Abstract
Purpose
Due to insufficient and conflicting prospective evidence, the recommendations on when to apply adjuvant radiochemotherapy in early-stage cervical cancer vary between international guidelines. In this population-based study, we evaluated the outcome of patients with early-stage cervical cancer based on risk factors and the adjuvant therapy they received.
Methods
The effect of primary therapy (surgery and radiochemotherapy RCT, surgery and radiotherapy RT, and surgery alone) on overall survival (OS) and recurrence-free survival (RFS) was evaluated in the complete cohort of 442 patients and in subgroups according to risk profile and nodal status.
Results
In low-risk patients, there was no difference in OS (p = 0.276) depending on whether patients received adjuvant therapy or not. Concerning RFS, patients with RT (including one patient with RCT) exhibited a significantly worse outcome compared to the group with surgery alone (p = 0.015). In intermediate-risk patients, the administration of adjuvant RT significantly benefited RFS when compared to surgery only in multivariate analysis (p = 0.031). Concerning OS, no significant influence for adjuvant treatment could be seen (p = 0.354). Though trends towards better OS and RFS could be observed in patients of the high-risk group—both in RCT and RT groups compared to surgery alone—the effects did not prove to be significant.
Conclusion
Our study reaffirms the evidence against the use of adjuvant radio(chemo)therapy in low-risk early-stage cervical cancer. In intermediate-, and less pronounced in high-risk patients, however, it seems to be beneficial. The role of adjuvant radio(chemo)therapy in early cervical cancer should be further investigated in prospective randomized trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical cancer is the second most common cause of cancer death in women worldwide. Even though its prevalence is considerably higher in third-world countries compared to the developed world, approximately 5000 patients in Germany are diagnosed with cervical cancer each year [1]. Thanks to screening programs in western countries, cervical cancer is increasingly diagnosed at earlier stages. The standard treatment for early-stage cervical cancer varies between countries.
While the national comprehensive cancer network (NCCN) favors radical hysterectomy over primary radio(chemo)therapy only in tumors smaller than 4 cm, the current German S3 guideline recommends radical hysterectomy for all-stage IB-IIA tumors in the absence of multiple risk factors, such as lymph node metastasis [2, 3]. Combined therapy, such as radical hysterectomy and lymphadenectomy plus radiochemotherapy, was shown to induce substantial morbidity. Therefore, a careful selection of patients should take place [4, 5].
International guidelines do not completely agree on the risk factors that indicate the need for adjuvant therapy. Guideline recommendations are largely based on the results of two large randomized trials conducted approximately 20 years ago [6,7,8]. Sedlis et al. demonstrated that adjuvant radiotherapy decreases recurrence rates in patients with certain risk factors, called the “Sedlis criteria” [7]. They consist of deep stromal invasion, capillary and lymphatic space involvement or a tumor diameter of more than 4 cm [2, 7]. In a trial by Peters et al., the combination of chemotherapy and radiotherapy proved to be superior to radiotherapy alone in patients with involved lymph nodes, residual tumor or parametric invasion [6].
Based on the available data, nodal involvement and residual tumor constitute undisputed risk factors that require adjuvant treatment. The recurrence rate in patients with lymph node metastases is increased up to 40% compared to nodal negative patients [9, 10]. Apart from that, the “Sedlis criteria” still constitute intermediate risk factors that guide adjuvant treatment decisions. A more recent analysis confirmed the role of tumor size, deep stromal invasion and lymphovascular space invasion on recurrence rates, but also established adenocarcinoma/adenosquamous histology as a risk factor [11]. While adjuvant therapy is considered necessary for parametric invasion by NCCN guidelines, this does not represent an indicator towards further treatment in the German guideline [2, 3]. This is supported by the findings of Uno et al. that demonstrated similar pelvic control rates for patients with and without parametric invasion [12]. The question whether patients with intermediate risk factors benefit from the addition of chemotherapy to adjuvant radiotherapy is currently addressed in the ongoing GOG0263 study (NCT01101451) [13]. Nevertheless, further investigation into the adjuvant treatment in early cervical cancer is desperately needed.
In this population-based study, we evaluated the outcome of patients with early-stage cervical cancer based on risk factors and the adjuvant therapy they received.
Materials and methods
Database and cohort
We retrospectively analyzed data obtained from the Clinical Cancer Registry of the Tumor Center—Institute for Quality Management and Health Services Research, University of Regensburg, Germany, which are described elsewhere [14,15,16].
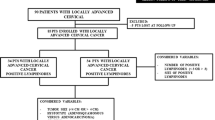
Using the database, 1613 patients with histologically confirmed cervical cancer diagnosed between January 2003 and December 2015 were identified. We included only FIGO stages IB–IIA. We further restrained the cohort to patients that underwent radical hysterectomy. Further exclusion criteria were simultaneous or prior malignant disease of other origin, histology other than squamous carcinoma, adenocarcinoma or adenosquamous carcinoma and insufficient documentation (≤ one medical record available, Fig. 1). The remaining cohort consisted of 442 patients.
Risk groups and therapy groups
The effect of primary therapy (surgery and radiochemotherapy RCT, surgery and radiotherapy RT, and surgery alone) on overall survival OS and recurrence-free survival RFS was evaluated in the complete cohort of 442 patients and in subgroups according to risk profile and nodal status. Three risk groups were defined as follows: The low-risk group included patients without residual tumor (R0), negative nodal status (N0), and exhibiting no further histopathological risk factors (no lymph vessel invasion L0, no vein invasion V0, tumor size ≤ 4 cm, grading G1/G2). Patients in the intermediate-risk group had a diagnosis of R0 and N0 and only one or two of the four histopathological findings L1, V1, tumor size > 4 cm, G3. The high-risk group was defined as showing at least R1 or N1 or negative residual and nodal status R0/N0 combined with three or four of the histopathological findings L1, V1, tumor size > 4 cm, G3. The outcome comparisons were performed for the treatment groups surgery alone, surgery plus R(C)T (RCT or RT), RCT, and RT, respectively.
Statistical analyses
Continuous data are presented as mean, median, minimum, maximum values and standard deviation. Categorical data are described using absolute frequencies and relative percentages. Statistical comparisons were made using t test for continuous data in case of normal distribution; otherwise, Mann–Whitney U test was performed. Pearson’s Chi-square test was used for testing independence of categorical variables; in case of small numbers, Fisher’s exact test was applied.
Data on life and recurrence status were obtained from medical records, death certificates, and registration offices. Overall survival (OS) and recurrence-free survival (RFS) were estimated by means of Kaplan–Meier method and Cox regression model from the date of cancer diagnosis until the date of death of any cause, until the date of first recurrence report, or last date recorded alive, respectively. A cut-off date was set at 12/31/2019. In multivariate regression analyses, adjustments were made for potential confounding parameters: age at diagnosis, Charlson Comorbidity Index [17], histology, grading, FIGO stage, tumor size, nodal status, lymph vessel invasion, blood vessel invasion, and residual tumor status. Hazard ratios (HR) were considered significant if the corresponding 95% confidence interval (95% CI) excluded 1. All t tests were calculated two-sided. p values < 0.05 were considered statistically significant. All calculations were performed using IBM SPSS Statistics Version 25.0 (IBM Corp., Armonk, NY, USA).
Results
Description of patient cohort
398 patients (90.0%) with stage IB and 44 patients (10.0%) with stage IIA cervical cancer were included in the study (Table 1). Median follow-up was 7.8 years (95% CI 7.0–8.6), mean follow-up 8.7 years (95% CI 8.3–9.1). Median age at diagnosis was 48.0 years (mean 50.1 SD 13.1 years). In most patients (86.0%), Charlson comorbidity score did not surpass 2, meaning that they suffered from no other disease than cervical cancer. Squamous cell cancer (72.4%) was the prevailing histology, followed by adenocarcinoma (21.9%) and adenosquamous carcinoma (5.7%). Grading was predominantly G2 (45.1%) or G3 (49.3%), whereas G1 and GX were present in only 5.4% and 0.2% of cases, respectively. Lymphatic and vascular invasion was present in 41.0% and 9.3%, respectively. Surgical lymph node staging was performed in 96.2% of patients, 79.9% of whom were N0, 16.7% N1 and 3.5% not documented. 31.4% of patients had less, and 61.5% had more than 25 lymph nodes removed with a median of 30 (range 3–117). In 3.2% of patients, the number of nodes removed is unknown.
Tumor size was smaller than 4 cm in 71.9% and larger than 4 cm in 20.6% of cases. For the remaining 7.5% of cases, information was irretrievable. The actual tumor size in mm was only recorded for 63.4% of the patients. Considering a cut-off 2 cm, 23.1% of the patients showed a tumor size smaller, and 40.3% a tumor size larger than 2 cm. In most patients, resection status was R0 (94.6%). Residual tumor (R1 or R2) was present in 3.4%. Resection status was unclear in 2.0% of patients. 201 (45.5%), 124 (28.1%) and 117 (26.5%) patients were classified as low, intermediate and high risk, respectively. 249 patients received surgery alone (56.3%), 74 received adjuvant radiotherapy (RT, 16.7%) and 119 received radiochemotherapy (RCT, 26.9%). The patients with either RCT or RT added up to 193 [R(C)T, 43.7%]. The patients’ characteristics in the differentiated groups surgery plus RCT, surgery plus RT, and surgery only are depicted in Table 1. Distribution of patients’ characteristics according to treatment groups surgery and R(C)T vs surgery only is shown in Table 1.
Recommended and performed adjuvant treatment according to risk group
In the low-risk group, adjuvant therapy was recommended according to information from discharge letters in only 41 of 201 patients (20.4%), of whom 31 (75.6%) actually received R(C)T (30 patients with RT only and one patient with RCT). Six patients refused therapy, no reason for deviation from the treatment recommendation was found for the remaining four patients.
From 81 (65.3%) recommended adjuvant treatments in the 124 patients of the intermediate-risk group, 65 (80.2%) were actually performed (43 RCT, 22 RT), 11 were rejected by the patients, no information was available for five cases.
As expected, the highest rate of recommendations for adjuvant treatment (93.1%, N = 109) was observed in the 117 high-risk patients, which is in accordance to the German S3 guideline. The contraindications named in the discharge letters of eight patients without recommendation were uniformly advanced age and comorbidities, either alone or combined. From the 109 planned treatments, 97 (89.0%) were implemented as RCT (in 75 cases) and RT (in 22 cases), 7 were rejected by the patient, and in 5 cases, no reason for deviation was given.
Outcome
Complete cohort
In the complete cohort of 442 patients, the 5-year rates were 86.6% for OS, and 77.1% for RFS (Table 2). Patients with adjuvant R(C)T yielded a 5-year OS of 83.8% compared to 88.8% in patients with surgery only (log rank p = 0.121). The difference was smaller and not significant when comparing RFS, showing 5-year rates of 75.8% and 77.9%, respectively (p = 0.366). After adjusting for age at diagnosis, Charlson Comorbidity Index, histology, grading, FIGO stage, tumor size, nodal status, lymph vessel invasion, blood vessel invasion, and residual tumor status in multivariate analyses, adjuvant therapy did not prove to be advantageous for OS (HR 0.774, 95% CI 0.453–1.322, p = 0.348, Table S2, Table 2) and RFS (HR 0.882, 95% CI 0.562–1.383, p = 0.584, Table S3, Table 3). Comparing patients with surgery plus RCT and surgery plus RT separately to patients with surgery only, likewise no significant benefit was detected. The HR for OS in surgery group vs RCT group was 0.875 (95%-CI 0.438–1.746, p = 0.705), compared to the RT group, the HR for OS was 0.722 ((95% CI 0.396–1.313, p = 0.285). Concerning RFS, the HR for RCT compared to surgery only was 0.766 (95% CI 0.426–1.375, p = 0.372), for RT compared to surgery, it was 0.958 (95% CI 0.586–1.566, p = 0.864, Table 4).
In multivariate Cox regression analyses, the following patient characteristics proved to be significant risk factors for OS: age at diagnosis (p < 0.001), comorbidity defined by Charlson comorbidity score (p = 0.049), FIGO stage (p = 0.027), nodal status (p = 0.015), and residual status (p < 0.001). Lymph and blood vessel invasions, on the other hand, were not significantly associated with OS (p = 0.277 and p = 0.192, respectively) (Table S2).
RFS was significantly associated with age at diagnosis (p < 0.001), tumor size (p = 0.021) and residual tumor (p = 0.028) in multivariate analysis. There was a trend towards significance for FIGO Stage (p = 0.051) and nodal status (p = 0.083) (Table S3).
Risk groups
In the low-risk group, the 5-year OS rate was 91.1%, in the intermediate group 88.3%, and in the high-risk group 77.2%, exhibiting a significant difference of OS between low- and high-risk groups with p = 0.001. The corresponding 5-year rates for RFS were 78.2%, 83.5%, and 68.1%, showing no difference between low and intermediate risks (p = 0.963), and a small but non-significant difference between low and high risks (p = 0.076).
Table 2 and Fig. 2 show the results from Kaplan–Meier survival analyses in dependence of adjuvant R(C)T in the named subgroups. The corresponding results from univariate and multivariate regression analyses are listed in Table 3. Table 4 shows the results for the more detailed subgroups, the adjuvant therapy group divided into RCT and RT.
Low risk
In the low-risk group, the majority of patients was treated by surgery only (N = 170; 84.6%). Only 1 (< 1%) and 30 patients (14.9%) received RCT and RT, respectively. There was no difference in OS from Kaplan–Meier (p = 0.611 and multivariate regression analysis (p = 0.276) depending on whether patients received adjuvant therapy or not. Concerning RFS, patients with RT (including one person with RCT) exhibited a significantly worse outcome compared to the group with surgery only both in univariate and multivariate analysis: 5-year RFS rates were 66.2% vs 80.3% (p = 0.025), HR was 2.348 (95% CI 1.183–4.660, p = 0.015).
Intermediate risk
In the intermediate-risk group, treatment approaches were distributed more evenly. Fifty-nine patients (47.6%) received surgery only, 22 patients (17.7%) were treated by adjuvant RT and 43 (34.7%) by adjuvant RCT. Neither in univariate nor multivariate analysis, OS correlated with whether patients received adjuvant treatment (p = 0.370 and p = 0.354, respectively). Furthermore, it was not significantly correlated with the type of adjuvant treatment (p = 0.741 for RCT vs surgery, p = 0.094 for RT vs surgery). A benefit for RFS, however, was significantly associated with the administration of adjuvant RT when compared to surgery only in multivariate analysis (HR 0.258, 95% CI 0.075–0.885, p = 0.031). This was not the case in the RCT group.
High risk
In the high-risk group, only 20 patients (17.1%) did not receive adjuvant treatment. 22 (18.8%) and 75 patients (67.0%) were treated by adjuvant RT and RCT, respectively. There was no significant difference in OS comparing patients that received adjuvant therapy and those who did not in univariate (p = 0.737) or multivariate analysis (p = 0.268). Type of adjuvant treatment RCT or RT was not associated with an increased OS (Table 4). No therapy group, be it combined or differentiated, yielded a better RFS than the surgery only group (Tables 3, 4). Though trends towards better OS and RFS could be seen in patients of the high-risk group—both in RCT and RT groups compared to surgery alone—the effects did not prove to be significant.
Tumor size
The intenseness of therapy increased with tumor size: among 91 patients with a tumor size larger than 4 cm, 65 (71.4%) received R(C)T. In patients with tumors smaller than 4 cm, the portion was only 35.5% (113 from 318). Patients with tumors larger than 2 cm received R(C)T in 51.1% (91 of 178 cases), as opposed to patients with tumors smaller than 2 cm (31.4%, 32 of 102 cases).
Considering OS and RFS, no significant benefit was observed from R(C)T in patients with small and large tumors, be it the threshold of 4 cm or 2 cm. However, a tendency towards a positive effect on OS and RFS was seen in patients with larger tumors—greater than 2 cm—with a HR for R(C)T vs surgery only of 0.581 (95% CI 0. 258–1.305, p = 0.188) and 0.569 (95% CI 0.292–1.105, p = 0.096), respectively. This tendency was less obvious when considering the tumor size limit of 4 cm.
Nodal status
Node negative patients (N0) were preferably treated by surgery only (N = 233 of 356, 65.4%), RCT and RT being less common (N = 62, 17.4%, N = 61, 17.1%, respectively). In 73 patients with positive regional lymph nodes (N1), surgery plus RCT was the most dominant treatment (N = 55, 75.3%), followed by surgery plus RT (N = 12, 16.4%) and surgery only (N = 6, 8.2%).
As far as treatment modalities are concerned, no significant differences in OS and RFS could be derived in both the N0 and N1 groups from univariate and multivariate survival analyses (Tables 3 and 4). However, the HR for R(C)T compared to surgery only in N1 patients was 0.468 (95% CI 0.114–1.911, p = 0.290) and 1.043 (95% CI 0.632–1.720, p = 0.870) in N0 patients, respectively.
Discussion
Cervical cancer is a significant cause of morbidity and mortality. Despite its high prevalence, data on optimal treatment approaches are scarce. Previously, we demonstrated an improvement in OS through the addition of adjuvant RCT to surgery in FIGO stage IIB disease regardless of lymph node status [16]. In this study, we evaluated the effect of adjuvant treatment in early-stage cancer depending on the presence of risk factors. Only two randomized prospective studies exist on the subject. Sedlis et al. randomized FIGO IB patients without residual tumor or involved lymph nodes but with two or more intermediate risk factors later named the “Sedlis criteria” to receive observation or RT following radical surgery. Adjuvant radiotherapy led to a reduction of recurrence rates at the cost of an approximately 4% higher rate of grade 3/4 adverse events. There was no increase in OS but an improvement of long-term PFS [7, 8, 18]. Another randomized controlled trial by Peters et al. compared adjuvant radiotherapy to adjuvant radiochemotherapy. Patients with clinical-stage IA(2), IB and IIA carcinoma with parametric invasion, residual tumor and/or lymph node involvement were included in the study. PFS as well as OS was significantly improved by the addition of chemotherapy [6]. A retrospective analysis of the clinical and histopathological data of the study discovered that the absolute benefit of the addition of chemotherapy was less evident among patients with only one involved lymph node and smaller primary tumors (< 2 cm) [18]. Hence, some questions concerning adjuvant treatment in early-stage cancer remain. It is unclear whether patients with only one intermediate risk factor benefit from RT and whether additional chemotherapy is beneficial in patients apart from those with nodal involvement, residual tumor or parametric invasion. Furthermore, risk factors may not be limited to the ones mentioned above. Due to these uncertainties, international guidelines on the topic are inconsistent. The risk group stratification in this study was based on the recommendations of the German S3 guideline. A tumor size of 4 cm or more, deep stromal invasion and lymphatic as well as vascular space invasion indicate intermediate risk when at least two of them are present. Residual tumor and lymph node metastases are regarded as high-risk factors. Our investigation demonstrates a gradual decrease of OS rates from low to high risk, the effect being less pronounced for RFS. Particularly, tumor size, residual tumor and nodal status had a significant impact on OS and apart from nodal status also on RFS. The effect of blood and lymph vessel invasion on OS and RFS, on the other hand, could not be confirmed in this cohort.
Our retrospective analyses did not show a benefit of adjuvant therapy in patients without risk factors. This is in line with international guideline recommendations [2, 3]. This question was addressed in only one other retrospective study reaching a comparable conclusion [19]. We even perceived a significantly inferior RFS in low-risk patients treated with adjuvant radio(chemo-)therapy. The reason for this effect remains unclear. A potential explanation is the presence of other risk factors than the ones evaluated. These might have prompted physicians to propose adjuvant treatment to the patients and could lead to a negative selection bias for R(C)T. Taking into account the substantial morbidity induced by the combination of surgery and adjuvant R(C)T patients without risk factors should be spared from this treatment [4, 7].
The administration of adjuvant therapy in intermediate-risk patients did not lead to an improvement in OS, but improved RFS. This was restricted to radiotherapy and did not apply to treatment with adjuvant radiochemotherapy. Similar results were obtained in the high-risk group with three or more intermediate risk factors or lymph node involvement or residual tumor. Though the Hazard ratios suggest an improvement in OS and RFS in patients of the high-risk group—both in RCT and RT groups compared to surgery alone—the effects did not prove to be significant. In our patient cohort, the additional beneficial effect of chemotherapy to radiotherapy in high-risk patients proposed by the Intergroup 0107/GOG 109 trial could not be reproduced. This observation may result from a number of factors [6]. First, patient numbers are not powered to detect minor differences in survival, especially in the light of an uneven patient distribution across treatment groups. Second, the administered chemotherapy regimen will probably vary from the one used in the Intergroup 0107/GOG 109 trial in some patients without well-founded evidence for its equivalence. Moreover, a survival advantage is only expected for patients with residual tumor and lymph node involvement, not for the other patients classified as high risk due to 3 or more risk factors. Recent findings suggest that lymph node involvement is the most relevant factor predicting the survival benefit of RCT, while patients with positive margins and/or parametric invasion alone might not benefit from RCT [20]. In addition, the benefit for patients with only one involved lymph node seems to be lower than initially expected [18]. In our cohort, the effect of adjuvant treatment on OS and RFS in lymph node positive patients was not significant. However, a closer look on the hazard ratios proposes that the death rate and the rate of patients experiencing recurrence or death are approximately halved in patients receiving adjuvant radio(chemo)therapy.
Stratified for tumor size, we did not perceive a significant benefit for adjuvant therapy in patients with primary tumors smaller or larger than 2 cm. However, a tendency towards a positive effect on OS and RFS was seen in patients with tumors > 2 cm.
As mentioned above for the cohort of high-risk patients in general, the effect of adjuvant therapy in node positive patients may be masked by the low number of patients in this group. Since this study included only tumor stages IB–IIA, the influence of parametric invasion was not assessed. Previously, we demonstrated that FIGO IIB cervical cancer patients benefit from a combination of surgery and radiochemotherapy, whereas no benefit was seen for surgery and adjuvant radiotherapy without chemotherapy [16]. In combination, the results from both studies suggest parametric invasion being the most relevant risk factor for the effect of RCT.
Conclusion
In summary, our data reaffirm the evidence from previous studies against the use of adjuvant therapy in low-risk early-stage cervical cancer [7, 8, 18]. In intermediate- and less pronounced in high-risk patients, however, it seems to provide an advantage over treatment by surgery alone. The relevance of different risk factors should be further investigated in prospective randomized trials.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to preservation of privacy but are available from the corresponding author on reasonable request.
References
Robert Koch Institut Bericht zum Krebsgeschehen in Deutschland (2016) https://edoc.rki.de/bitstream/handle/176904/3264/28oaKVmif0wDk.pdf?sequence=1&isAllowed=y. Accessed 09 Apr 2020
National Comprehensive Cancer Network® NCCN Clinical Practice Guidelines in Oncology Cervical cancer: Version 3.2019 — January 10, 2019. https://www.nccn.org/professionals/physician_gls/pdf/cervical_blocks.pdf. Accessed 17 Mar 2019
Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF S3-Leitlinie Diagnostik, Therapie und Nachsorge der Patientin mit Zervixkarzinom: Langversion, 1.0, 2014, AWMF-Registernummer: 032/033OL. http://leitlinienprogramm-onkologie.de/Leitlinien.7.0.html,. Accessed 17 Mar 2019
Landoni F, Maneo A, Colombo A et al (1997) Randomised study of radical surgery versus radiotherapy for stage Ib–IIa cervical cancer. Lancet 350(9077):535–540. https://doi.org/10.1016/S0140-6736(97)02250-2
Falcetta FS, Medeiros LR, Edelweiss MI et al (2016) Adjuvant platinum-based chemotherapy for early stage cervical cancer. Cochrane Database Syst Rev 11:CD005342. https://doi.org/10.1002/14651858.CD005342.pub4
Peters WA, Liu PY, Barrett RJ et al (2000) Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J ClinOncol 18(8):1606–1613. https://doi.org/10.1200/JCO.2000.18.8.1606
Sedlis A, Bundy BN, Rotman MZ et al (1999) A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a gynecologic oncology group study. GynecolOncol 73(2):177–183. https://doi.org/10.1006/gyno.1999.5387
Rotman M, Sedlis A, Piedmonte MR et al (2006) A phase III randomized trial of postoperative pelvic irradiation in stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J RadiatOncolBiol Phys 65(1):169–176. https://doi.org/10.1016/j.ijrobp.2005.10.019
Takekuma M, Kasamatsu Y, Kado N et al (2017) The issues regarding postoperative adjuvant therapy and prognostic risk factors for patients with stage I–II cervical cancer: a review. J ObstetGynaecol Res 43(4):617–626. https://doi.org/10.1111/jog.13282
Lahousen M, Haas J, Pickel H et al (1999) Chemotherapy versus radiotherapy versus observation for high-risk cervical carcinoma after radical hysterectomy: a randomized, prospective. Multicenter Trial GynecolOncol 73(2):196–201. https://doi.org/10.1006/gyno.1999.5343
Ryu SY, Kim MH, Nam BH et al (2014) Intermediate-risk grouping of cervical cancer patients treated with radical hysterectomy: a Korean Gynecologic Oncology Group study. Br J Cancer 110(2):278–285. https://doi.org/10.1038/bjc.2013.716
Uno T, Ito H, Isobe K et al (2005) Postoperative pelvic radiotherapy for cervical cancer patients with positive parametrial invasion. GynecolOncol 96(2):335–340. https://doi.org/10.1016/j.ygyno.2004.09.061
Gynecologic Oncology Group radiation therapy with or without chemotherapy in patients with stage I–IIA cervical cancer who previously underwent surgery. https://clinicaltrials.gov/ct2/show/NCT01101451. Accessed 10 Sep 2020
Scharl S, Papathemelis T, Kronberger K et al (2018) Does post-operative radiochemotherapy improve survival in high-grade endometrial cancer patients? Results of a population-based cohort analysis of a cancer registry. Arch GynecolObstet 297(5):1245–1253. https://doi.org/10.1007/s00404-018-4708-6
Papathemelis T, Scharl S, Kronberger K et al (2017) Survival benefit of pelvic and paraaortic lymphadenectomy in high-grade endometrial carcinoma: a retrospective population-based cohort analysis. J Cancer Res ClinOncol 143(12):2555–2562. https://doi.org/10.1007/s00432-017-2508-1
Papathemelis T, Knobloch S, Gerken M et al (2019) Impact of nodal status and treatment strategy on overall survival in advanced stage cervical cancer. J Cancer Res ClinOncol. https://doi.org/10.1007/s00432-019-02890-7
Charlson ME, Pompei P, Ales KL et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Monk BJ, Wang J, Im S et al (2005) Rethinking the use of radiation and chemotherapy after radical hysterectomy: a clinical-pathologic analysis of a Gynecologic Oncology Group/Southwest Oncology Group/Radiation Therapy Oncology Group trial. GynecolOncol 96(3):721–728. https://doi.org/10.1016/j.ygyno.2004.11.007
Sun F, Li Y, Liu J et al (2014) Impact of postoperative adjuvant therapy on prognosis of low-risk cervical cancer: analysis of 208 cases. Nan Fang Yi Ke Da XueXueBao 34(3):401–405
Trifiletti DM, Swisher-McClure S, Showalter TN et al (2015) Postoperative chemoradiation therapy in high-risk cervical cancer: re-evaluating the findings of gynecologic oncology group study 109 in a large, population-based cohort. Int J RadiatOncolBiol Phys 93(5):1032–1044. https://doi.org/10.1016/j.ijrobp.2015.09.001
Funding
No funding obtained.
Author information
Authors and Affiliations
Contributions
Protocol/project development: MK-S, MG, SS, CB,TP. Data collection: CB, MG. Data analysis/interpretation: CB, MG, SS. Manuscript writing: SS. Manuscript editing: MK-S, AS, MA, AI, ECI,OO,OK,TP.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments. This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Scharl, S., Becher, C., Gerken, M. et al. Is there a benefit for adjuvant radio(chemo)therapy in early cervical cancer? Results from a population-based study. Arch Gynecol Obstet 304, 759–771 (2021). https://doi.org/10.1007/s00404-021-05989-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-021-05989-w