Abstract
Purpose
To evaluate Foley catheter and controlled release dinoprostone insert compared to foley catheter alone on induction to delivery interval and maternal satisfaction.
Methods
A randomized trial was conducted in a university hospital in Malaysia from December 2018 to May 2019. Term nulliparas with unfavorable cervix (Bishop score ≤ 5) scheduled for labor induction were randomized to Foley catheter and controlled release dinoprostone insert simultaneously or Foley catheter alone. Primary outcomes were induction to delivery interval (hours) and maternal satisfaction on birth experience (assessed by 11-point Visual Numerical Rating Scale VNRS 0–10, higher score more satisfied).
Results
Induction to vaginal delivery intervals was mean ± standard deviation 22.5 ± 10.4 vs. 35.1 ± 14.9 h, P = < 0.001 but maternal satisfaction on birth experience was not significantly different median[interquartile range] VNRS 8[7–9] vs. 8[7–9], P = 0.12 for Foley catheter-controlled-release dinoprostone and Foley catheter alone arms, respectively. Cesarean delivery rates were 35/102(34.3%) vs. 50/101(49.5%), P = 0.02 RR 0.7 95% CI 0.5–0.9 NNTb 6.3 95% CI 3.5–39.4, pain score at 6 h after catheter insertion 5[2–8] vs. 1[1–3], P < 0.001, Bishop score at trial devices removal 9[9–10] vs. 8[7–9], P = 0.001, requirement for oxytocin induction or augmentation 39/102(38.2%) vs. 76/101(75.2%) NNTb 3 95% CI 2.0–4.1, P < 0.001 and amniotomy rates 73/99(73.7%) vs. 81/95(85.3%), P = 0.052 RR 0.9 85% CI 0.8–1.0 in Foley catheter-controlled-release dinoprostone and Foley catheter alone arms respectively.
Conclusion
In nulliparas with unripe cervixes at term, combined Foley catheter and controlled release dinoprostone vaginal insert compared to Foley catheter alone reduces the induction to vaginal delivery interval and cesarean delivery rate but satisfaction was not significantly increased.
Clinical trial registration
ISRCTN2282883, 03/12/2018, “prospectively registered” (https://doi.org/10.1186/ISRCTN12282883).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Induction of labor is a common obstetric procedure; National Health Service England maternity statistic shows that labor induction rate has increased from 20.3% in 2006–2007 to 29.4% in 2016–2017 [1]. The ARRIVE trial shows that induction of labor at 39 weeks in low-risk nulliparous women results in a significantly lower cesarean delivery rate [2]: this seminal study could drive the labor induction rate even higher.
The cervix is composed predominately of the extracellular matrix proteins, collagen, elastin, glycosaminoglycans and smooth muscle cells. During ripening and labor, softening and dilatation are brought on by proteolytic enzymes degrading and rearranging collagen and apoptosis of smooth muscle cells [3]. While the mechanism of ripening is not fully understood, the cervix also undergoes. temporary hyperplasia with a multitude of collagenous cells to support the birthing process[4]. Mechanical methods for cervical ripening include transcervical (single or double) balloon insufflation, laminaria tents, synthetic Dilapan into the cervical canal and catheter injection of fluid into the extra-amniotic space [5]. Evidence on non-pharmacological or physiological methods of cervical ripening such as acupuncture, homeopathy, sexual intercourse, hypnosis, castor oil and breast stimulation are insufficient to guide practice [6].
The five most commonly used methods for cervical ripening in labor induction are Foley catheter, vaginal misoprostol, oral misoprostol, vaginal dinoprostone, and intracervical dinoprostone—among pregnant women with intact membranes [7]. Dinoprostone is available as vaginal or intracervical gels, vaginal tablets and controlled release vaginal inserts for labor induction.
Labor induction in nulliparous women with unripe cervixes is a challenge; their elective induction at term that required prostaglandin ripening results in vaginal delivery rate of about 58% as compared with an over 97% vaginal delivery rate in multiparas [8]. The World Health Organization (WHO) strongly recommends the use of balloon catheter for induction of labor [9].
The controlled-release dinoprostone insert is easily retrieved should tachysystole occur thereby allowing rapid resolution of uterine contractions and normalization of associated fetal heart rate abnormalities [10] which arguably make the insert conveniently safer compared to bolus prostaglandin regimens.
Concurrent Foley catheter and controlled release dinoprostone vaginal insert is also uniquely complementary. Both Foley catheter [11, 12] and controlled-release dinoprostone vaginal insert [13] once inserted are typically left passively in-situ for 24-h. Interim vaginal examinations can be restricted and minimized to only if a specific indication arises, leading to increased maternal satisfaction during the ripening process [14].
These observations motivated our selection of combined Foley catheter and controlled release dinoprostone vaginal insert to compare with Foley catheter alone in the induction of labor of nulliparas with unripe cervixes at term. We sought to evaluate the Foley catheter-vaginal insert combination compared to Foley catheter alone on hastening vaginal birth and improve maternal satisfaction on their birth experience.
Material and methods
This single center, prospective, randomized controlled trial was approved by the Medical Ethics Committee of University Malaya Medical Centre (date of approval November 15, 2018; reference number 201882–6564) and registered in ISRCTN registry on December 3, 2018 (registration number ISRCTN2282883 (https://doi.org/10.1186/ISRCTN12282883). The trial was conducted in University Malaya Medical Centre with the first participant recruited on December 12, 2018 and the last on May 8, 2019. This trial was registered in the Malaysian National Medical Research Register NMRR: 18-3139-44981.
Participants
Women were assessed for eligibility when they are referred or attended for their scheduled labor induction in the Obstetrics Unit, University Malaya Medical Centre, Kuala Lumpur, Malaysia. Women were included if they were nulliparas (no prior pregnancy beyond 20 weeks gestation), age ≥ 18 years, singleton pregnancy, term gestation (≥ 37 weeks) at enrollment, cephalic presentation, intact membranes, Bishop score < 6, reassuring pre-induction fetal heart rate tracing and contraction < 2 in 10 min. We excluded women with known gross fetal anomaly, known allergy to latex or dinoprostone and inability to consent or need for interpreter. Potential participants were approached, provided with the patient information sheet and had verbal enquiries answered by the recruiting investigator (co-author NL). Written informed consent was obtained from all participants. Participants’ characteristics were transcribed onto the case report forms.
Recruitment and randomization
Pre-induction cardiotocography (CTG) and assessment of cervical ripening was done for all participants before randomization. Participants were excluded from this study if CTG was non-reassuring. The randomization sequence was generated using random number generator at random.org in random block of 4 or 8 by investigator (co-author PCT) who is not involved in the recruitment process. The random allocation sequence was placed in a sealed numbered opaque envelope for strict number order assignment to participants. Randomization was by opening the remaining lowest numbered sealed envelope. Participants were randomized to Foley catheter and controlled release dinoprostone vaginal insert 10 mg or Foley catheter alone.
Interventions
A size 18F Foley catheter was inserted into the cervix under aseptic technique. Participants were positioned in the dorsal recumbent position with thighs comfortably abducted, knees flexed and feet flat on the normal hospital bed. A sterile Cusco’s speculum was inserted into the vagina to visualize the cervix then a 18F Foley catheter was inserted through the cervix with a sponge holder. The balloon of the catheter was inflated with 60 ml of sterile water in three aliquots of 20 ml and gentle traction was applied till the balloon met resistance. The external end of the Foley catheter was blocked with a spigot and then taped to the inner thigh without additional tension [15].
In the combined arm, following Foley catheter insertion, the controlled release dinoprostone insert 10 mg was immediately placed through the Cusco’s speculum into the posterior fornix of the vagina by sponge holder. The insert was laid transversely with tape cut to sufficient length to run outside the introitus to permit retrieval if needed.
The time of induction initiation was documented. Fetal heart rate monitoring was performed after the intervention for at least 20 min or until the fetus status was reassuring and every 6 h thereafter if there was no indication for more frequent monitoring. At 6-h after the Foley catheter insertion, participants were evaluated for pain using a visual numerical rating scale VNRS scored from 0 to 10 (high score greater pain). The Foley catheters were removed if any of the following occurred—expulsion, abnormal fetal heart rate tracing, spontaneous rupture of membranes, uterine tachysystole (more than five contraction in ten minutes over 30 min) or uterine hyperstimulation and after 24 h since placement. The controlled release dinoprostone were removed immediately in the event of onset of labor, spontaneous rupture of membranes or at amniotomy, uterine tachysystole or hyperstimulation, non-reassuring fetal heart rate tracing, maternal systemic adverse dinoprostone effect such as nausea, vomiting, hypotension or tachycardia and after 24 h since placement.
The time of the Foley catheter and controlled release dinoprostone insert removal and Bishop score after removal of trial devices were recorded. Amniotomy was performed after Foley catheter removal when the cervical dilatation was at least 2–3 cm with the fetal head station ≤ − 2 (time of amniotomy or spontaneous rupture of membrane (SROM) were recorded). Titrated intravenous infusion of oxytocin for induction was initiated immediately after membranes rupture unless already in established labor (augmentation as needed for poor progress) and oxytocin commencement was recorded.
For participants who did not achieve favorable cervix state of at least 2 cm of cervical dilatation to permit amniotomy, subsequent management was at the discretion of their care provider. Standard care was provided during labor to all participants and management decisions were made by care providers according to usual practice in the best interest of the participants. If not already removed for interim issues or expelled, all trial devices were removed 24 h after insertion.
Trial devices
The Foley catheters were purchased from a commercial hospital supplies purveyor at a cost of USD 1.00 each. The controlled release dinoprostone vaginal insert used in the trial was Cervidil®, Ferring Pharmaceuticals Inc., New Jersey, USA: a standard formulary item at our center. All trial devices were supplied free of charge.
Blinding
Participants and care providers were not masked to the interventions as it was not technically feasible.
Outcome measures
Primary outcomes were intervention to vaginal delivery interval and maternal satisfaction on “your birth experience since the beginning of induction of labor to the birth of your baby” using an 11-point VNRS scored from 0 to 10 (high score, greater satisfaction).
Secondary outcome measures included pain at 6-h after induction started, Cesarean delivery and indication for operative delivery, time of amniotomy, amniotic fluid color, intrapartum oxytocin induction or augmentation, delivery blood loss, maternal temperature ≥ 38 °C from induction to delivery, birthweight, umbilical cord arterial pH, Apgar scores and neonatal intensive care unit admission and indication. After delivery, all participants were given a short questionnaire form to complete. Participants were asked on “recommend the induction method I had to my friend who has to undergo induction of labor” with 5 grade Likert scale response.
Sample size calculation
We powered our study for 2 primary outcomes, induction to vaginal delivery interval and maternal satisfaction on birth experience. PS program version 3.1.2 [16] was used to calculate the sample size.
Induction to vaginal delivery interval:
Yossi’s et al. [17] reports induction to delivery interval for their nulliparas Foley arm of 25.1 h with standard deviation of 13.7 h. For this study, we assumed a 6 h reduction in time interval from induction to vaginal delivery with standard deviation of 13.7 h, then taking alpha of 0.05, power of 80%, 1 to 1 randomization ratio, and applying student t test, 83 women were needed in each arm. We assumed a 10% drop-out, 103 women were needed in each arm and total number participants planned was N = 206.
Maternal satisfaction score with birth process:
For satisfaction score, assuming a 1-point difference in the VNRS (scored from 0 to 10) satisfaction score and that the standard deviation of the satisfaction score is 2, then taking alpha 0.05, power 80%, 1 to 1 randomization ratio and applying Chi Square test, 64 women are needed in each arm (N = 128). Mann Whitney U test was applied instead of the Student test for ordinal data, sample size uplifted by 15%; so, 148 is needed (128 × 1.15). Then factoring a 10% drop-out rate, a total of (148/0.9). N = 165 participants in total were needed.
We planned to enroll 206 women into our trial to be powered to evaluate both primary outcomes.
Statistical analysis
Data were entered into a statistical software package SPSS (Version 23, IBM, SPSS Statistics). The Student t test was used to analyze means with normal data distribution, the Mann–Whitney U test for non-normally distributed data or ordinal data and Chi-square test for categorical data. Two-sided P values were reported and P < 0.05 was regarded as significant. Analysis was on intention-to-treat basis.
Ethical aspects
Women who chose not to participate received standard care. Participants who decided to withdraw may do so without having to give a reason and their care was not affected.
Results
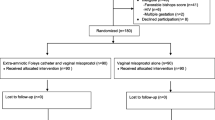
Figure 1 depicts the recruitment flow of participants through the study. A total of 208 nulliparas at term admitted for labor induction were identified or referred by care providers and approached; two women were excluded (one had a favorable Bishop score and the other declined to participate). 206 women consented to participate and were randomized; 103 to each intervention arm. Two participants assigned to Foley catheter alone had spontaneous membranes rupture discovered just prior to catheter insertion and one assigned to the concurrent Foley catheter and controlled release dinoprostone vaginal insert arm withdrew before her Foley catheter insertion as she changed her mind about labor induction. We included all participants for analysis based on the intention-to-treat principle excluding these three women as their data was not collected. We stopped recruitment on reaching the 206 participants sample size target. Data from 203 participants (Foley and controlled release dinoprostone vaginal insert n = 102, Foley alone n = 101) participants were analyzed.
Table 1 shows the characteristics of the trial participants stratified according to treatment allocation. Characteristics were not different across trial arms: specifically, indications for labor inductions were similar.
Table 2 shows the primary outcomes: induction to vaginal delivery intervals were (mean ± standard deviation) 22.5 ± 10.4 vs. 35.1 ± 14.9 h, P < 0.001 (a significant reduction of 12.6 h for the combined arm). Maternal satisfaction on birth experience was not significantly increased median [interquartile range] VNRS 8 [7–9] vs. 8 [7–9], P = 0.12 for the combined and Foley catheter alone arms respectively.
Table 3 lists the secondary outcomes. Cesarean delivery rate was lower by an absolute 15.2% 35/102 (34.3%) vs. 50/101 (49.5%), P = 0.02 RR 0.7 95% CI 0.5–0.9 NNTb 6.3 95% CI 3.5–39.4, pain score at 6 h after induction was raised 5 [2–8] vs. 1 [1–3], P < 0.001, Bishop score at devices removal or expulsion was higher 9 [9–10] vs. 8 [7–9], P = 0.001, requirement for oxytocin induction or augmentation increased 39/102 (38.2%) vs. 76/101 (75.2%) NNTb 3 95% CI 2.0–4.1, P < 0.001 and amniotomy rate was borderline trend toward elevation 73/99 (73.7%) vs. 81/95 (85.3%), P = 0.052 RR 0.9 85% CI 0.8–1.0 in combined compared to Foley catheter alone arm. Apgar score at 5-min was 10 [10–10] vs. 10 [10–10], P = 0.03: there was no neonate with the clinically important score of Apgar < 7 at 5 min [18]. Indications for cesarean delivery were similar across trial arms. Other maternal and neonatal outcomes were not significantly different.
There were no important harms of maternal intensive care admission, severe sepsis, Foley catheter disintegration, urinary retention, or fetal injury identified in the course of the trial.
Post hoc analyses
Sensitivity analysis to evaluate the induction to delivery interval for all deliveries revealed result of 23.7 ± 12.2 vs. 35.1 ± 14.9 h P < 0.001 (Table 2), a mean decrease of 15.9 h for combined arm, similar in magnitude to the 12.6 h reduction for vaginal delivery, indicating the finding is robust on this labor metric. Sub-analysis on indications of cesarean delivery showed no significant difference overall, on labor dystocia (includes failure to progress, failed induction of labor and failed instrumentation) vs. the rest of indications and non-reassuring fetal status vs. the rest of indications across trial arms though the latter analysis with result of 16/35 (45.7%) vs. 13/50 (26.0%), P = 0.07 RR 1.8 95% CI 1.0–3.2 was a borderline result (Table 3).
Discussion
Induction to vaginal delivery interval was shortened and cesarean delivery rate reduced but maternal satisfaction based on “birth experience since the beginning of induction of labor to the birth of your baby” was not increased: the latter finding was unexpected as there is reasonable expectation that satisfaction would be driven by a more rapid birth process following labor induction [19] even without the bonus of a reduction in cesarean delivery. The abovementioned satisfaction metric using VNRS is also consistent with another satisfaction metric we collected as the secondary outcome of “recommend the induction method I had to my friend who has to undergo induction of labor” assessed with a 5-point Likert scale response which also showed no difference across trial arms.
Pain 6−h after the start of induction was significantly more intense, Bishop score was higher at trial devices removal or expulsion, spontaneous membrane rupture was more likely and titrated oxytocin infusion for labor induction or augmentation rate decreased in the combined compared to Foley catheter alone arm. These findings are consistent with the established narrative that the Foley catheter typically ripens the cervix with far less uterine stimulation making the Foley suited for outpatient use [20] compared to prostaglandins. Amniotomy and oxytocin are frequently required to push along the ripening and labor induction when Foley is used alone [21].
A November 2020 network meta-analysis (30 trials with 6465 women) reports time to vaginal delivery when comparing Foley catheter alone to combined Foley with misoprostol or combined with dinoprostone were not significantly different but is longer compared to combined with oxytocin [22] and a borderline result (mean duration, − 2.9 h; 95% confidence interval, − 5.7 to 0.0; P = 0.05) combined Foley with any prostaglandins. Combination regimens of Foley catheter and dinoprostone [23,24,25] in the meta-analysis [22] all dinoprostone used was in the gel form.
Meta-analyses of combined-concurrent Foley catheter with prostaglandins or oxytocin [22, 26,27,28] compared to Foley catheter alone for labor induction and also trials evaluating concurrent Foley catheter and bolus prostaglandin [29,30,31] do not demonstrate a significant positive impact on cesarean delivery, so the significant reduction we found maybe a statistical Type 1 error. Confirmation by further study is warranted.
There is a solitary pilot trial [32] that reported in 2020 on identical interventions of Foley catheter with controlled-release dinoprostone vaginal insert compared to Foley alone that involved 100 women (50 nulliparas and 50 multiparas); in their nulliparas subgroup median (25-75th percentile) time to vaginal delivery was 21.2 [16.6–38.0] (combined arm, n = 26) vs. 31.3 [23.3–46.9] hours (Foley alone n = 24), (Wilcoxon P = 0.05). The pilot trial’s was underpowered but impact magnitude was similar to our powered and significant finding of a reduction in the induction.
Strengths and limitations
Our study was a powered randomized trial comparing combination regimen of Foley catheter and controlled release dinoprostone vaginal insert compared to Foley catheter alone for labor induction. A larger than expected impact magnitude decrease in induction to vaginal delivery interval from the base assumption further assisted in increasing our trial’s statistical power. Our analysis was intention to treat with only three women with data unavailability after randomization; our sample size calculation factored in a far higher 10% drop-out rate than what was observed.
As to limitations, this study was conducted at a single center which potentially limits the generalizability of our results. Masking of observers and labor care providers was not attempted as it was considered impractical. Maternal satisfaction on birth experience were evaluated using VNRS which is a single dimensional and ad hoc scoring while validated questionnaires on anxiety and trauma during childbirth could have been used for a more comprehensive assessment. The clinically important and original finding on reduction on cesarean delivery rate was a secondary outcome that was not appropriately powered at trial protocol work up.
Conclusion
In nulliparous women with unfavorable cervixes at term, combined-concurrent use of Foley catheter and controlled-released dinoprostone vaginal insert hastens vaginal birth and reduces the cesarean delivery rate but maternal satisfaction was not increased.
Data availability
All data generated or analyzed during this study are included in this published article and the datasets used are available from the corresponding author on reasonable request and subject to approval of our ethics board.
Abbreviations
- CTG:
-
Cardiotocography
- VNRS:
-
Visual numerical rating scale
References
NHS D (2017) NHS Maternity Statistics, England 2016–2017. NHS Digital 2017
Grobman WA et al (2018) Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med 379(6):513–523
Leppert PC (1995) Anatomy and physiology of cervical ripening. Clin Obstet Gynecol 38(2):267–279
Evbuomwan O, Chowdhury YS (2021) Physiology, cervical dilation, in StatPearls. Treasure Island (FL)
de Vaan MD et al (2019) Mechanical methods for induction of labour. Cochrane Database Syst Rev 10:CD001233
Lim CE, Ng RW, Xu K (2013) Non-hormonal methods for induction of labour. Curr Opin Obstet Gynecol 25(6):441–447
Chen W et al (2016) A systematic review and network meta-analysis comparing the use of Foley catheters, misoprostol, and dinoprostone for cervical ripening in the induction of labour. BJOG 123(3):346–354
Laughon SK et al (2012) Induction of labor in a contemporary obstetric cohort. Am J Obstet Gynecol 206(6):486 e1–9
WHO (2011) in WHO Recommendations for Induction of Labour. Geneva
Rugarn O et al (2017) Induction of labour with retrievable prostaglandin vaginal inserts: outcomes following retrieval due to an intrapartum adverse event. BJOG 124(5):796–803
Wickramasinghe R, Senanayake H, De Silva C (2020) Intrauterine Foley catheter for 48 versus 24 hours for cervical ripening: a randomized controlled trial. Int J Gynaecol Obstet 149(2):225–230
Gu N et al (2015) Foley catheter for induction of labor at term: an open-label, randomized controlled trial. PLoS One 10(8):e0136856
Brennan MC et al (2011) Retention of dinoprostone vaginal insert beyond 12 hours for induction of labor. Am J Perinatol 28(6):479–484
Win S. et al (2019) Vaginal assessment and expedited amniotomy in oral misoprostol labor induction in nulliparas: a randomized trial. Am J Obstet Gynecol 220(4):387 e1–387 e12.
Chia HM et al (2020) Speculum versus digital insertion of Foley catheter for induction of labor in Nulliparas with unripe cervix: a randomized controlled trial. BMC Pregnancy Childbirth 20(1):330
Dupont WD, Plummer WD, Jr. (1990) Power and sample size calculations. A review and computer program. Control Clin Trials 11(2):116–128
Mizrachi Y et al (2016) Induction of labor in nulliparous women with unfavorable cervix: a comparison of Foley catheter and vaginal prostaglandin E2. Arch Gynecol Obstet 294(4):725–730
Committee Opinion No (2015) 644: The Apgar Score. Obstet Gynecol 126(4):e52–e55
Tan PC et al (2007) Concurrent oxytocin with dinoprostone pessary versus dinoprostone pessary in labour induction of nulliparas with an unfavourable cervix: a randomised placebo-controlled trial. BJOG 114(7):824–832
Diederen M et al (2018) Safety of the balloon catheter for cervical ripening in outpatient care: complications during the period from insertion to expulsion of a balloon catheter in the process of labour induction: a systematic review. BJOG 125(9):1086–1095
Orhue AA (1995) Induction of labour at term in primigravidae with low Bishop’s score: a comparison of three methods. Eur J Obstet Gynecol Reprod Biol 58(2):119–125
Orr L et al (2020) Combination of Foley and prostaglandins versus Foley and oxytocin for cervical ripening: a network meta-analysis. Am J Obstet Gynecol 223(5):743 e1–743 e17
Barrilleaux PS et al (2002) Cervical ripening and induction of labor with misoprostol, dinoprostone gel, and a Foley catheter: a randomized trial of 3 techniques. Am J Obstet Gynecol 186(6):1124–1129
Chowdhary A et al (2019) Comparison of intracervical Foley catheter used alone or combined with a single dose of dinoprostone gel for cervical ripening: a randomised study. J Obstet Gynaecol 39(4):461–467
Greybush M et al (2001) Preinduction cervical ripening techniques compared. J Reprod Med 46(1):11–17
Zhu L et al (2018) Intracervical Foley catheter balloon versus dinoprostone insert for induction cervical ripening: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 97(48):e13251
Zeng X et al (2015) Efficiency of dinoprostone insert for cervical ripening and induction of labor in women of full-term pregnancy compared with dinoprostone gel: a meta-analysis. Drug Discov Ther 9(3):165–172
Chen W et al (2015) Meta-analysis of Foley catheter plus misoprostol versus misoprostol alone for cervical ripening. Int J Gynaecol Obstet 129(3):193–198
Al-Ibraheemi Z et al (2018) Misoprostol with foley bulb compared with misoprostol alone for cervical ripening: a randomized controlled trial. Obstet Gynecol 131(1):23–29
Levine LD et al (2016) Mechanical and pharmacologic methods of labor induction: a randomized controlled trial. Obstet Gynecol 128(6):1357–1364
Aduloju OP et al (2016) Combined Foley’s catheter with vaginal misoprostol for pre-induction cervical ripening: a randomised controlled trial. Aust N Z J Obstet Gynaecol 56(6):578–584
Edwards RK et al (2021) Controlled release dinoprostone insert and foley compared to foley alone: a randomized pilot trial. Am J Perinatol 38(S 01):e57–e63
Acknowledgements
We thank the women who participated in this trial and their care providers. The Department of Obstetrics and Gynecology, Faculty of Medicine, University of Malaya provided the resources to fund internally this research.
Funding
This research was internally funded by the Department of Obstetrics and Gynecology, University of Malaya. Identifier: UMSCOG/RF00142018/01.
Author information
Authors and Affiliations
Contributions
NV: project development (support), data analysis and interpretation (support), manuscript drafting and editing (main). NL: project development (main), data collection (main), data analysis and interpretation (support). PCT: project development (main), data analysis and interpretation (main), manuscript drafting and editing (main). JGSH: data analysis and interpretation (main), manuscript drafting and editing (main). MH: data interpretation (support), manuscript review and amendments (support). BKL: data interpretation (support), manuscript review and amendments (main). All authors read and approved the final manuscript. All authors assert ownership over and responsibility for the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
All authors have no conflict of interest to declare that are relevant to the content of this manuscript.
Ethics approval
This randomized controlled trial was approved by the Medical Ethics Committee of University Malaya Medical Centre (date of approval November 15, 2018; reference number 201882–6564) and registered in ISRCTN registry on December 3, 2018 (registration number ISRCTN2282883 (https://doi.org/10.1186/ISRCTN12282883). The trial was conducted in University Malaya Medical Centre with the first participant recruited on December 12, 2018 and the last on May 8, 2019. This trial was registered in the Malaysian National Medical Research Register NMRR: 18–3139-44981.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vallikkannu, N., Laboh, N., Tan, P.C. et al. Foley catheter and controlled release dinoprostone versus foley catheter labor induction in nulliparas: a randomized trial. Arch Gynecol Obstet 306, 1027–1036 (2022). https://doi.org/10.1007/s00404-021-06383-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-021-06383-2