Abstract
Purpose
This study aimed to estimate the incidence of fatal amniotic fluid embolism, describe its risk factors, and analyze perinatal outcomes.
Methods
Maternity cases and newborn records of amniotic fluid embolism were collected from the Zhejiang Maternal Surveillance System from October 2006 to October 2019. This study strictly limited the diagnostic criteria for AFE and excluded suspicious cases in order to minimize false-positive AFE cases. The risk factors of fatal amniotic fluid embolism and the relationship between perinatal prognosis and AFE were investigated using logistic regression analysis, estimating the adjusted odds ratios (aORs) and 95% confidence intervals (CIs).
Results
149 cases of amniotic fluid embolism were registered, of which 80 cases were fatal. The estimated fatal AFE incidence was 0.99 per 100,000. The occurrence of fatal AFE was significantly correlated with spontaneous vaginal delivery (aOR 12.3, 95% CI 3.3–39.2) and cardiac arrest (aOR 64.8, 95% CI 14.6–287.8). The average diagnosis time of fatal AFE is 85.51 min, and the peak period of female death is 1–12 h after the onset of the disease, accounting for 60% (48/80) of cases. Fatal amniotic embolism is a cause of intrauterine fetal death and fetal death during delivery (aOR 11.957, 95% CI 1.457–96.919; aOR 13.152, 95% CI 1.636–105.723). Of the 149 confirmed AFE cases, 11 cases of stillbirth occurred, 12 cases were stillborn, and 7 cases of neonatal death were reported. The perinatal mortality rate was 202 per 1000.
Conclusions
Early detection, diagnosis, and treatment of amniotic fluid embolism are essential to avoiding fatal AFE. Clinicians should fully evaluate the pros and cons of choosing the delivery method for pregnant women. When cardiac arrest occurs in women with amniotic fluid embolism, obstetricians should be particularly careful and provide timely and effective treatment to minimize the fatality rate. The outcome of AFE is not only related to maternal survival but also plays a decisive role in the prognosis of the infant over the perinatal period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amniotic fluid embolism (AFE) is a rare complication during pregnancy and is one of the main causes of maternal death and adverse perinatal outcomes. Depending on reports, the maternal mortality rate in developed countries is 3.5 per 100,000, of which the incidence of maternal death due to amniotic fluid embolism is 5–24.3% [1,2,3]. Because different countries have different diagnostic criteria and statistical methods for AFE, there are definite differences in the calculated incidence and mortality of AFE. However, the mortality rate of AFE did not show a clear downward trend over time. In 1941, Steiner and Lushbaugh studied the autopsy results of 32 patients with amniotic fluid embolism. They suggested that the fetal components (fetal squamous cells and mucin) in the maternal blood circulation were the main cause of death in patients with amniotic fluid embolism [4]. Interestingly, the large amount of research accumulated by many scholars over the past 50 years all demonstrate the viewpoints put forward by Steiner and Lushbaugh [1, 3]. However, the pathogenesis and pathophysiology of amniotic fluid embolism are still incompletely understood. The currently accepted hypothesis involves the mechanical obstruction of the maternal circulation by amniotic fluid components when the maternal–fetal barrier is overcome. The maternal body produces an immune response to fetal antigens, and a cascade of reactions occurs, resulting in a systemic inflammatory response syndrome, which in turn causes a series of manifestations such as pulmonary hypertension, pulmonary edema, severe hypoxemia, respiratory failure, circulatory failure, cardiac arrest, disseminated intravascular coagulation (DIC) and multiple organ dysfunction syndrome (MODS) [5,6,7].
Due to the uncertainty of the molecular biological mechanism of amniotic fluid embolism, in recent years, researchers have focused on the analysis and identification of high-risk factors for amniotic fluid embolism. Still, most of the literature lacks an analysis of the relationship between AFE and neonatal outcomes. Only a few articles mentioned the time trend of the occurrence of AFE. Furthermore, they lacked a detailed description of the time trend, the incidence of AFE and the correlation between maternal outcomes [8].
This study aims to estimate the risk factors of fatal amniotic fluid embolism and describe its association with perinatal outcomes and the possible temporal patterns of adverse AFE outcomes.
Methods
A population-based case-cohort study was carried out through the Zhejiang Provincial Maternal Surveillance System. All cases of maternal amniotic embolism occurring in various cities are reported to county-level institutions by township or district, reported to municipal and provincial maternal and child health institutions after layer-by-layer verification, and finally incorporated into the national annual report on maternal health and health summary to form the relevant clinical data. Ascertainment of cases of AFE was based on International Classification of Disease, tenth revision (ICD-10) code O88.1.
The inclusion criteria for this study were cases in Zhejiang Province that met the above diagnostic criteria for AFE. Confirmed cases of AFE met at least the clinical diagnosis or the pathological diagnosis; the standard is presented in Fig. 1. The exclusion criteria in this study were the diagnostic criteria for false-positive cases. False-positive AFE cases include clinical false-positive cases and paradoxical cases. Clinical false-positive cases were defined as a controversial clinical diagnosis without pathological examination despite being diagnosed and registered as AFE. Through a retrospective analysis of the clinical symptoms, signs, examination results and management of the case, it was finally concluded that the case was misdiagnosed. Paradoxical cases were characterized by the absence of fetal component in maternal blood on autopsy despite being clinically diagnosed as AFE and entered into the monitoring system. If the clinical diagnosis does not match the pathological result, the case was identified as a non-AFE case.
To reduce the false-positive diagnosis of AFE, each case of amniotic fluid embolism needs to provide detailed information about the pregnant and lying-in women and newborns, perinatal management, and all the diagnosis and treatment of this pregnancy. Through rigorous collection and statistics, suspicious cases were selected, and false-positive cases were eliminated to improve the research accuracy. Therefore, all samples were collected randomly and rigorously.
The statistical methods of this study adopt descriptive analysis and regression analysis. The descriptive analysis uses frequency, percentage, variance and standard deviation to illustrate the incidence of amniotic fluid embolism, mortality and the proportion of fatal AFE. Logistic regression was used to calculate and analyze all the real and available data included in the Zhejiang Provincial Maternal Surveillance System from 2006 to 2019. Unadjusted odds ratios (ORs), adjusted odds ratios, and 95% confidence intervals were calculated to study the risk factors of fatal AFE and the potential factors affecting the prognosis of newborns. The potential interactions in the regression model were verified by adding interaction terms between all variables in the model and subsequent likelihood-ratio testing on removal. P < 0.05 was considered statistically significant. All data were analyzed using SPSS 23 software.
Result
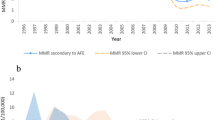
Among the 8,080,147 total deliveries in the monitoring system, 688 cases of maternal death were recorded, resulting in a maternal mortality rate of 8.52 per 100,000. According to database statistics, a total of 149 cases were qualified for AFE diagnosis, including 69 cases of non-fatal AFE and 80 cases of fatal AFE. The incidence of AFE was 1.844 per 100,000. The 80 patients who died of AFE accounted for 11.63% of maternal deaths, and the incidence of fatal AFE was 0.99 per 100,000. Figure 2 shows the annual incidence of fatal AFE (1/100,000), maternal mortality (1/100,000) and the proportion of AFE in the death population (%) during the 14 years from 2006 to 2019. Although the maternal mortality rate decreases year by year, the incidence of fatal AFE has not shown a significant decrease over time.
Characteristics of women with non-fatal AFE and fatal AFE
The characteristics of 149 women with amniotic fluid embolism are shown in Table 1. Amniotic fluid embolism occurred in 84 women before or during delivery, whereas 65 patients developed the condition after delivery. Among these postpartum AFE cases, forty-four women (55%) experienced poor outcomes.
Risk factors analysis for confirmed cases of fatal AFE
Table 2 describes the risk factors for fatal amniotic fluid embolism. In order to illustrate the OR, aOR and 95% CI of each risk factor in the table, the risk factors were divided into four categories: general characteristics, complications during pregnancy, intrapartum and postpartum complications, and perinatal management. There was a significant association with maternal age (OR 2.217, 95% CI 1.122–4.377 in women aged 35 and over compared with women aged under 35). The adjusted analysis revealed that maternal age was not significantly related to fatal amniotic fluid embolism (aOR 1.142, 95% CI 0.518–2.516). We have concluded that the mode of delivery, postpartum complications,meconium contamination degree III maternal cardiac arrest and postpartum AFE can increase the risk of fatal amniotic fluid embolism. On the contrary, potent drugs that promote uterine contraction and pregnancy complications such as abnormal uterine pressure, pre-eclampsia, placenta previa, premature rupture of membranes and placental abruption are not related to the increased risk of fatal amniotic fluid embolism. In addition, fatal amniotic fluid embolism is significantly correlated with delivery methods, especially with spontaneous vaginal delivery (aOR 20.917, 95% CI 3.213–136.175). Meconium contamination degree III is a positive factor in fatal AFE (aOR 6.5, 95% CI 1.8–18.8).
Prodrome of amniotic fluid embolism
Fifty-three women had clinical manifestations of cardiac arrest when amniotic fluid embolism occurred, and 50 of them (62.5%) eventually developed fatal amniotic fluid embolism (aOR 70.817, 95% CI 12.822–391.135 in women with cardiac arrest). More than half of women (53/80, 66.2%) had prodromal symptoms when the fatal amniotic fluid embolism occurred (Table 3). 24.5% (13/53) of the symptoms involved a single system, whereas 75.5% (40/53) involved multiple systems. Of the 53 fatal AFE women's prodromal symptoms, the cardiovascular system was most affected, accounting for 83.75 percent. The main symptoms included tachycardia, arrhythmia, low blood pressure, cardiac arrest, of which the first symptoms of cardiac arrest there were 50 cases. Hemodynamic changes were the second most common type of symptoms, accounting for 31.3%, mainly manifested as postpartum hemorrhage and DIC. The respiratory system was involved in 19.3% of cases, mainly manifested as dyspnea, shortness of breath, cyanosis, respiratory failure or ARDS. The nervous system accounted for 20.6%, mainly manifested as convulsions, amaurosis and so on.
Temporal pattern of fatal AFE
Table 4 shows the diagnosis interval and death interval of fatal AFE. The average diagnosis time of fatal AFE was 85.51 min (0–627 min). 48.75% (39/80) of the patients exhibited rapid disease progression, and the clinical manifestations were diagnosed immediately. 23 cases were diagnosed with a delay of more than 1 h, and 9 cases were diagnosed with a delay of 30–60 min. Nine cases were diagnosed within 30 min after the appearance of clinical manifestations. The average time from the onset of clinical manifestations to maternal death due to amniotic fluid embolism was 33.56 h (0–552 h), of which 20% (16/80) died within 1 h after the onset, and 60% (48/80) of deaths occurred in 1–12 h. The 1–12 h after the occurrence of AFE are the peak period of death.
Association of fatal AFE with perinatal outcomes
Table 5 shows the results of perinatal outcomes studies related to amniotic embolism. Of the 149 known cases of amniotic fluid embolism, there were 11 cases of stillbirth, 12 cases were stillborn and 7 cases of neonatal deaths were recorded (the causes of the adverse outcomes above excluded abnormalities in the fetus itself, considering that they were induced by amniotic fluid embolism), and the perinatal mortality rate was 202 per 1000. Amniotic embolism is a notable cause of intrauterine fetal death and fetal death during delivery (aOR 11.957, 95% CI 1.457–96.919; aOR 13.152, 95% CI 1.636–105.723). The adjusted analysis showed that the different outcomes of maternal amniotic embolism were not clearly related to the severity of neonatal asphyxia and neonatal death.
Discussion
The study traced all cases of amniotic fluid embolism registered in the system for 14 years and standardized the diagnostic criteria, which resulted in a significant decrease in the number of confirmed cases of AFE. It is not difficult to see that there is a certain degree of over-reporting of registered cases of AFE. Due to the standardization of diagnostic criteria, the false-positive diagnosis of non-fatal amniotic fluid embolism has been greatly reduced. In contrast, the confirmed cases of fatal AFE have not changed significantly, which ultimately leads to an upward trend in AFE mortality. According to the data analysis of the study, the proportion of maternal deaths caused by amniotic fluid embolism is 11.63%, which is the same as the maternal mortality (5–15%) caused by amniotic fluid embolism in developed countries [2, 7, 9, 10].
Unlike most studies on the risk factors of amniotic fluid embolism, this study further explored the potential risk factors of fatal amniotic fluid embolism. Women with spontaneous vaginal delivery were 12 times more likely to develop fatal AFE than women undergoing cesarean section. In contrast, instrumental vaginal delivery was 2.8 times that of cesarean section, demonstrating that the delivery method is indeed causal with the adverse outcome of AFE. Surprisingly, most studies have proposed that cesarean section is a high-risk factor for increased risk of AFE [11, 12], but for the fatal AFE, cesarean section is a protective factor. The typical clinical manifestations of amniotic fluid embolism are the sudden occurrence of hypoxemia, hypotension and coagulation dysfunction. Some studies have reported that abnormal coagulation in AFE patients is characterized by reduced levels of fibrinogen and coagulation factorV and abnormally increased D-dimer levels [13]. Therefore, the early detection, diagnosis and treatment of AFE are essential to preventing the occurrence of DIC and eventually death. Compared with non-cesarean section patients, the vital signs of women who underwent surgery were monitored more closely during and after surgery. A drop in the patient’s oxygen saturation and blood pressure is detected immediately, allowing for prompt evaluation of the patient's condition. In addition, the initial presentation of amniotic fluid embolism can be manifested in the following situations. When amniotic fluid embolism occurs during cesarean section, severe hemorrhage at the incision site and uterine weakness may be encountered. If the bleeding is difficult to control, hysterectomy can be performed quickly if necessary, delaying other coagulation disorders and preventing DIC. Although the cesarean section is a protective factor for fatal AFE, it is a risk factor for the occurrence of AFE. Therefore, the risks and benefits of delivery by cesarian section should be fully assessed. For women with spontaneous vaginal delivery and instrumental vaginal delivery, the evaluation of bleeding and vital signs monitoring during labor and after delivery should be strengthened to reduce the missed diagnosis of AFE and the incidence of fatal AFE.
66.2% of women with fatal AFE showed one or more conscious symptoms when amniotic fluid embolism occurred, such as arrhythmia, cardiac arrest, dyspnea, bleeding, convulsions, etc. Women with amniotic fluid embolism who developed cardiac arrest were 64.8 times more likely to die than those without the condition. Therefore, cardiac arrest is a major risk factor for fatal AFE. Shock is a pathological state of cellular hypoxia caused by insufficient tissue perfusion, and cardiac arrest is a key clinical manifestation of hemodynamic disorders. Amniotic fluid embolism is unpredictable. Due to rapid disease progression, clinicians should identify the characteristic presentation of the disease progression in time and initiate early active treatment and carry out anti-shock treatment as soon as possible to restore maternal hemodynamic stability.
This study indicates that postpartum hemorrhage is a potential high-risk factor that increases the risk of fatal AFE (aOR 5.28, 95% CI 1.8–15.9). However, it is currently impossible to determine whether postpartum hemorrhage is a precursor of amniotic fluid embolism or a possible result of the amniotic fluid embolism. In addition, the poor prognosis of AFE is related to its time of occurrence (aOR 1.3,95% CI 1.2–4.5). The abnormal uterine cavity pressure caused by twins, polyhydramnios, and macrosomia is considered to be an influencing factor in the occurrence of amniotic fluid embolism [10, 11, 14]. However, this study found no correlation between common pregnancy complications and the pregnancy outcome of AFE women. Pregnancy complications include preeclampsia, placenta previa, placental abruption and premature rupture of membranes. However, patients with Meconium contamination degree III showed a 6.5 times higher probability of developing an adverse outcome. In addition, women over the age of 35 were defined as the advanced age group and less than 35 years old as the non-advanced age group. After adjustment, we found that age was not a potential risk factor for fatal AFE. The median age was 38.5 ± 2.24 in the elderly women group and 29.3736 ± 3.22 in the non-elderly women group. Women in the aging group are generally younger, which resulted in little difference in age and similar body functions between the two groups.
At present, few studies have analyzed and statistically studied the interval between AFE occurrence and clinical diagnosis. Among them, MS Kramer reported no significant temporal trend in the occurrence of AFE, despite strong temporal trends in medical induction, cesarean delivery, advanced maternal age and other identified risk factors[8]. Our study found that the maternal mortality rate within 1 h of the occurrence of AFE was 20% (16/80), while the 1–12 h mortality rate of patients with AFE was as high as 60% (48/80), which was the peak period of death.
Numerous studies have focused only on the effects of AFE on the mother while ignoring the prognosis of newborns. A Canadian study on the risk factors of amniotic fluid embolism found that neonatal death is part of the serious consequences of AFE. Still, the study did not provide detailed statistics and descriptions of neonatal outcomes [12]. It is clear that the outcome of the perinatal infant is negatively affected by lethal AFE. Furthermore, the probability of a stillbirth and stillborn in women with fatal AFE is about 11–13 times that of women with non-fatal AFE. The different outcome of AFE is not only related to maternal survival but also plays a critical role in the prognosis of the perinatal infant. Timely and high-quality supportive care has a positive impact on the prognosis of pregnant women and newborns with amniotic fluid embolism.
This study has both strengths and limitations. Its strengths include the large sample for studying AFE through hospital discharges in China. Due to the large sample size, this study goes a step further to analyze the risk factors for fatal AFE, rather than limiting the scope to simple AFE. Therefore, our findings are more targeted and instructive. In addition, our study provides unprecedented assessments of different perinatal outcomes.
China's territorial area is 9,634,057 square kilometers, ranking third in the world. The spatial distribution of its eastern and western regions is significantly different, and the medical level varies significantly between different regions. One limitation of this study is that the collected cases are limited to the eastern Zhejiang Province, which has a superior medical level. In contrast, the economy and medical level are lagging in the western region, and a comparable survey is currently unfeasible. Therefore, the results are more applicable to countries with similar resource settings.
Future research should include maternal AFE cases across multiple regions. More clinical research and evidence-based medicine are needed to provide a clinical basis for the diagnosis, treatment, and prognostic evaluation of amniotic fluid embolism.
References
Wakasa T, Ishibashi-Ueda H, Takeuchi M (2021) Maternal death analysis based on data from the nationwide registration system in Japan (2010–2018). Pathol Int 71:223–231. https://doi.org/10.1111/pin.13076
Bonnet MP, Zlotnik D, Saucedo M, Chassard D, Bouvier-Colle MH, Deneux-Tharaux C (2018) Maternal death due to amniotic fluid embolism: a national study in France. Anesth Analg 126:175–182. https://doi.org/10.1213/ANE.0000000000002511
Kanayama N, Inori J, Ishibashi-Ueda H, Takeuchi M, Nakayama M, Kimura S, Matsuda Y, Yoshimatsu J, Ikeda T (2011) Maternal death analysis from the Japanese autopsy registry for recent 16 years: significance of amniotic fluid embolism. J Obstet Gynaecol Res 37:58–63. https://doi.org/10.1111/j.1447-0756.2010.01319.x
Steiner PE, Lushbaugh CC, Frank HA (1949) Fatal obstetric shock from pulmonary emboli of amniotic fluid. Am J Obstet Gynecol 58:802–805. https://doi.org/10.1016/s0002-9378(16)39241-9
Clark SL (2014) Amniotic fluid embolism. Obstet Gynecol 123:337–348. https://doi.org/10.1097/AOG.0000000000000107
Hiroshi KM (2015) Amniotic fluid embolism: anaphylactic reactions with idiosyncratic adverse response. Obstet Gynecol Survey 70:511–517
Kanayama N, Tamura N (2014) Amniotic fluid embolism: pathophysiology and new strategies for management. J Obstet Gynaecol Res 40:1507–1517. https://doi.org/10.1111/jog.12428
Kramer MS, Rouleau J, Liu S, Bartholomew S, Joseph KS (2012) Amniotic fluid embolism: incidence, risk factors, and impact on perinatal outcome. BJOG 119:874–879. https://doi.org/10.1111/j.1471-0528.2012.03323.x
Tamura N, Farhana M, Oda T, Itoh H, Kanayama N (2017) Amniotic fluid embolism: pathophysiology from the perspective of pathology. J Obstet Gynaecol Res 43:627–632. https://doi.org/10.1111/jog.13284
Conde-Agudelo A, Romero R (2009) Amniotic fluid embolism: an evidence-based review. Am J Obstet Gynecol 201(445):e441–e413. https://doi.org/10.1016/j.ajog.2009.04.052
Shen F, Wang L, Yang W, Chen Y (2016) From appearance to essence: 10 years review of atypical amniotic fluid embolism. Arch Gynecol Obstet 293:329–334. https://doi.org/10.1007/s00404-015-3785-z
Kramer MS, Abenhaim H, Dahhou M, Rouleau J, Berg C (2013) Incidence, risk factors, and consequences of amniotic fluid embolism. Paediatr Perinat Epidemiol 27:436–441. https://doi.org/10.1111/ppe.12066
Schröder L, Hellmund A, Gembruch U, Merz WM (2020) Amniotic fluid embolism-associated coagulopathy: a single-center observational study. Arch Gynecol Obstet 301:923–929. https://doi.org/10.1007/s00404-020-05466-w
Steven L, Clark M (2010) Amniotic fluid embolism. Clin Obstet Gynecol 53:322–328
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, C., Xu, D. & Luo, Q. Fatal amniotic fluid embolism: incidence, risk factors and influence on perinatal outcome. Arch Gynecol Obstet 307, 1187–1194 (2023). https://doi.org/10.1007/s00404-022-06535-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06535-y