Abstract
Study objective
To investigate the short-term outcomes of laparoscopic ureteroneocystostomy in patients with ureteral endometriosis (UE).
Design
Retrospective cohort study of consecutive patients who underwent surgery for the ureter endometriosis with hydronephrosis.
Setting
A private hospital that provide primary, secondary and tertiary care.
Patients
30 consecutive patients with UE who underwent laparoscopic ureteroneocystostomy at our institution between May 2008 and April 2020.
Interventions
Laparoscopic ureteroneocystostomy, if necessary, hysterectomy, salpingo-oophorectomy, cystectomy, partial bladder resection, or partial bowel resection were performed.
Measurements and main results
The most common chief complaint was pelvic pain (40%). Endometriosis affected only the left ureter in 56.7% of patients, only the right ureter in 33.3%, and both ureters in 6.7%. Involvement of the ipsilateral ovary was confirmed in 64.3%. The most frequent location of UE was 1–3 cm above the UVJ (46.7%). A psoas hitch was performed in 7 patients (23.3%), and the Boari flap was used in 9 patients (30%). Hysterectomy was performed in 12 patients (40%), and 6 of them had a concomitant bilateral salpingo-oophorectomy (20%). In addition, 3 patients (10%) underwent partial bowel resection, and 2 patients (6.7%) underwent partial bladder resection. After surgery, 24 of 27 patients (80.0%) were free of sever hydronephrosis after surgery. Hydronephrosis recurred in a single patient (3.3%), but the grade of hydronephrosis improved significantly after surgery (P < 0.001). At 6 months of follow up, 4 patients (13.3%) experienced urinary tract infections and 2 patients (6.7%) reported dysuria. Patients reported a regression of dysmenorrhea symptoms (P < 0.001).
Conclusion
This study shows that ureteroneocystostomy provides good results in terms of relapses and symptom control in patients with ureteral endometriosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endometriosis, defined as the presence of endometrial glands and stroma outside the uterus [1], has an estimated prevalence of 5–15% in women of reproductive age [2]. Urinary tract involvement occurs in 1% of affected women; it involves the ureter in 10% [3]. Ureteral endometriosis (UE) is typically unilateral, with a predisposition for the left side in 64% of affected women [4]. Examination of resected ureteral segments reveals 2 types of involvement: 38.5% demonstrate endometriosis inside the muscular layer of the ureter (intrinsic UE), and 61.5% show adventitial infiltration (extrinsic UE) [5].
Dysmenorrhea is extremely common in patients with UE, reported by 70.6%, while 52.3% report pelvic pain [6]. The presence of UE is frequently associated with endometriosis of the bladder (47%) and the bowel (43%) [7]. Hydronephrosis is present in 61% of patients with UE [6]. Combined Oral contraceptives and progestins are first-line therapies for pain associated with UE [8], but surgical treatment is necessary to salvage the renal system when UE causes ureteral obstruction [9]. Both conservative procedures (for example, ureterolysis) and more radical surgery (ureteroneocystostomy) are commonly performed [10]. Ureteroneocystostomy has a lower recurrence rate but a higher surgical complication rate than ureterolysis [11].
In recent years, minimally invasive techniques have been used successfully to manage UE [12]. Laparoscopic ureteroneocystostomy requires either extravesical reimplantation of the ureter, a psoas hitch, or a Boari flap, depending on the length of the proximal residual ureter [13]. However, the outcomes of laparoscopic ureteroneocystostomy in patients with severe UE are unknown. The purpose of this study is to investigate the short-term outcomes of laparoscopic ureteroneocystostomy.
Materials and methods
This retrospective study assessed 30 consecutive patients with UE who underwent laparoscopic ureteroneocystostomy at our institution between May 2008 and April 2020. All patients were referred to our center with a diagnosis of endometriosis and suspected UE. Inclusion criteria for ureteroneocystostomy included moderate to severe hydronephrosis with radiologic evidence of ureteral stricture, and/or symptomatic bladder endometriosis. Exclusion criteria were a history or presence of ovarian cancer related to endometriosis and ureteral stricture with complete loss of renal function. We performed ureteroneocystostomy in patients with hydronephrosis who had radiologic evidence of a ureteral stricture and in patients with endometriotic bladder lesions requiring resection of the ureterovesical junction (UVJ). Patients were evaluated and treated by a multidisciplinary team comprising gynecologists, urologists, gastroenterological surgeons, radiologists, and pathologists.
We used the revised American Fertility Society classification of endometriosis, endorsed by the American Society for Reproductive Medicine (ASRM), to define the stages of endometriosis in each patient. The intensity of dysmenorrhea was assessed using a Visual Analogue Scale (VAS) at 1 month before treatment and 6 months after treatment. The grade of hydronephrosis was recorded at 1 month before treatment and 6 months after treatment. It was evaluated by the Society of Fetal Urology grading system.
Preoperative evaluation included a physical examination, blood testing, transabdominal and transvaginal pelvic ultrasonography, chest radiography, pelvic computed tomography, and pelvic magnetic resonance imaging. Postoperative evaluation of the hydronephrosis was done by transabdominal ultrasonography. A preoperative double-J catheter was placed when patient complained pelvic pain or hydronephrosis was severe. All patients were informed of the risks and possible complications of surgery. Written informed consent was obtained from all patients prior to surgery. This retrospective study was performed in accordance with the Declaration of Helsinki was and approved by the Institutional Review Board of our hospital (IRB number: 877). All patients provided written informed consent for inclusion in this study.
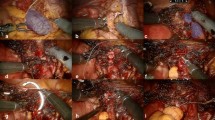
All surgical procedures were performed by a single surgeon (MA), experienced in laparoscopic ureteroneocystostomy, with the assistance of the gynecologists on his surgical team. Hysterectomy, salpingo-oophorectomy, cystectomy, partial bladder resection, or segmental bowel resection were also performed when necessary. Ureteroneocystostomy and bowel resection were performed with the support of urologists and gastroenterological surgeons, respectively. Laparoscopic surgery was performed under pneumoperitoneum with intra-abdominal pressure of 8 mm Hg. A 10-mm laparoscope was placed at the umbilical position, with 3–5-mm suprapubic trocars used for instrumentation (Fig. 1). Extravesical laparoscopic ureteroneocystostomy was performed in 7 defined steps:
-
1.
The ureter was separated from its surrounding tissue using monopolar or scissors forceps.
-
2.
After locating the margin of the bladder by instilling 200 mL of saline, the medial and lateral paravesical spaces and the retropubic space of Retzius were dissected. The bladder was then mobilized.
-
3.
The length of the proximal residual ureter was assessed, and it was determined whether a psoas hitch or a Boari flap was needed to avoid anastomotic tension. If it was not needed, we proceeded with extravesical reimplantation.
-
4.
The ureter was sutured to the bladder mucosa using 3-0 synthetic absorbable suture (Fig. 2a, b).
-
5.
A double-J catheter was placed into the ureter when half of the anastomotic suturing was completed.
-
6.
The Lich–Gregoir technique was used to close the bladder muscle over the ureter, using 4-0 synthetic absorbable suture to prevent vesicoureteral reflux (Fig. 2c, d).
-
7.
An ureteral catheter was placed into the bladder.
For ureteric defects longer than 5–7 cm, a length that cannot be bridged using the psoas hitch technique alone, we added a Boari flap to reduce the distance between the ureter and the bladder, thereby reducing anastomotic tension. After completing the first 2 steps of the extravesical ureteroneocystostomy, the Boari flap method was performed as follows:
-
1.
Anastomotic tension was assessed between the ureter and the area of the bladder with maximum mobilization (Fig. 3a).
-
2.
The bladder wall was incised and a rectangular flap, 3–4 cm in width, was created (Fig. 3b).
-
3.
A submucosal tunnel was created in the flap to allow the ureter to run between the mucosa and the detrusor muscle (Fig. 3c).
-
4.
The bladder flap was fixed to the psoas muscle using size 0 synthetic absorbable suture (Fig. 3d).
-
5.
The ureter was pulled through the submucosal tunnel and anastomosis sutured was performed (Fig. 3e), with a double-J catheter placed when half of the anastomosis was finished.
-
6.
The bladder was closed in 2 layers (mucosa and detrusor), using 3-0 synthetic absorbable suture (Fig. 3f).
We removed the ureteral catheter on postoperative day 5–7. The double-J catheter was removed 2–3 weeks after surgery.
Statistical analysis
Statistical analysis was performed using Excel functions (Microsoft Office 2016 for Mac version 16). We used the Wilcoxon matched pairs signed rank test to compare preoperative and postoperative endometriosis-related pain. The Wilcoxon signed rank test was used to compare preoperative and postoperative grade of hydronephrosis. The chi-square test was used to compare the characteristics of patients with intrinsic and extrinsic UE. Statistical significance was defined as a P value less than 0.05.
Results
A total of 30 patients with radiologically diagnosed, histologically confirmed UE were included in this retrospective analysis. The median patient age was 38.5 years (range, 25 to 51 years); the vast majority (80%) of patients were in their 30 s and 40 s. Table 1 lists the patient characteristics. A total of 56.7% of patients were nulliparous, 20% were primiparous, 16.7% had a history of 2 prior deliveries, and 6.7% had undergone 3 deliveries. The most common chief complaint was pelvic pain (40%). An annual health check at workplace brought 5 patients with hydronephrosis and 4 patients with ovarian cysts to our hospital for further examination. An annual health check at workplace includes physical examination, blood testing, transabdominal and transvaginal pelvic ultrasonography. About one-third of patients underwent hormonal therapy with combined oral contraceptives or progestins before surgery.
Table 2 shows the preoperative findings in our patient cohort. Moderate (Grade 2, 3) to severe (Grade 4) hydronephrosis was present in 26 patients (86%), and a double-J stent was placed in 18 patients (60%) before surgery. The grade of preoperative hydronephrosis was unclear in three patients (10%) since preoperative double-J stent was placed at other hospital. One patient (3.3%) with grade 0 (normal) hydronephrosis underwent ureteroneocystostomy because of bladder endometriosis. Endometriosis affected only the left ureter in 56.7% of patients, only the right ureter in 33.3%, and both ureters in 6.7%. Involvement of the ipsilateral ovary was confirmed in 64.3%. Cancer antigen 125 (CA 125) levels were abnormal in 43.3%.
Table 3 shows the procedures performed and the intraoperative findings. Twelve of the 30 patients (40%) undergoing ureteroneocystostomy underwent concurrent hysterectomy; 6 of these (20%) also underwent bilateral salpingo-oophorectomy (BSO). In addition, 3 patients (10%) underwent partial bowel resection, and 2 patients (6.7%) underwent partial bladder resection. Stage I endometriosis was present in 3.3% of patients, stage II in 10%, stage III in 13.3%, and stage IV was present in 50%. The most frequent location of UE was 1–3 cm above the UVJ (46.7%). A psoas hitch was performed in 7 patients (23.3%), and the Boari flap was used in 9 patients (30%).
A comparison of UE lesions with ureteroneocystostomy procedures is listed in Table 4. Of the 13 patients with UE lesions 1–3 cm above the UVJ, extravesical reimplantation alone was performed in 9 patients (69.2%), a psoas hitch was used in 3 (23.1%), and a Boari flap was used in 1 patient (7.7%). The patient who received a Boari flap also underwent partial bladder resection. Of the 11 patients with UE lesions 4–6 cm above the UVJ, extravesical reimplantation was performed in 5 patients (45.5%), a psoas hitch was used in 3 (27.3%), and a Boari flap was used in 3 (27.3%). Of the 6 patients with UE lesions more than 7 cm above the UVJ, a psoas hitch was used in 1 patient (16.7%), and a Boari flap was used in 5 (83.3%).
Table 5 shows the postoperative findings. 29 of 30 patients (96.6%) were free of sever to mild hydronephrosis (Grade 2–4) after surgery, 27 patients had no hydronephrosis (Grade 0) and 2 patients had mild hydronephrosis (Grade 1). Hydronephrosis recurred in a single patient (3.3%), but the grade of hydronephrosis improved significantly after surgery (P < 0.001, Fig. 4). Patients reported that the intensity of their dysmenorrhea improved significantly 6 months after surgery (P < 0.001, Fig. 5). Histological examination showed that 60% of UE lesions were intrinsic. At 6 months of follow up, 4 patients (13.3%) experienced urinary tract infections and 2 patients (6.7%) reported dysuria. About two-thirds of patients required hormonal therapy with combined oral contraceptives or progestins after surgery to prevent relapses. Of the 18 patients who retained their uterus, 1 had a single subsequent delivery and 2 patients had 2 subsequent deliveries.
Discussion
The presence of dysmenorrhea in women of reproductive age raises the possibility of UE. Up to 80% of patients with UE do not have any urinary symptoms [14], making the diagnosis difficult. Five of the patients (16.7%) in our study had hydronephrosis detected during annual health check at workplace. Such patients are at risk for decreased renal function if their hydronephrosis remains undetected. The most frequent chief complaint noted by our patients was pelvic pain (reported by 40%). Uccella et al. report that 52.3% of patients with UE report pelvic pain [6]. To avoid missing a potentially serious diagnosis such as UE, abdominal ultrasonography should be performed in all women with pelvic pain.
Pharmacologic therapy does nothing to ameliorate the fibrotic, narrowed ureter that results from UE; it can only relieve the pain related to endometriosis [15]. Surgical treatment is necessary to remove any areas of ureteral stenosis, but the recurrence rate of hydronephrosis after ureterolysis is as high as 12% [16]. In contrast, the recurrence rate of hydronephrosis after ureteroneocystostomy is 3.1% [17]. This difference in recurrence may be results from the fact that intrinsic UE occurs hydronephrosis more frequently than extrinsic UE [18]. However, it is difficult to differentiate intrinsic and extrinsic UE preoperatively.
Since intrinsic UE directly involves the muscularis of the ureter, removing the endometriotic lesion surrounding the ureter is inadequate treatment. In our study, 60% of lesions were of the intrinsic type. Patients with intrinsic UE tend to have endometrial lesions of the bladder and rectum (Supplemental table). Sillou et al. report that an assessment of the ureteric circumference at the area of the endometriotic lesion can predict whether lesions are intrinsic [19]. However, this is only speculation. If we cannot rule out intrinsic UE preoperatively, ureteroneocystostomy is a better surgical treatment than ureterolysis in terms of preventing recurrence. We found a recurrence rate of hydronephrosis was 3.3% after ureteroneocystostomy, similar to the rate reported by Ceccaroni et al. [17]. Our experience confirmed that reducing anastomotic tension is a critical part of ureteroneocystostomy [20]. Our results show that when the distance between the UE lesion and the UVJ is greater than 7 cm, either a psoas hitch or a Boari flap is necessary. Boari flaps are not necessary when the UE lesion is within 3 cm of the UVJ, except when partial bladder resection is performed. Both of these methods can be performed with robotic assistance [21].
Improving the pain associated with endometriosis is an important factor in patient satisfaction with whatever procedure is performed. We found that the intensity of dysmenorrhea improves significantly after ureteroneocystostomy. Alves et al. and Bastu et al. also report that the intensity of dysmenorrhea, dyspareunia, and chronic pelvic pain improves significantly after surgery [22, 23]. The laparoscopic treatment with a diode laser is also effective to relieve chronic pain [24]. The grade of hydronephrosis improves significantly after surgery, and it is important for renal function. Huang et al. also describe postoperative remission of hydronephrosis [25].
This study is one of the largest documented series of patients with UE undergoing laparoscopic ureteroneocystostomy. Since ureteral endometriosis is a rare disease, the number of patients and data is limited; therefore, further studies are necessary. The uniqueness of our study is that we refer to the most frequent location of UE and specific surgical procedure of ureteroneocystostomy, such as Psoas hitch or Boari flap.
Our study is limited in that we did not compare outcomes between the laparoscopic approach and laparotomy. Robot-assisted ureteroneocystostomy has better surgical outcomes (less postoperative pain and decreased blood loss) than open surgery [26]. This indicates the advantage of minimally invasive surgery, including laparoscopic ureteroneocystostomy.
In conclusion, our study indicates that laparoscopic ureteroneocystostomy has the benefits for preventing recurrent hydronephrosis for patients with UE.
References
Vercellini P, Viganò P, Somigliana E, Fedele L (2014) Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 10(5):261–275
Abrao MS, Dias JA, Bellelis P, Podgaec S, Bautzer CR, Gromatsky C (2009) Endometriosis of the ureter and bladder are not associated diseases. Fertil Steril [Internet] 91(5):1662–1667. https://doi.org/10.1016/j.fertnstert.2008.02.143
Berlanda N, Vercellini P, Carmignani L, Aimi G, Amicarelli F, Fedele L (2009) Ureteral and vesical endometriosis. Two different clinical entities sharing the same pathogenesis. Int J Gynecol Obstet 64(9):615–623
Vercellini P, Pisacreta A, Pesole A, Vicentini S, Stellato G, Crosignani PG (2000) Is ureteral endometriosis an asymmetric disease? BJOG An Int J Obstet Gynaecol 107(4):559–561
Antonelli A, Simeone C, Frego E, Minini G, Bianchi U, Cunico SC (2004) Surgical treatment of ureteral obstruction from endometriosis: our experience with thirteen cases. Int Urogynecol J 15(6):407–412
Uccella S, Cromi A, Casarin J, Bogani G, Pinelli C, Serati M et al (2014) Laparoscopy for ureteral endometriosis: Surgical details, long-term follow-up, and fertility outcomes. Fertil Steril [Internet]. https://doi.org/10.1016/j.fertnstert.2014.03.055
Bosev D, Nicoll LM, Bhagan L, Lemyre M, Payne CK, Gill H et al (2009) Laparoscopic management of ureteral endometriosis: the Stanford University Hospital Experience With 96 Consecutive Cases. J Urol [Internet] 182(6):2748–2752. https://doi.org/10.1016/j.juro.2009.08.019
Barra F, Scala C, Ferrero S (2018) Current understanding on pharmacokinetics, clinical efficacy and safety of progestins for treating pain associated to endometriosis. Expert Opin Drug Metab Toxicol [Internet] 14(4):399–415. https://doi.org/10.1080/17425255.2018.1461840
Nezhat C, Paka C, Gomaa M, Schipper E (2012) Silent loss of kidney secondary to ureteral endometriosis. J Soc Laparoendosc Surg 16(3):451–455
Gennaro KH, Gordetsky J, Rais-Bahrami S, Selph JP (2017) Ureteral endometriosis: preoperative risk factors predicting extensive urologic surgical intervention. Urology [Internet] 100:228–233. https://doi.org/10.1016/j.urology.2016.08.016
Barra F, Scala C, Biscaldi E, Vellone VG, Ceccaroni M, Terrone C et al (2018) Ureteral endometriosis: a systematic review of epidemiology, pathogenesis, diagnosis, treatment, risk of malignant transformation and fertility. Hum Reprod Update 24(6):710–730
Lusuardi L, Hager M, Sieberer M, Schätz T, Kloss B, Hruby S et al (2012) Laparoscopic treatment of intrinsic endometriosis of the urinary tract and proposal of a treatment scheme for ureteral endometriosis. Urology [Internet] 80(5):1033–1038. https://doi.org/10.1016/j.urology.2012.07.036
Stein R, Rubenwolf P, Ziesel C, Kamal MM, Thüroff JW (2013) Psoas hitch and Boari flap ureteroneocystostomy. BJU Int 112(1):137–155
Knabben L, Imboden S, Fellmann B, Nirgianakis K, Kuhn A, Mueller MD (2015) Urinary tract endometriosis in patients with deep infiltrating endometriosis: prevalence, symptoms, management, and proposal for a new clinical classification. Fertil Steril 103(1):147–152
Maccagnano C, Pellucchi F, Rocchini L, Ghezzi M, Scattoni V, Montorsi F et al (2013) Ureteral endometriosis: proposal for a diagnostic and therapeutic algorithm with a review of the literature. Urol Int 91(1):1–9
Mereu L, Gagliardi ML, Clarizia R, Mainardi P, Landi S, Minelli L (2010) Laparoscopic management of ureteral endometriosis in case of moderate-severe hydroureteronephrosis. Fertil Steril [Internet] 93(1):46–51. https://doi.org/10.1016/j.fertnstert.2008.09.076
Ceccaroni M, Ceccarello M, Caleffi G, Clarizia R, Scarperi S, Pastorello M et al (2019) Total laparoscopic ureteroneocystostomy for ureteral endometriosis: a single-center experience of 160 consecutive patients. J Minim Invasive Gynecol [Internet] 26(1):78–86. https://doi.org/10.1016/j.jmig.2018.03.031
Seracchioli R, Raimondo D, Di Donato N, Leonardi D, Spagnolo E, Paradisi R et al (2015) Histological evaluation of ureteral involvement in women with deep infiltrating endometriosis: analysis of a large series. Hum Reprod 30(4):833–839
Sillou S, Poirée S, Millischer AE, Chapron C, Hélénon O (2015) Urinary endometriosis: MR Imaging appearance with surgical and histological correlations. Diagn Interv Imaging [Internet] 96(4):373–381. https://doi.org/10.1016/j.diii.2014.11.010
Nezhat CH, Malik S, Nezhat F, Nezhat C (2004) Laparoscopic ureteroneocystostomy and vesicopsoas hitch for infiltrative endometriosis. J Soc Laparoendosc Surg. 8(3–7):3
Yang C, Jones L, Rivera ME, Verlee GT, Deane LA (2011) Robotic-assisted ureteral reimplantation with Boari flap and psoas hitch: a single-institution experience. J Laparoendosc Adv Surg Tech 21(9):829–833
Alves J, Puga M, Fernandes R, Pinton A, Miranda I, Kovoor E et al (2017) Laparoscopic management of ureteral endometriosis and hydronephrosis associated with endometriosis. J Minim Invasive Gynecol 24(3):466–472
Bastu E, Celik HG, Kocyigit Y, Yozgatli D, Yasa C, Ozaltin S et al (2020) Improvement in quality of life and pain scores after laparoscopic management of deep endometriosis: a retrospective cohort study. Arch Gynecol Obstet [Internet] 302(1):165–172. https://doi.org/10.1007/s00404-020-05583-6
Angioni S, Nappi L, Sorrentino F, Peiretti M, Daniilidis A, Pontis A et al (2021) Laparoscopic treatment of deep endometriosis with a diode laser: our experience. Arch Gynecol Obstet [Internet] 304(5):1221–1231. https://doi.org/10.1007/s00404-021-06154-z
Huang JZ, Guo HL, Li JB, Chen SQ (2017) Management of ureteral endometriosis with hydronephrosis: Experience from a tertiary medical center. J Obstet Gynaecol Res 43(10):1555–1562
Isac W, Kaouk J, Altunrende F, Rizkala E, Autorino R, Hillyer SP, Laydner H, Long JA, Kassab A, Khalifeh A, Panumatrassamee K, Eyraud R, Falcone T, Haber GP, Stein RJ (2013) Robot-assisted ureteroneocystostomy: technique and comparative outcomes. J Endourol 27(3):318–323. https://doi.org/10.1089/end.2012.0196
Funding
None.
Author information
Authors and Affiliations
Contributions
TY: protocol/project development, data collection, data analysis, manuscript writing, statistical analysis, and revision. TH: protocol/project development, data analysis, and manuscript writing. SY: protocol/project development and manuscript writing. KK: data collection. SS: data collection. MS: data collection. YY: data analysis and statistical analysis. MA: protocol/project development and responsible surgeon.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest to report.
Ethical approval
This retrospective study was performed in accordance with the Declaration of Helsinki was and approved by the Institutional Review Board of our hospital (IRB number: 877).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yamada, T., Hada, T., Yanai, S. et al. Rate of recurrent hydronephrosis after laparoscopic ureteroneocystostomy for ureteral endometriosis. Arch Gynecol Obstet 306, 133–140 (2022). https://doi.org/10.1007/s00404-022-06462-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06462-y