Abstract
Objective
The objective of this study was to determine if high-dose antibiotic prophylaxis with cefazolin decreases the risk of surgical site infection (SSI) after a cesarean delivery.
Methods
We performed a retrospective cohort study of women who underwent a cesarean section. Two preoperative antibiotic regimens were compared: low dose versus high dose. The primary outcome was SSI. A sample size of 343 patients per group was calculated for a 50% reduction in risk for SSI.
Results
Seven hundred and thirty women were included with an incidence of SSI of 5%. Women who received the high-dose antibiotic regimen had lower rates of risk factors for SSI. The only exception was skin incision closure with staples. The rate of SSI did not differ between the low-dose and high-dose groups, even after adjusting for confounding variables [aOR 1.78, 95% CI (0.82–3.9)].
Conclusions
Higher doses of antibiotic prophylaxis did not decrease the rates of SSI after cesarean delivery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cesarean section remains the most common type of surgical procedure worldwide. In the United States alone, around 30% of pregnant women undergo cesarean delivery despite ACOG prevention policies [1].
Compared to vaginal delivery, women undergoing cesarean section have a fivefold to tenfold increased risk of complications related to infections [2] such as endometritis and wound complications (disruption and SSI). The latter increases the burden on patients’ morbidity and the costs of maternal health care [3]. With the advent of preoperative antibiotic surgical prophylaxis, SSI rates have decreased. A recent Cochrane Systematic Review demonstrated that postoperative complications from cesarean section, such as SSI, endometritis, and serious maternal infection, were all decreased by 60–70% in pregnant women who received preoperative antibiotic prophylaxis [4]. In addition, preoperative antibiotic administration was not associated with any neonatal adverse events [5].
Current guidelines recommend cefazolin, a first-generation cephalosporin with bactericidal activity, as the preoperative antibiotic of choice. Its mechanism of action involves binding penicillin-binding proteins to the inner cell wall, hindering cell wall synthesis, and leading ultimately to bacterial cell lysis. The use of cefazolin has been widely accepted based on its broad-spectrum activity (gram-positive, group B streptococcus and gram-negative) and its low cost. It distributes widely in most organ tissues with a short peak time (highest serum concentration within minutes) and with a halflife of about 1.8 h [6]. ACOG states that for surgical prophylaxis, a single 1 g dose of cefazolin intravenous should be administered within 60 min prior to cesarean section and that a higher dose should be considered in women with a body weight greater than 100 kg or BMI greater than 30 kg/m2 [7]. Recently, a higher dose of cefazolin has been suggested, with the hypothesis that higher levels above the minimal inhibitory concentration (MIC) lead to lower postoperative maternal infection complications in the obstetrical population. Two randomized control trials comparing 2 g versus 3 g cefazolin regimens in obese patients. The studies’ primary outcomes consisted of drug concentration in adipose tissue. Young et al. showed that both regimens reached a minimal inhibitory concentration in adipose tissue for gram-positive and gram-negative bacteria [8]. On the other hand, Maggio et al. showed that a higher dose did not increase the adipose tissue concentration of cefazolin [9]. No significant difference was found in drug concentration in maternal adipose tissue among groups [8, 9]. Higher plasma and tissue antimicrobial levels have also been reported in patients receiving a prophylactic 4 g cefazolin regimen [10]. The aforementioned trials have important pitfalls, since the outcomes did not include maternal infectious complications and were underpowered. In an attempt to address this concern, Ahmadzia et al. performed a retrospective study in obese patients comparing the two regimens of prophylactic cefazolin with the primary outcome being the infectious comorbidity. Despite being underpowered, the authors concluded that a high-dose regimen did not affect SSI rates [11]. In light of these conflicting results, there is a need for further studies with SSI, rather than drug tissue levels, as the primary outcome. In this study, we performed a chart review comparing two regimens of cefazolin prophylaxis with the primary outcome being SSI.
We believe that high-dose antibiotic prophylaxis may decrease the risk of postoperative wound disruption and infection in mothers undergoing cesarean delivery. Our objective is to validate this hypothesis by performing a retrospective cohort of patients delivered by cesarean at our institution who received high-dose preoperative antibiotic prophylaxis.
Methods
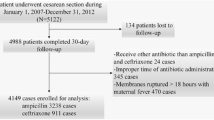
After review and determination by the University of Texas Medical Branch Galveston Hospital Institutional Review Board, our research project (IRB protocol #16-0204) was found to meet the exemption criteria and review was waived. We then conducted a retrospective cohort study at our medical facility, a tertiary center serving a patient population indigenous to the southeast area of Texas. All patients’ information was de-identified per our IRB policy and the authors did not have access to such information during or after data collection. Our obstetric population has mostly Medicaid insurance and is mainly Hispanic. During the year 2016, a high-dose regimen of cefazolin (2 g, if BMI < 30 or 3 g if BMI ≥ 30) was implemented in our institution for cesarean section prophylaxis. Before this period, we used a regimen consistent with current ACOG recommendations (1 g, if BMI < 30 or 2 g if BMI ≥ 30). Patients were stratified into two groups by preoperative dosage of cefazolin. Those groups were either high-dose regimen or low-dose regimen.
In our medical center, in-training physicians perform cesarean sections with the assistance of faculty. Throughout both the high-dose and low-dose periods, the procedures were consistent; all cases had hair clipped at incision before surgery initiation, and chlorohexidine was used for skin preparation. Most patients had Pfanneistiel skin incision, low transverse hysterotomy, spontaneous placenta extraction, single-layer hysterotomy closure, and either staple or suture skin closure. Surgical antibiotic prophylaxis was given intravenously and within 60 min prior to skin incision in compliance with our national guidelines (ACOG).
Medical records were reviewed and data were collected by the authors on maternal demographics, characteristics, indications of cesarean delivery, duration of hospital stay, and antibiotic usage. These data included age, parity, ethnicity, mode of delivery, date of admission or discharge, preoperative antibiotic prophylaxis, type of skin closure, and estimated blood loss. We also collected information on medical, obstetrical, intraoperative, and postoperative complications, including wound infections and disruptions.
The primary outcome was SSI occurring within 30 days post surgery, based on the Centers for Disease Control and Prevention criteria [12]. Wound infection was defined as cellulitis, abscess, or purulent drainage evidenced by the need of debridement, wound revision, or antibiotics. Wound disruption was defined as subcutaneous skin separation secondary to seroma or hematoma or wounds that were later found to have fascia dehiscence. We excluded patients who had chorioamnionitis since this group of patients was already treated intrapartum mostly with ampicillin plus gentamicin; if cesarean delivery was pursued, either clindamycin or metronidazole alone was added preoperatively. Chorioamnionitis was defined as temperature greater or equal to 37.8 °C in addition to more than or equal to 2 of the following: fetal tachycardia, uterine tenderness, foul odor discharge, maternal leukocytosis, or maternal tachycardia. We also excluded patients that received alternative antibiotic regimens due to allergy or noncompliance to the protocol regimen of interest.
Data analysis
Analysis was performed using STATA (StataCorp 14.0, Dallas, TX). The prevalence of SSI in our institution ranges from 7 to 10%. A sample size of 343 patients per group was estimated for a 50% reduction in risk for SSI. The last 367 patients before implementation and first 365 patients after implementation of the high-dose antibiotic protocol were consecutively selected for the study. For nonparametric data, Wilcoxon rank-sum was used. For categorical data points, we used Chi-squared test or Fisher’s exact test. For continuous parametric variables, unpaired t test was used. Data are reported as mean ± SEM or median with interquartile range [IQR] as appropriate. Statistical analysis was performed using univariable and multivariable logistic regressions and a P < 0.05 was considered statistically significant.
Results
A total of 730 women were included between 2015 and 2016. The mean age was 29 years. Most of our patients were obese (BMI > 30 kg/m2; 65–73%), Hispanic (59–60%), had skin closure by staples, and underwent an elective repeat cesarean section (68%). The prevalence of SSI after cesarean section during both periods was between 4 and 5%. The specific baseline characteristics between low-dose and high-dose antibiotic groups are shown in Table 1. The rate of SSI did not differ between the low-dose and high-dose groups [14/367 (4%) vs. 19/365 (5%), P = 0.38]. There was significant difference between groups for several baseline characteristics such as BMI, gestational diabetes, incision skin closure, estimated blood loss, and cesarean delivery rates after vaginal attempt. On univariable analysis, women who had the high-dose regimen had a lower BMI (35.6 ± 0.40 vs. 33.1 ± 0.42; P < 0.0001), lower gestational diabetes (13 vs. 8%; P = 0.04), lower estimated blood loss greater than 1000 mL (1111 ± 32 vs. 1049 ± 11; P = 0.0001), and lower skin incision closure with staples (37 vs. 49%; P = 0.04). SSI rates did not differ among groups (4 vs. 5%; P = 0.38; Table 1).
After adjusting significant confounding variables, the rates of SSI were not significantly different between high-dose and low-dose regimen groups [aOR 1.78, 95% CI (0.82–3.9)]. Patients who had their skin incisions closed by staples and had an EBL greater than 1000 mL were associated with higher rates of SSI [aOR 3.03, 95% CI (1.03–8.94) and aOR 3.99, 95% CI (1.89–8.94), respectively; Table 2].
Comment
In 2013, the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Surgical Infection Society, and the Society for Healthcare Epidemiology of America jointly revised the clinical practice guidelines for antimicrobial prophylaxis in surgery [13]. In the 2013 update, the authors suggest that institutions should consider doubling the dose of cefazolin for patients weighing more than 80 kg and using 3 g for patients weighing more than 120 kg based on low cost and favorable safety profile. These recommendations were based on expert opinion and on scarce data in general surgical—rather than obstetrical—patients.
The optimal dose of cefazolin remains controversial due to insufficient supporting data. Available evidence from two out of three clinical trials [8,9,10] show that obese women undergoing cesarean section did not have different mean inhibitory concentration of antimicrobial agents in adipose tissue despite receiving a higher dose of antibiotic prophylaxis. Another retrospective study comparing the two regimens of prophylactic cefazolin in morbidly obese patients was underpowered and did not find a difference in SSI rates in mothers who received the high-dose regimen [11].
We performed a large retrospective cohort study comparing two different regimens of cefazolin for antimicrobial prophylaxis in cesarean sections with SSI as a primary outcome. A total of 730 women were included in the study based on our power sample size calculation. This single-center study with a patient population consisted of mainly Hispanics insured by Medicaid included all deliveries irrespective of the subjects’ BMIs. Surgical procedures were homogenous throughout both periods of interest. Mothers receiving the high-dose regimen were less obese, less diabetic, had lower cesarean rates after failed attempt of vaginal delivery, had lower estimated blood loss, and were less likely to have skin closure with staples. High-dose antibiotic regimen did not affect SSI rates, even after adjusting for the abovementioned confounding variables. In fact, patients who received higher dose antibiotics showed a trend toward higher SSI or wound complications. Our findings generally support what prior clinical trials have found, but ours is the first inclusive of the general obstetric population with SSI as a primary outcome. Our study also shows that the 2013 expert consensus is not beneficial in the pregnant population.
Being retrospective in nature, our study has the following limitations despite being carefully designed. First, our results may be biased secondary to collecting the wrong patient information (information bias or measurement errors); second, since patients were not randomized, known SSI risk factors can be confounding our results. To address the latter, we performed a univariable model analysis and identified significant confounders that were later included in the final multivariable analysis logistic model. Third, using outpatient medical records to identify SSI, we may have missed some patients with SSI due to a patient’s failing to reveal the clinic providers of being diagnosed with SSI in another hospital or loss to follow up, falsely decreased our SSI rates. Unfortunately, the incidence of SSI in our study was below the estimated rate used for sample size calculation, making it not adequately powered. Despite being underpowered, patients with the high-antibiotic regimen had a trend toward higher SSI rates, rather than the opposite. In addition, known risk factors for SSI were significantly lower in the high-antibiotic group, favoring lower SSI rates against the observed trend. This leads us to believe that even if a larger sample size is undertaken, our findings would not be impacted significantly. We would instead expect that trend to be more significant.
Our findings support the conclusion from a prior study by Ahmadzia et al. [11]: a higher dose of cefazolin for preoperative surgical prophylaxis does not improve SSI rates despite its low cost and safety profile. Current ACOG guidelines should be followed until further level I clinical trial evidence is available. Obstetricians should be aware that increased blood loss and skin closure with staples are important determinants of SSI postoperatively.
References
Betran AP, Ye J, Moller AB, Zhang J, Gulmezoglu AM, Torloni MR (2016) The increasing trend in caesarean section rates: global, regional and national estimates: 1990–2014. PLoS ONE 11:e0148343
Gibbs RS (1980) Clinical risk factors for puerperal infection. Obstet Gynecol 55:178s–184s
Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ (1999) The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol 20:725–730
Smaill FM, Grivell RM (2014) Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev 2014:Cd007482
Mackeen AD, Packard RE, Ota E, Berghella V, Baxter JK (2014) Timing of intravenous prophylactic antibiotics for preventing postpartum infectious morbidity in women undergoing cesarean delivery. Cochrane Database Syst Rev 2014:9516
Currier JS, Tosteson TD, Platt R (1993) Cefazolin compared with cefoxitin for cesarean section prophylaxis: the use of a two-stage study design. J Clin Epidemiol 46:625–630
ACOG Practice Bulletin No (2011) 120: Use of prophylactic antibiotics in labor and delivery. Obstet Gynecol 117:1472–1483
Young OM, Shaik IH, Twedt R et al (2015) Pharmacokinetics of cefazolin prophylaxis in obese gravidae at time of cesarean delivery. Am J Obstet Gynecol 213(541):e1–e7
Maggio L, Nicolau DP, DaCosta M, Rouse DJ, Hughes BL (2015) Cefazolin prophylaxis in obese women undergoing cesarean delivery: a randomized controlled trial. Obstet Gynecol 125:1205–1210
Stitely M, Sweet M, Slain D et al (2013) Plasma and tissue cefazolin concentrations in obese patients undergoing cesarean delivery and receiving differing pre-operative doses of drug. Surg Infect (Larchmt) 14:455–459
Ahmadzia HK, Patel EM, Joshi D et al (2015) Obstetric surgical site infections: 2 grams compared with 3 grams of cefazolin in morbidly obese women. Obstet Gynecol 126:708–715
Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG (1992) CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control 20:271–274
Bratzler DW, Dellinger EP, Olsen KM et al (2013) Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 70:195–283
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
MLR project development, data collection, manuscript writing, data analysis. CO project development, data collection. TR project development, data collection. MA project development, data collection. NS project development, data collection. JV project development, data collection. GO project development, manuscript writing, data analysis. GS project development, manuscript writing, data analysis. AS project development, data collection, manuscript writing, data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Research involving human participants
The protocol was reviewed by the University of Texas Medical Branch Galveston Hospital Institutional Review Board, our research project (IRB protocol #16-0204) and found to meet the exemption criteria and review was waived.
Informed consent
No informed consent was required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
La Rosa, M., Omere, C., Redfern, T. et al. The impact of low-dose versus high-dose antibiotic prophylaxis regimens on surgical site infection rates after cesarean delivery. Arch Gynecol Obstet 301, 69–73 (2020). https://doi.org/10.1007/s00404-019-05370-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-019-05370-y