 73% for BV positive swabs (Table 3). The optimal cut-off value of 4.7 is also reflected in the ROC curve analysis (Fig. 2). Performing a qPCR-test after a pH value > 4.7 results in the utilisation of 60% fewer qPCR’s overall (Table 4). This method could reduce the price per correct BV diagnosis from €188.89 to €100.50 and total costs of this study from €8500.00 to €3417.00 (calculations shown in Table 4).
Discussion
To our knowledge, this is the first study that investigates the association between pH and BV as diagnosed by qPCR in an asymptomatic subfertile population. Additionally, this study explores the utility of pH as a cost-reducing measure in this population. The estimated price of €50, per qPCR forms a barrier to its clinical adoption despite superior performance versus traditional methods, especially given the backdrop of rising healthcare costs in the Netherlands. Using a value pH ≥ 4.7 as a step-up for qPCR succeeds at lowering total study costs by 60%. The cut-off pH ≥ 4.7 was chosen because it maximised diagnostic value and is in line with previous research on the use of pH as an indicator for BV diagnosed using more traditional methods [15, 16].
This study confirms the high prevalence of BV in a multi-ethnic subfertile population (45% non-Caucasian) with 26% of the women testing positive by qPCR. This percentage is in accordance with the studies of Haahr et al. and Borgdorff et al. These authors report a BV prevalence of 28% and 32% in a respectively 10% non-Caucasian and 90% non-Caucasian population [4, 12].
Lifestyle factors such as smoking, diet, vaginal douching and multiple sexual partners are known to effect BV. No information about vaginal douching and multiple sexual partners have been gathered in this study [13, 17]. Measured factors (smoking, BMI) in this study did not significantly alter the association between pH and BV, probably because of the small study size. Furthermore, hormonal treatment could be of influence on BV status as suggested in a few prior studies investigating the microbiome during IVF [19, 20]. In this study, nine women became positive during treatment, an interesting finding requiring further investigation.
Unexplained subfertility had a possible association with a positive qPCR (p = 0.16), suggesting that BV might be connected to subfertility in this group. Other studies describe that BV might be an unrecognised factor behind unexplained subfertility, but further research on this topic is necessary [7, 21]. If there is a strong association between BV and unknown causes of subfertility, treatment of BV in these cases could be fundamental and potentially lead to more successful outcomes.
A limitation of this study is the sample size, which was too small to determine the influence of relevant factors such as ethnicity or cause of subfertility on the cut-off pH value. More than half of the population was of high social economic status, which could indicate self-selection bias. A second limitation could be the measurement tool for pH only registered values ranging from 4.0 to 7.0. Physiological vaginal pH values can go lower than 4.0 and in some extreme cases of BV surpass 7.0 [22]. Capping the extreme pH values leads to an underestimation of the association between pH and BV. Thirdly, measurement errors might be introduced due to a degree of subjectivity in the visual colour coding of the pH strips.
The utility of these findings to the clinical practice is not yet fully known. Trials should further investigate the possible causal link between BV and fertility outcomes and whether treatment of BV leads to better fertility outcomes. The results of a prospective cohort study about the influence of BV in a general subfertility population (undergoing ART treatment) of this study group are soon to be expected. Another component of uncertainty is the absence of effective methods for the treatment of BV and a lack of knowledge about whether treatment leads to improved live birth rates. New treatment options for BV need to be investigated as well, such as novel anti-microbial agents, concomitant use of vaginal acidification and the use of probiotics or vaginal flora transplantation.
If treatment of BV will improve fertility outcomes, pH and qPCR testing will become a standard part of the initial fertility assessment. At this point, commercial BV or microbiome testing is already a daily practice in some fertility clinics, at high costs. If more evidence underwrites this association, the investment of a qPCR is small relative to the total cost of a failed IVF treatment. Nevertheless, pH could be used as a step up for qPCR to reduce costs even further or as a screening method in the initial fertility assessment. This study details a simple method in which the diagnostic power provided by qPCR can be leveraged at a 60% reduced cost, potentially removing some future hurdles for the implementation of qPCR in the daily fertility practice [17].
Conclusion
This study shows that the vaginal pH for a multi-ethnic, asymptomatic population of women attending fertility clinics is strongly associated with BV qPCR outcome. Using the pH cut-off point of 4.7 has a high sensitivity for the diagnosis of BV and can be achieved at a cost reduction of 60%.
References
Rostad B, Schmidt L, Sundby J, Schei B (2013) Has fertility declined from mid-1990s to mid-2000s? Acta Obstet Gynecol Scand (John Wiley & Sons, Ltd) 92:1284–1289
Hunault CC, Habbema JDF, Eijkemans MJC, Collins JA, Evers JLH, Veldete ER (2004) Two new prediction rules for spontaneous pregnancy leading to live birth among subfertile couples, based on the synthesis of three previous models. Hum Reprod 19:2019–2026
Greil AL, Slauson-Blevins K, McQuillan J (2010) The experience of infertility: a review of recent literature. Sociol Health Illn (Wiley & Sons, Ltd) 32:140–162
Haahr T, Jensen JS, Thomsen L, Duus L, Rygaard K, Humaidan P (2016) Abnormal vaginal microbiota may be associated with poor reproductive outcomes: a prospective study in IVF patients. Hum Reprod 31:795–803
Kindinger LM, Bennett PR, Lee YS, Marchesi JR, Smith A, Cacciatore S et al (2017) The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome. BioMed Central 5:6
Koedooder R, Singer M, Schoenmakers S, Savelkoul PHM, Morré SA, de Jonge JD et al (2019) The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: a prospective study. Hum Reprod 34:1042–1054
Salah RM, Allam AM, Magdy AM, Mohamed AS (2013) Bacterial vaginosis and infertility: cause or association? Eur J Obstet Gynecol Reprod Biol (Elsevier) 167:59–63
Haahr T, Zacho J, Bräuner M, Shathmigha K, Skov Jensen J, Humaidan P (2019) Reproductive outcome of patients undergoing in vitro fertilisation treatment and diagnosed with bacterial vaginosis or abnormal vaginal microbiota: a systematic PRISMA review and meta-analysis. . BJOG: Int J Obstet Gynaecol (John Wiley & Sons, Ltd) 126:200–207
Ralph SG, Rutherford AJ, Wilson JD (1999) Influence of bacterial vaginosis on conception and miscarriage in the first trimester: cohort study. BMJ (BMJ Group) 319:220–223
Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM et al (2006) High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis 193:1478–1486
Ravel J, Moreno I, Simón C (2021) Bacterial vaginosis and its association with infertility, endometritis, and pelvic inflammatory disease. Am J Obstet Gynecol 224:251–257
Borgdorff H, van der Veer C, van Houdt R, Alberts CJ, de Vries HJ, Bruisten SM et al (2017) The association between ethnicity and vaginal microbiota composition in Amsterdam, the Netherlands Fredricks DN, editor. PLoS ONE (Public Library of Science) 12:e0181135
Hellberg D, Nilsson S, Mårdh PA (2000) Bacterial vaginosis and smoking. Int J STD AIDS (SAGE Publications, Sage UK: London, England) 11:603–606
van den Munckhof EHA, van Sitter RL, Boers KE, Lamont RF, Te Witt R, le Cessie S et al (2019) Comparison of Amsel criteria, Nugent score, culture and two CE-IVD marked quantitative real-time PCRs with microbiota analysis for the diagnosis of bacterial vaginosis. Eur J Clin Microbiol Infect Dis (Springer, Berlin, Heidelberg) 38:959–966
Simoes JA, Discacciati MG, Brolazo EM, Portugal PM, Dini DV, Dantas MCM (2006) Clinical diagnosis of bacterial vaginosis. Int J Gynaecol Obstet (Wiley) 94:28–32
Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK (1983) Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 74:14–22
García-Velasco JA, Budding D, Campe H, Malfertheiner SF, Hamamah S, Santjohanser C et al (2020) The reproductive microbiome—clinical practice recommendations for fertility specialists. Reprod Biomed Online 41:443–453
Nouioui I, Carro L, García-López M, Meier-Kolthoff JP, Woyke T, Kyrpides NC et al (2018) Genome-based taxonomic classification of the phylum Actinobacteria. Front Microbiol 9:2007
Hyman RW, Herndon CN, Jiang H, Palm C, Fukushima M, Bernstein D et al (2012) The dynamics of the vaginal microbiome during infertility therapy with in vitro fertilization-embryo transfer. J Assist Reprod Genet 29:105–115
Carosso A, Revelli A, Gennarelli G, Canosa S, Cosma S, Borella F et al (2020) Controlled ovarian stimulation and progesterone supplementation affect vaginal and endometrial microbiota in IVF cycles: a pilot study. J Assist Reprod Genet 37:2315–2326
Spandorfer SD, Neuer A, Giraldo PC, Rosenwaks Z, Witkin SS (2001) Relationship of abnormal vaginal flora, proinflammatory cytokines and idiopathic infertility in women undergoing IVF. J Reprod Med J Reprod Med 46:806–810
O’Hanlon DE, Moench TR, Cone RA (2013) Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS ONE 8:e80074
Acknowledgements
Thanks to Professor Dr. S. le Cessie of the department of Biomedical Data Sciences at the Leiden University Medical Center for her advice on all statistical matters but particularly the GEE analysis. A special thanks to the HMC fertility doctors and their team for including patients and retrieving samples.
Funding
This article was funded by a research grant from the Haaglanden Medical Centre. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have stated explicitly that they have no conflict of interest in connection with this article.
Author information
Authors and Affiliations
Contributions
MMvdT: protocol/project development, data collection and management, data analysis, manuscript writing. SvdS: data collection, data analysis, manuscript writing. EHAvdM: protocol/project development, manuscript editing. KEB: protocol/project development, manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
This article was funded by a research grant from the Haaglanden Medical Centre. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have stated explicitly that they have no conflict of interest in connection to this article.
Compliance with ethical standards
The trial was approved by the local ethics board (medical ethics committee Leiden-Delft-Den Haag, reference Z21.031). Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van den Tweel, M.M., van der Struijs, S., van den Munckhof, E.H.A. et al. The relationship between vaginal pH and bacterial vaginosis as diagnosed using qPCR in an asymptomatic subfertile population. Arch Gynecol Obstet 306, 1787–1793 (2022). https://doi.org/10.1007/s00404-022-06764-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06764-1