Abstract
Purpose
Management of high-grade cervical intraepithelial neoplasia [CIN grade 2 or 3 (CIN2–3)] diagnosed during pregnancy is controversial. Monitoring with colposcopy and cytology every 8–12 weeks is advised by the most current guidelines.
Study design
This study analyzes the course of disease in pregnant women with abnormal cytologies or clinically suspicious cervixes.
Results
In total, 139 pregnant women, at a median age of 31 years (range 19–49), treated at the Colposcopy Unit of the University Medical Center Hamburg-Eppendorf between 2011 and 2017 were identified. During pregnancy, at least one biopsy was performed on 70.5% of patients. In 84.7% of cases, CIN2–3 (CIN2 n = 14 (14.3%), CIN3 n = 69 (70.4%)) was detected, 7.1% (n = 7) of women were diagnosed with CIN1, while no dysplasia was found in 8.2% (n = 8) of cases. No interventions were necessary during pregnancy. Despite explicit invitation, only 72.3% of women with CIN2–3 attended postpartal consultations. While 61.7% showed persistent lesions, 5% were diagnosed with CIN1 and 33.3% with complete remission. During pregnancy, 68.7% of women with prepartal CIN2–3 were tested for HPV infection. Later, 49.1% were followed up postpartally by means of HPV testing and histology. HPV clearance was observed in 36.4% of women with complete histological remission. Postpartum conization was performed on 44.6% of patients with prepartal CIN2–3 diagnosis. CIN2–3 was histologically confirmed in 97.3% cases. Progression from persistent CIN3 to microinvasive carcinoma was observed in a single case.
Conclusions
High-grade CIN lesions, diagnosed during pregnancy, show a high rate of regression postpartum; whereas, progression to carcinoma is rare. Close and continuous monitoring rarely has any therapeutic consequences. Compliance for postpartal follow-up needs to be improved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the course of pregnancy, about 1.3 and 2.7 in 1000 women will be affected by some degree of cervical intraepithelial neoplasia (CIN) [1, 2]. The lesions are caused by human papilloma virus (HPV), which is the most common sexually transmitted infection in females, with a lifetime prevalence of up to 75% [3]. During productive HPV infection, low-grade cervical abnormalities may be clinically detectable through means of screening [e.g., low-grade squamous intraepithelial lesions (LSIL) or CIN grade 1 (CIN1)]. These infections are usually transient and resolve themselves within 1–2 years [3, 4] without intervention. A minority of HPV infections, however, persist beyond 12 months, increasing the risk of carcinogenic progression to cervical precancerous lesions [high-grade squamous intraepithelial lesions (HSIL), CIN grade 2 or 3 (CIN2–3)] and potentially even cancer when left untreated [3, 4]. Of the women infected with high-risk HPV (HR-HPV), approximately 6–26.7% (depending on HPV type: HPV16 26.7%, HPV18 19.1%, HPV 31 14.3%, HPV 33 14.9%, HR-others 6.0%) will develop CIN3 within a time period of up to 12 years [5].
The prevalence of abnormal cytological findings in the population of pregnant women is similar to that of their age-matched non-pregnant peers, and ranges from 2 to 7% [1, 6, 7]. According to the literature, the overall risk of developing cervical cancer (CC) in pregnancy after biopsy-proven CIN2–3 is fairly low at 0–0.4% [8]. However, CC is the most common gynecological malignancy diagnosed during pregnancy and is estimated to occur in 1.5–12 of every 100,000 pregnancies [1, 9]. Nearly, 3% of newly diagnosed CC cases occur in pregnant women, most likely due to being one of the few cancers for which screening is part of any routine prenatal work-up [10].
While there is a considerable amount of research on the outcome of obstetrical scenarios in patients with a history of treatment for cervical neoplasia, the available data on the course of intraepithelial neoplasia during and after pregnancy are heterogeneous. Likewise, little is known about the postpartum course of HSIL.
The first diagnostic step after abnormal cytology during pregnancy is usually colposcopy. It presents a conservative diagnostic option at a time when as little interference as possible is preferable. However, there is always the risk of discounting a possible lesion as a pregnancy-related manifestation, or simply underestimating an issue at hand [11]. Therefore, a thorough approach must consist of cytology, accompanied by testing for HR-HPV. In the case of abnormal colposcopy, further work-up is required, including a colposcopy-guided biopsy (CGB).
Overall, the management of intraepithelial lesions during pregnancy has changed over the years from an aggressive, biopsy and treatment-based course of action to a more conservative and expectant approach [9, 12,13,14,15,16,17,18]. According to the current guidelines of the American Society of Colposcopy and Cervical Pathology (ASCCP), observational management in pregnancy with follow-ups every 12 weeks is recommended [17]. The actual treatment of CIN may be deferred to the period after delivery if CC is excluded [12,13,14,15,16,17,18].
With this in mind, the objective of this study was to analyze the course of high-grade cervical intraepithelial lesions (CIN2–3) during and after pregnancy.
Methods
Patients
All pregnant women referred to the Colposcopy Unit of the University Medical Center, Hamburg-Eppendorf with suspicious clinical findings or abnormal cervical cytology between July 2011 and January 2017 were analyzed retrospectively. Informed consent was obtained from all patients to review their medical records following approval of preserving data required for the retrospective character of the study by the Ethics Committee of the Medical Board Hamburg (reference no. 190504).
Patient history was acquired in a standardized questionnaire. As part of the work-up, all patients received a gynecological examination, HPV testing (PapilloCheck®), in-house cytology as well as targeted colposcopy-guided biopsy of the uterine cervix.
Cervical samples for cytology and HPV testing were taken from the ectocervix and endocervix by use of soft cervical swabs (PapCone®, Otto Bock PUR Life Science GmbH, Duderstadt, Germany). Cell material was then suspended in SurePath preservative medium (Becton Dickinson Co., Franklin Lakes, NJ, USA) and stored at 4 °C.
While different classification systems are used internationally, cervical cytology in Germany is classified using the Munich Nomenclature based on guidelines published by the German Society of Colposcopy and Cervical Pathology (AG-CPC) [16, 19]. To make the data accessible to an international readership, the Munich Nomenclature was transcribed into the Bethesda classification as suggested by the AG-CPC (Online Resource 1) [19]. The original data were accumulated using both classification systems. Different cytological screening methods (conventional and liquid-based cytology) lead to the decision to concentrate on histological findings and HPV status for the remainder of our paper.
Samples were tested for HPV by use of the PapilloCheck® (Greiner Bio One—GBO) test, which detects and differentiates 24 HPV types.
In a final step, the postpartal findings were analyzed to compare CIN persistence, regression and progression. Regression was defined as a lower-grade lesion detected in the post-partum period compared to the initial visit. Persistent disease was defined as CIN of the same grade as found at initial diagnosis. Disease progression was defined as histological evidence of a higher CIN grade or cancer at a subsequent visit when compared to the initial consultation.
Statistical analysis
Statistics were primarily based on descriptive data. Data are presented in terms of means ± standard deviations and median (minimum–maximum) for continuous data or counts and percentages for categorical data. Correlation of different outcome variables (remission vs. no remission, regression vs. no regression, persistence vs. no persistence and progression vs. no progression) were assessed by use of the Chi-square test and Kappa tests. Due to missing data, the nominator varies with regard to different items. Analyses were performed using SPSS 24 or Excel. P values < 0.5 were considered to be statistically significant.
Results
Study cohort
A total of 139 pregnant women were included in the study (Table 1). The mean age of these patients was 31 years. Initial consultation at our colposcopy unit took place at a median gestational age of 16 weeks (range 2–38). Over the course of their pregnancy 90/139 (64.7%), women were seen at our center for a second consultation, 48/139 (34.5%) for a third, 15/139 (10.8%) a fourth and 5/139 (3.6%) a fifth. The mean interval between prepartal visits was 61.4 days. Prior to their pregnancy 57 (47.1%), women had been diagnosed with abnormal cervical cytologies, out of which 5 (3.6%) women reported to have undergone previous conization because of CIN2 or CIN3. Only one woman had been HPV vaccinated after previous conization. HSIL was diagnosed in three out of five patients and two were referred with suspicious clinical findings. All women were closely monitored during pregnancy and none of them were HPV positive or required cervical biopsies during pregnancy.
Prepartal results
External cytology results at admittance were compared with in-house colposcopy findings. Out of 104 women diagnosed with HSIL in external cytology, 68 (65.4%) had undergone documented in-house colposcopies (Table 2). During the course of their pregnancy, 56/83 (67.4%) women with prepartal CIN2–3 diagnoses were tested for HPV infections. An amount of 50 (89.3%) patients had high-risk HPV infections.
Among enrolled patients, ten women were HPV vaccinated. Out of those, five were vaccinated after a history of suspect cytology, previous to the time of data acquirement. During the time of pregnancy, these women were never diagnosed with high-grade CIN. An additional five out of ten were also HPV vaccinated previous to their participation in the study; however, due to missing data, we are not able to provide more information about their cytological or histological status during this period.
Prepartal CGB was performed in 98/139 (70.5%) cases (Table 3). While 8 (8.2%) cases showed no signs of dysplasia, 7 (7.1%) women were diagnosed with CIN1, 14 (14.3%) patients with CIN2 and 69 (70.4%) with CIN3. Invasive disease was not suspected in any case. CGB did not lead to any major complications. Excisional procedures were not performed during the course of pregnancy. The mean gestational age at CIN3 diagnosis at our clinic was 16.8 weeks (range 5–30 weeks).
Further, concordance rates of prepartal histology and cytology at referral were compared and displayed in Table 3. Out of 83/139 (59.7%) women diagnosed with CIN2–3 in pregnancy, 70 (84.3%) women had previously been referred with external cytologies. Finally, 63/70 (90%) patients diagnosed with CIN2–3 during pregnancy were referred due to HSIL in cytology; likewise, 4/83 (4.8%) patients with LSIL and 3/83 (3.6%) were referred with AS-CUS (Table 3). On closer examination of the discrepancy between histological and cytological findings with regards to the 4/83 (4.8%) patients with CIN2–3 lesions after LSIL cytologies, 3/4 (75.0%) had previously shown major changes in in-house colposcopy; while, 1/4 (25%) patients presented with minor changes in colposcopy. Likewise, 2/3 (66.7%) women with AS-CUS at referral showed signs of minor changes at in-house colposcopy; whereas, 1/3 (33.3%) had no documented colposcopy findings.
Comparing in-house prepartal histology and prepartal colposcopy resulted in a colpo-histopathological concordance of 29/39 (74.4%), an "underestimation" of 6/39 (15.4%) and an "overestimation" of 4/39 (10.3%) with regards to colposcopy and histology both performed at our own clinic (Table 2).
Postpartal results
90/139 (64.7%) women were seen for postpartum follow-up (FU). In 88/90 (97.8%) cases, mode of delivery was documented: 65 (73.9%) patients experienced spontaneous delivery, 20 (22.7%) women underwent caesarean section, 2 (2.3%) patients had an abortion, and one patient (1.1%) had a termination of pregnancy independent from positive CIN diagnosis.
After delivery, 60/83 (72.3%) women, diagnosed with CIN2–3 during pregnancy, attended a postpartal FU (Table 2). All were submitted to CGB. At their postpartum consultation, 37/60 (61.7%) women diagnosed with prepartal CIN2–3 presented with persistent lesions, 3/60 (5%) women showed CIN1 and 20/60 (33.3%) patients showed complete remission (Table 2). In a regression analysis, the influence of age and HR-HPV infection on disease prognosis of CIN2–3 after delivery was analyzed (Table 4).
Further, 28/56 (50%) women diagnosed with CIN2–3 combined with a positive HPV test in their prepartal period were followed up after pregnancy by means of both a repeated HPV test as well as histology.
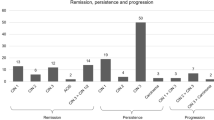
While HPV clearance was recorded in none of 17/28 (60.7%) cases with persistent or partially regressive histological findings postpartum, 4/11(36.4%) women with complete histological remission presented with HPV clearance (Fig. 1).
Postpartum conization was performed in 37/83 (44.6%) patients with prepartal CIN2–3. Histology confirmed CIN3 in 31/37 (83.7%) cases and CIN2 for 5/37 (13.5%) women. In 1/37 (2.7%) patients diagnosed with persistent CIN3 8 weeks after caesarean section, the cone revealed a CIN3 with progression to a microinvasive carcinoma (age 37, HPV type 16+, L0V0).
Discussion
According to the American College of Obstetricians and Gynecologists and international guidelines, the primary aim of cytological screening and colposcopy performed during pregnancy is the exclusion of invasive cancer [12,13,14,15,16,17,18]. Therefore, as with non-pregnant patients, abnormal cytology during pregnancy leads to the recommendation of colposcopy and if necessary further CGB of high-grade lesions [17, 20]. To adhere to this guideline, one should be aware of the issues associated with diagnostics in such cases. Cytometrically, CIN lesions in pregnancy are considered identical to those of non-pregnant women. However, they can be more difficult to interpret correctly, especially during the second half of pregnancy, leading to an overall higher rate of falsely positive results [7, 21, 22]. In addition, colposcopical accuracy in pregnancy may be limited by hormonal changes resulting in cervical hyperemia, hyperplasia of endocervical glands, mucus overproduction, prolapsing vaginal walls and contact bleeding [21,22,23,24,25]. Although the squamocolumnar junction and the transformation zone are better exposed in pregnancy due to physiological eversion, changes such as oedema, cyanosis, friability, increased pelvic congestion and vaginal wall protrusion may present as objective limitations to subjective colposcopical interpretation [8, 23]. While the most common cytology in the study cohort was HSIL, only 60.6% (63/104) of these cytological findings were later verified as CIN2–3 in biopsy, thus confirming the issue of overdiagnosis in this particular study group. In these circumstances, increasing the accuracy of diagnostic procedures becomes a priority and, thus, pregnant women with suspected pathologies of the cervix should be assessed and consequently treated in specialized units.
Correlating colposcopy with histopathological diagnosis in non-pregnant women shows a relatively high reliability which increases in correlation with a higher CIN grade, resulting in an accuracy of 68% for CIN1, 73.3% for CIN2, 81.4% for CIN3 and 88.9% for invasive cancer [26]. Likewise, correlating colposcopy with histopathological diagnosis in our pregnant cohort shows similar results with a colpo-histopathological concordance rate of 33% for CIN1 and 81.8% for CIN2–3 lesions (Table 2). Fader et al. report a colpo-histopathological concordance rate of 62.9%. Ciavattini et al. describe their rate at 68.1% with a better reliability in the first half of pregnancy [9, 23]. Baldauf et al. show an even higher colpo-histopathological concordance rate of 72.6%, with an overestimation and underestimation rate of 17.6% and 9.8%, respectively, interestingly enough not influenced by pregnancy [27].
With reference to the over- or underestimation of colposcopy in 25.6% of cases included in this study, one should consider the clinical approach to securing a biopsy of the portio during pregnancy. The objective of biopsy is to confirm or otherwise rule out a microinvasive carcinoma within a suspicious area, not, however, to secure CIN2–3. The latter may be just as adequately diagnosed by means of expert colposcopy. This altered approach to the diagnostic procedure in pregnancy may explain the obvious divergence in the findings of this study.
According to recent ASCCP guidelines, pregnant women with high-grade lesions in cytology and corresponding colposcopical findings, with or without additional biopsy, should be followed up with repeat colposcopy at intervals no shorter than 12 weeks at the discretion of the clinician in charge [17]. However, currently there are in fact no data to support the necessity for repeat evaluations [17, 23]. On the contrary, continuous monitoring may lead to insecurity on the side of the pregnant patient. This insecurity may in turn give rise to a decrease in patient compliance, vital to a functioning diagnostic and therapeutic approach during and beyond pregnancy. Further, repeat diagnostics appear contradictory, when taking into account the low risk for disease progression during pregnancy, if CC has previously been excluded.
A systematic review and meta-analysis regarding the clinical course of untreated CIN2 under active surveillance in a non-pregnant cohort shows evidence of CIN2 regression being more common than expected. The review summarizing 36 studies (all together over 3000 women) was able to show that half of the CIN2 lesions regress spontaneously within 2 years of surveillance. In young women, in particular, expectant management is, therefore, a valid option, as up to 60% of CIN2 lesions regress under active surveillance, while progression is extremely rare. Pooled rates for regression within 12 months of CIN2 diagnosis in non-pregnant women stand at 46%, while progression rates lie around 14% [28]. Overall, the risk of progression is particularly low in women negative for HR-HPV or HPV 16/18 at baseline; whereas, for those who test positive, the regression rate lies at 40% within 2 years [28].
Although there is a consensus concerning the diagnosis, evolution and treatment of CIN2–3/HSIL for non-pregnant women, this is not the case for their counterparts. Data on the prepartal period are particularly scarce. Further additional research is required concerning the transition to the postpartal period and the changes necessary to the diagnostic and therapeutic approach under these conditions. A retrospective analysis and review of literature revealed heterogeneous data concerning the regression rates of CIN2–3 after pregnancy with 16.7% to almost 69.3% [9, 26, 29,30,31,32,33,34] and persistence rates between 26.8% and 70% [11, 35,36,37,38] (Table 5). Moreover, a comparison of non-pregnant women with their pregnant counterparts, both diagnosed with CIN lesions (CIN1–3), revealed a significantly higher tendency for spontaneous regression (56.9% vs. 31.4%, p = 0.144) within the pregnant cohort [35].
There are many theories with regards to the rather high regression of HSIL or CIN2–3 lesions associated with pregnancy in literature [8, 17, 30, 32, 39]. Some authors found that vaginal delivery is associated with higher regression rates at around 67% when compared to 13% after caesarean sections [32, 39]. It has been speculated that cervical trauma, especially occurring during the second and third stages of labor and also delivery itself, may lead to an inflammatory reaction of the cervix epithelium, which may in turn promote repair mechanisms [30]. In addition, the loss of dysplastic cell material during cervical ripening and the passage through the birth canal have been considered beneficial for regression of dysplasia [30]. A further theory suggests that transient ischemic changes during vaginal delivery may be relevant [8]. In contrast, other authors have not found any association between mode of delivery and regression rates [29, 35, 38]. Yost et al. found a rather high regression rate of HSIL in 70% of cases independent from the mode of delivery [29].
This study did not find any significant differences for regression rates with regard to vaginal delivery versus caesarean section. Accordingly, the mode of delivery should not be influenced by CIN status and likewise, does not influence the later. However, in cases of cervical carcinoma, recent data indicate that primary cesarean section improves prognosis [40]. Progression rates of CIN2–3 lesions to invasive carcinoma in pregnancy vary from 2 to 28% [35].
In this study’s cohort, one patient suffering from a persistent CIN3 lesion after caesarean section was later diagnosed with transition to a microinvasive carcinoma in cone biopsy. Overall, out of 64/139 (46%) patients in this cohort, who underwent at least one prepartal and one postpartal histology (Table 2), regression of CIN2–3 was proven in 38% of cases; while, the progression rate stood at 1.6%, showing a satisfying overall correlation with a pooled analysis of studies reporting histopathological outcomes (Table 5).
Besides remodeling and repair theories of the cervix after pregnancy contributing to disease regression, high rates of postpartum regression may just as well reflect the natural course of cervical HPV infections [23]. Likewise, the range of these findings might be explained by disparities of HPV prevalence, types and clearance rates as well as overestimation in pregnancy (Fig. 1). In most cases, retrospective study designs, undocumented HPV statuses and patients lost to FU limit further interpretation. As data were obtained from a specialized colposcopy unit, many patients were referred for initial colposcopy and recommendations, but not always followed in the unit. Nevertheless, the study cohort remained large enough to deduce meaningful observations.
While it might be complicated to change the retrospective design of these studies, the two latter issues need to be rectified. Whereas the testing for HPV status and type should be increased in the future, the matter of postpartal FU remains. This issue could be improved by taking away the emphasis placed on close and frequent monitoring during pregnancy; while, in turn, stressing the need for a postpartal thorough work-up comprised of colposcopy, cytology and histology accompanied by HPV testing. What is more, better communication not only between responsible physicians in clinics and private practices, but also between both latter parties and their patients might help to focus attention on the new mother´s health along with that of her child with regards to FU consultations.
Similarly, the link between genital HPV infections and CC has become well established and indeed cofactors which modify the risk for HPV positive women are well known (e.g., immunosuppression or immunodeficiency) [41, 42]. Further research has revealed that pregnancy itself is associated with a modestly increased prevalence of cervical HPV infection. The prevalence odds ratio for the association of pregnancy and HPV infection is 2.2 (95% CI 1.1–4.5) [37]. The prevalence of HPV increases with progression of gestational age (HPV prevalence of 18.9% among non-pregnant women vs. 27.3% in those in the first 12 weeks of pregnancy and 39.7% in those who were past the 12th week of pregnancy). This association appears to be independent of age and other major HPV risk factors of the mother [43].
In this cohort, HPV clearance was recorded in 4/14 (28.6%) women with complete histological remission. In the case of persistent HPV infection after pregnancy, additional long-term FU, even in cases of primary regression of CIN, might be necessary to detect recurrent disease.
Finally, HR-HPV prevalence in pregnancy in combination with CIN2–3 leads to the recommendation of close pre- and especially postpartum FUs. For gynecologists, it is of fundamental importance to recognize the opportunity presented in pregnancy of making standard diagnostic procedures available to especially those women who do not otherwise partake in routine check-ups. A limitation of this study is its retrospective design, its limited number of cases and its lack of additional longitudinal FU data. However, on the other hand, it is one of the first studies to evaluate the course of HPV infection in pregnant patients before and after delivery.
Conclusion
High-grade CIN lesions diagnosed during pregnancy show high rates of postpartal regression, while progression to carcinoma is rare. Although widely considered a safer approach, close and continuous monitoring, potentially problematic for the pregnancy, rarely results in therapeutic consequences. At the same time, prepartal consultations present an opportunity to include women into screening procedures that should be continued after delivery.
References
Insinga RP, Glass AG, Rush BB (2004) Diagnoses and outcomes in cervical cancer screening: a population-based study. Am J Obstet Gynecol 191(1):105–113 (Epub 2004/08/06)
Al-Halal H, Kezouh A, Abenhaim HA (2013) Incidence and obstetrical outcomes of cervical intraepithelial neoplasia and cervical cancer in pregnancy: a population-based study on 8.8 million births. Arch Gynecol Obstet. 287(2):245–250 (Epub 2012/10/12)
Gravitt PE, Winer RL (2017) Natural history of HPV infection across the lifespan: role of viral latency. Viruses. 9(10):267 (Epub 2017/09/22)
Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE (2011) Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst 103(5):368–383 (Epub 2011/02/02)
Kjaer SK, Frederiksen K, Munk C, Iftner T (2010) Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 102(19):1478–1488 (Epub 2010/09/16)
Douvier S, Filipuzzi L, Sagot P (2003) Management of cervical intra-epithelial neoplasm during pregnancy. Gynecologie, Obstetrique and Fertilite. 31(10):851–855 (Epub 2003/12/04. Prise en charge d'une neoplasie intra-epitheliale du col de l'uterus en cours de grossesse)
Morimura Y, Fujimori K, Soeda S, Hashimoto T, Takano Y, Yamada H et al (2002) Cervical cytology during pregnancy-comparison with non-pregnant women and management of pregnant women with abnormal cytology. Fukushima J Med Sci 48(1):27–37 (Epub 2002/10/09)
Origoni M, Salvatore S, Perino A, Cucinella G, Candiani M (2014) Cervical intraepithelial neoplasia (CIN) in pregnancy: the state of the art. Eur Rev Med Pharmacol Sci 18(6):851–860 (Epub 2014/04/08)
Fader AN, Alward EK, Niederhauser A, Chirico C, Lesnock JL, Zwiesler DJ et al (2010) Cervical dysplasia in pregnancy: a multi-institutional evaluation. Am J Obstet Gynecol. 203(2):113e1–113e6 (Epub 2010/06/05)
McIntyre-Seltman K, Lesnock JL (2008) Cervical cancer screening in pregnancy. Obstet Gynecol Clin N Am. 35(4):645–658 (Epub 2008/12/09)
Vlahos G, Rodolakis A, Diakomanolis E, Stefanidis K, Haidopoulos D, Abela K et al (2002) Conservative management of cervical intraepithelial neoplasia (CIN(2–3)) in pregnant women. Gynecol Obstet Investig 54(2):78–81 (Epub 2003/02/05)
Bentley J (2012) Colposcopic management of abnormal cervical cytology and histology. J Obstet Gynaecol Can. 34(12):1188–1202 (Epub 2012/12/13)
Hillemanns P, Friese K, Dannecker C, Klug S, Seifert U, Iftner T, Hädicke J, Löning T, Horn L, Schmidt D, Ikenberg H, Steiner M, Freitag U, Siebert U, Sroczynski G, Sauerbrei W, Beckmann MW, Gebhardt M, Friedrich M, Münstedt K, Schneider A, Kaufmann A, Petry KU, Schäfer APA, Pawlita M, Weis J, Mehnert A, Fehr M, Grimm C, Reich O, Arbyn M, Kleijnen J, Wesselmann S, Nothacker M, Follmann M, Langer T, Jentschke M (2019) Prevention of cervical cancer: guideline of the DGGG and the DKG (S3Level, AWMF Register Number 015/027OL, December 2017) - Part 2 on Triage. Treatment and Follow-up. Geburtshilfe Frauenheilkd 79(2):160–176. https://doi.org/10.1055/a-0828-7722
Jordan J, Arbyn M, Martin-Hirsch P, Schenck U, Baldauf JJ, Da Silva D et al (2008) European guidelines for quality assurance in cervical cancer screening: recommendations for clinical management of abnormal cervical cytology, part 1. Cytopathology 19(6):342–354 (Epub 2008/12/02)
Jordan J, Martin-Hirsch P, Arbyn M, Schenck U, Baldauf JJ, Da Silva D et al (2009) European guidelines for clinical management of abnormal cervical cytology, part 2. Cytopathology 20(1):5–16 (Epub 2009/01/10)
Kuehn W, Gieseking F (2015) Die aktuellen Empfehlungen der AG-CPC zur Kolposkopie 2015. Gyn Praktische Gynäkologie. 20:25–47
Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M et al (2013) 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 121(4):829–846 (Epub 2013/05/03)
WHO. Comprehensive cervical cancer control, a guide to essential practice. 2014;Second Edition:1–364.
Griesser H, Marquardt K, Jordan B et al (2013) Münchner Nomenklatur III: gynäkologische Zytodiagnostik der Zervix. Frauenarzt 54:1042–1048
Massad LS (2012) New guidelines on cervical cancer screening: more than just the end of annual Pap testing. J Lower Genit Tract Dis 16(3):172–174 (Epub 2012/07/24)
Michael CW, Esfahani FM (1997) Pregnancy-related changes: a retrospective review of 278 cervical smears. Diagn Cytopathol 17(2):99–107 (Epub 1997/08/01)
Economos K, Perez Veridiano N, Delke I, Collado ML, Tancer ML (1993) Abnormal cervical cytology in pregnancy: a 17-year experience. Obstet Gynecol 81(6):915–918 (Epub 1993/06/01)
Ciavattini A, Serri M, Di Giuseppe J, Liverani CA, Fallani MG, Tsiroglou D et al (2018) Reliability of colposcopy during pregnancy. Eur J Obstet Gynecol Reprod Biol 229:76–81 (Epub 2018/08/18)
Benedet JL, Selke PA, Nickerson KG (1987) Colposcopic evaluation of abnormal Papanicolaou smears in pregnancy. Am J Obstet Gynecol 157(4 Pt 1):932–937 (Epub 1987/10/01)
Valente PT, Schantz HD, Schultz M (1994) Cytologic atypia associated with microglandular hyperplasia. Diagn Cytopathol 10(4):326–331 (Epub 1994/01/01)
Coppolillo EF, De Vega HM, Brizuela J, Eliseth MC, Barata A, Perazzi BE (2013) High-grade cervical neoplasia during pregnancy: diagnosis, management and postpartum findings. Acta Obstet Gynecol Scand. 92(3):293–297 (Epub 2012/08/14)
Baldauf JJ, Dreyfus M, Ritter J, Philippe E (1995) Colposcopy and directed biopsy reliability during pregnancy: a cohort study. Eur J Obstet Gynecol Reprod Biol 62(1):31–36 (Epub 1995/09/01)
Tainio K, Athanasiou A, Tikkinen KAO, Aaltonen R, Cardenas J, Hernandes J et al (2018) Clinical course of untreated cervical intraepithelial neoplasia grade 2 under active surveillance: systematic review and meta-analysis. BMJ 360:k499 (Epub 2018/03/01)
Yost NP, Santoso JT, McIntire DD, Iliya FA (1999) Postpartum regression rates of antepartum cervical intraepithelial neoplasia II and III lesions. Obstet Gynecol 93(3):359–362 (Epub 1999/03/13)
Paraskevaidis E, Koliopoulos G, Kalantaridou S, Pappa L, Navrozoglou I, Zikopoulos K et al (2002) Management and evolution of cervical intraepithelial neoplasia during pregnancy and postpartum. Eur J Obstet Gynecol Reprod Biol 104(1):67–69 (Epub 2002/07/20)
Jain AG, Higgins RV, Boyle MJ (1997) Management of low-grade squamous intraepithelial lesions during pregnancy. Am J Obstet Gynecol 177(2):298–302 (Epub 1997/08/01)
Ahdoot D, Van Nostrand KM, Nguyen NJ, Tewari DS, Kurasaki T, DiSaia PJ et al (1998) The effect of route of delivery on regression of abnormal cervical cytologic findings in the postpartum period. Am J Obstet Gynecol 178(6):1116–1120 (Epub 1998/07/14)
Serati M, Salvatore S, Uccella S, Laterza RM, Cromi A, Ghezzi F et al (2009) Sexual function after radical hysterectomy for early-stage cervical cancer: is there a difference between laparoscopy and laparotomy? J Sex Med 6(9):2516–2522 (Epub 2009/06/25)
Lurain JR, Gallup DG (1979) Management of abnormal Papanicolaou smears in pregnancy. Obstet Gynecol 53(4):484–488 (Epub 1979/04/01)
Mailath-Pokorny M, Schwameis R, Grimm C, Reinthaller A, Polterauer S (2016) Natural history of cervical intraepithelial neoplasia in pregnancy: postpartum histo-pathologic outcome and review of the literature. BMC Pregnancy Childbirth 16:74 (Epub 2016/04/09)
Palle C, Bangsboll S, Andreasson B (2000) Cervical intraepithelial neoplasia in pregnancy. Acta Obstet Gynecol Scand 79(4):306–310 (Epub 2000/04/04)
Karrberg C, Brannstrom M, Strander B, Ladfors L, Radberg T (2013) Colposcopically directed cervical biopsy during pregnancy; minor surgical and obstetrical complications and high rates of persistence and regression. Acta Obstet Gynecol Scand 92(6):692–699 (Epub 2013/04/18)
Coppola A, Sorosky J, Casper R, Anderson B, Buller RE (1997) The clinical course of cervical carcinoma in situ diagnosed during pregnancy. Gynecol Oncol 67(2):162–165 (Epub 1997/12/31)
Siristatidis C, Vitoratos N, Michailidis E, Syciotis C, Panagiotopoulos N, Kassanos D et al (2002) The role of the mode of delivery in the alteration of intrapartum pathological cervical cytologic findings during the postpartum period. Eur J Gynaecol Oncol 23(4):358–360 (Epub 2002/09/07)
Zhang X, Gao YL, Yang Y (2015) Treatment and prognosis of cervical cancer associated with pregnancy: analysis of 20 cases from a Chinese tumor institution. J Zhejiang Univ Sci B 16(5):388–394 (Epub 2015/05/21)
Burd EM (2003) Human papillomavirus and cervical cancer. Clin Microbiol Rev 16(1):1–17 (Epub 2003/01/15)
Bosch FX, Bernaola IE (2006) The human papillomavirus vaccine and the incorporation of pediatrics in cervical cancer prevention. An Pediatr (Barc). 65(5):411–413 (Epub 2006/12/23. La vacuna frente al virus del papiloma humano y la incorporacion de la pediatria a la prevencion del cancer de cuello uterino)
Morrison EA, Gammon MD, Goldberg GL, Vermund SH, Burk RD (1996) Pregnancy and cervical infection with human papillomaviruses. Int J Gynaecol Obstet 54(2):125–130 (Epub 1996/08/01)
Author information
Authors and Affiliations
Contributions
DG: project development, study design, retrospective data collection, analysis of data and writing the manuscript (original draft); IL: project development, retrospective data collection, statistical analysis, writing and approving the manuscript, KP: project development, data collection, reviewing and approving the manuscript; AJ: project administration, reviewing and approving the manuscript; VM: project administration, review and approving the manuscript, supervision; SK: study review and approving the manuscript; EB: pathological review, writing and approving the manuscript, supervision; SL: statistical advice and analysis, review and approving the manuscript, methodology; BS: reviewing and approving the manuscript, supervision; LW: study design, organization of the dysplasia consultation unit, data collection, analysis of data, writing the manuscript (original draft).
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest. This study was not funded by anybody.
Ethical approval retrospective studies
This article does not contain studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study (Ethics Committee of the Medical Board Hamburg reference number 190504).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Grimm, D., Lang, I., Prieske, K. et al. Course of cervical intraepithelial neoplasia diagnosed during pregnancy. Arch Gynecol Obstet 301, 1503–1512 (2020). https://doi.org/10.1007/s00404-020-05518-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05518-1