Abstract
Purpose
To evaluate whether programmed intermittent epidural bolus (PIEB) reduces the incidence of maternal intra-partum fever compared with continuous epidural infusion (CEI) during labor.
Methods
Parturients were randomized to receive CEI (CEI group) or PIEB (PIEB group) with 10 ml per hour for epidural labor analgesia with 1500 subjects in each group. The maintaining dose of two groups is 0.08% ropivacaine with 0.4 μg/ml sufentanil, with patient-controlled epidural analgesia (PCEA) dose of 5 ml and lockout interval of 30 min. The incidence of maternal fever, pain score, epidural sensory levels, the number and proportion of PCEA demand, anesthetics consumption, satisfaction score, neonatal Apgar scale, and maternal and neonatal side effects were recorded.
Results
It was significantly lower of the incidence of maternal fever beginning at 4 h post-analgesia and continuing until delivery in the PIEB group than the CEI group (4 h: 2.6% vs. 4.2%; 5 h: 7.3% vs. 10.2%; delivery: 5.6% vs. 7.9%; 1 h post-delivery: 3.9% vs. 6.2%; 2 h post-delivery: 2.1 vs. 3.5%; total: 5.8% vs. 8.4% in PIEB and CEI, respectively). Compared with CEI group, pain scores at 3, 4, 5 h post-analgesia and delivery (3 h: 2 [1, 2] vs. 2 [1–3]; 4 h: 2 [2, 3] vs. 3 [2–4]; 5 h: 2 [2, 3] vs. 3 [2–4]; delivery: 3 [2–4] vs. 4 [3, 4] in PIEB and CEI, respectively), the number and proportion of PCEA demand (number: 0.7 ± 0.9 vs. 2.2 ± 1.9; proportion: 42.0% vs. 80.3% in PIEB and CEI, respectively), and anesthetics consumption significantly decreased in the PIEB group (Ropivacaine: 60 ± 13 mg vs. 76 ± 17 mg; Sufentanil: 26 ± 4 mg vs. 32 ± 6 mg in PIEB and CEI, respectively), without severe maternal and neonatal side effects and any difference in neonatal Apgar scale. The epidural sensory levels 2 h post-analgesia (2 h: 8[8, 9] vs. 9[8, 9] in PIEB and CEI) and satisfaction score (9 [9, 10] vs. 7 [6, 7] in PIEB and CEI) were significantly higher in the PIEB group compared with those in the CEI group.
Conclusions
PIEB with 10 ml of 0.08% ropivacaine and 0.4 μg/ml sufentanil hourly provided a lower incidence of intra-partum fever with a better analgesic effect compared with CEI, without any severe maternal and neonatal adverse reactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple lines of evidence show that epidural analgesia is associated with maternal intra-partum fever during labor [1,2,3,4]. Epidural- associated maternal intra-partum fever has been proved to be an independent risk factor for neonatal sepsis [5]. Maternal intra-partum fever is a critical predictor of neonatal morbidity, increases the risks of maternal cesarean section, neonatal low Apgar score, sepsis, and intensive care unit admission, and has drawn an increasingly broad range of interest [6, 7]. Up to date, the accurate mechanism of epidural- associated intra-partum hyperthermia is still undefined, but most of studies determine that the underling mechanism is noninfectious inflammation, especially serum IL-6 level elevation [8, 9].
PIEB provides better analgesia, less local anesthetic consumption, and better maternal satisfaction compared to continuous epidural infusion during labor analgesia [10, 11]. Furthermore, on-demand intermittent epidural injections appear to reduce the incidence of maternal intra-partum fever in the first 4 h of labor analgesia [12]. However, the relationship of PIEB and intra-partum fever is still unclear. Thus, we design this prospective, randomized, controlled and double-blind trial to determine the association of the incidence of maternal intra-partum fever and PIEB compared with CEI during labor.
Materials and methods
This study was approved by the Institutional Ethics Committee of the Affiliated Obstetrics and Gynecology Hospital of Nanjing Medical University (Protocol Number: NJMCHH-2012-A010). Written informed consents were obtained from all research participants according to the principles of Helsinki Declaration. This trial was registered at the Protocol Registration and Results System (register.clinicaltrials.gov) and the ClinicalTrials.gov ID is NCT01708668. The clinical trial was performed in the Affiliated Obstetrics and Gynecology Hospital of Nanjing Medical University, Nanjing, China between October 2012 and December 2017.
Inclusion criteria include: singleton, spontaneous labor, subjects who requesting epidural labor analgesia, age from 20 to 45 years, gestation week from 37 to 41, nulliparous, cervical dilation from 1 to 3 cm.
Exclusion criteria include: contraindications for epidural analgesia, a baseline temperature of ≥ 37.5 °C, allergic to opioids and/or local anesthetics, failed to performing epidural catheterization, organic dysfunction, those who were not willing to or could not finish the whole study at any time, unable to perform analgesia evaluation, using or used in the past 14 days of the monoamine oxidase inhibitors, alcohol addictive or narcotic dependent patients, subjects with a nonvertex presentation or scheduled induction of labor, multiple pregnancy, ASA physical status of 3 or higher, height less than 150 cm or more than 170 cm, morbid obesity (BMI more than 35), high-risk pregnancy (gestational diabetes mellitus, gestational hypertension, placenta previa, placental abruption, preeclampsia).
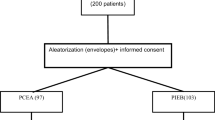
Three thousand parturients were randomly divided into receive CEI (CEI group) or PIEB (PIEB group) epidural labor analgesia, 1500 subjects in each group. Random numbers were generated by computer, and then they were sequentially sealed in the envelopes for grouping. Only the primary investigator opened the envelopes for randomization, other study investigators who observed, assessed, and collected the clinical data, obstetricians, midwifes, and the participants were blinded to group assignment.
As soon as the parturients were admitted to the labor room, the women were intravenously cannulated and continuously injected normal saline over labor and delivery course. A temperature of 22° ± 2 °C was maintained in the labor and delivery rooms. When the woman requested for labor analgesia and the cervical dilation ranged from 1 to 3 cm, epidural labor analgesia was initiated.
Epidural analgesia was initiated in the left lateral decubitus position at the L3–4 or L2–3 interspace. The epidural space was identified using the loss of resistance to saline technique with a 16-gage Tuohy epidural needle. A closed-end, multiorifice epidural catheter was inserted 3–4 cm into the epidural space through the Tuohy needle and secured. Test dose consisted of 3 ml of 1.5% lidocaine with 1:200,000 epinephrine. The parturients received an initial epidural loading bolus dose of 10 ml of 0.125% ropivacaine (AstraZeneca AB, Södertälje, Sweden) plus 0.4 μg/ml sufentanil (EuroCept B.V., Ankeveen, The Netherlands). Those patients would be excluded from the study if their visual analo scale (VAS, 0 = no pain; 10 = worst imaginable pain) scores were higher than 3 score 15 min after the bolus of an initial epidural loading dose. Fifteen min later, the following CEI was maintained at a constant speed of 10 ml h for CEI group. By comparison, hourly PIEB dose of 10 ml was given starting 75 min post the loading dose. PCEA dose was 5 ml with lockout time of 30 min. The maintaining and PCEA anesthetics were 0.08% ropivacaine plus 0.4 μg/ml sufentanil. The infusion pumps (ZZB-I for CEI and ZZB-II for PIEB; Jiangsu Aipeng Medical Science and Technolog Company Ltd., Nantong, China) were packed into an opaque, portable bag for blinding.
Demographic data were recorded. Maternal heart rate, noninvasive arterial blood pressure, respiratory rate, SpO2, tympanic temperature (First Temp Genius® thermometer, Sherwood Medical, St. Louis, MO), epidural sensory levels (loss to cold sensation), visual analo scale (VAS, 0 = no pain; 10 = worst imaginable pain), modified Bromage scale (MBS, 0 = no motor block; 1 = hip blocked; 2 = hip and knee blocked; 3 = hip, knee and ankle blocked) and fetal heart rate tracing were recorded immediately before initiation of analgesia and hourly thereafter until 2 h post-partum. Maternal tympanic temperature was measured by the ear canal with the thermometer according to the manufacturer’s guidelines. Maternal intra-partum fever was defined as a temperature greater than 38 °C. If the parturient still felt pain (VAS > 3) after pushing the PCEA button twice in a 60 min period, an additional manual incremental bolus of 5 ml of 0.15% ropivacaine was administered. The epidural infusion pumps were discontinued until 2 h after childbirth. Whenever the mother did not want to continue to receive the analgesia, they could quit the study freely.
After delivery, the mothers’ satisfaction score (0–10, 0 = dissatisfied, 10 = extremely satisfied) for labor epidural analgesia, consumption of analgesic drugs, number of epidural boluses, and placenta histopathological examination for inflammation (to observe the infectious inflammation role in intra-partum fever) were noted [13]. Obstetric characteristics such as group B streptococcus (GBS) colonization, number of vaginal examinations, oxytocin use, duration from rupture of the membranes to delivery, mode of membranes ruptured, the durations of labor courses, the duration of analgesia (epidural insertion to discontinuation of the pumps), and mode of delivery were collected. Neonatal rectal temperature (one hour post-delivery), weight, Apgar scores, and umbilical cord acid–base status were examined. Neonatal fever was defined as a temperature of more than 38 ºC. We observed the side effects as well.
Statistical analysis
Continuous data variables were analyzed between the groups using the Student’s t test or the Mann–Whitney U test as needed. Categorical variables were compared with χ2 or Fisher’s exact test where appropriate. The Statistical Package for Social Sciences for Windows version 13.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. It would be considered statistically significant if the p value was less than 0.05.
Results
Between October 2012 and December 2017, 3000 parturients were recruited to participate in the study, and 2865 cases were finally analyzed (Fig. 1). Forty subjects in the CEI group and 28 women in the PIEB group converted to cesarean section for obstetric reasons, and 49 cases in the CEI group and 18 patients in the PIEB group had insufficient analgesia, respectively. All these subjects were excluded from the study, with their babies’ Apgar scores ≥ 9 at 1 min and 5 min. There were no significant differences in maternal vital signs and fetal heart rate between the two groups. As for demographic data, the two groups were similar regarding maternal age, height, weight, BMI, gestational age, baseline temperature and cervical dilatation (Table 1). Since 2 h post-analgesia, partial mothers delivered their babies, which explained the total number of subjects decreased in Tables 2, 3 and 4. The incidence of maternal fever was statically lower in the PIEB group than the CEI group beginning at 4 h post-analgesia and continuing until delivery (Table 2).
The VAS pain scores were significantly lower at 3, 4, 5 h post-analgesia and delivery in the PIEB group compared with those in the CEI group (Table 3).
The epidural sensory levels were significantly higher beginning at 2 h post-analgesia and continuing until delivery in the PIEB group compared with those in the CEI group (Table 4). All patients had a MBS of 0 in the study. Epidural, obstetric, and neonatal outcomes are shown in Table 5, the number of epidural boluses, proportion of PCEA demand, consumptions of ropivacaine and sufentanil, and mothers’ satisfaction score in the PIEB group decreased significantly compared with the CEI group. No differences were found between the two groups with respect to other outcomes. Throughout the study, no severe maternal side effects were recorded, such as radycardia, hypotension, and desaturation. No neonatal fever was observed, and all neonatal Apgar scores were more than 8. We did not find statistical significant difference between the two groups in umbilical cord acid–base status, which was within the normal range in all neonates.
Discussion
Although the exact mechanism of intra-partum maternal fever during epidural labor analgesia remains unclear, intra-partum maternal hyperthermia was verified in many present studies, with the ratio of intra-partum maternal fever varying from 1 to 46% depending on different research methods [14,15,16,17,18,19,20,21,22,23,24,25,26]. In the present trial, we witnessed an incidence of intra-partum maternal fever of 8.4% (119 in 1411) in CEI group, 5.8% (85 in 1454) in PIEB group, and 7.1% (204 in 2865) in all subjects, respectively. The total incidence (7.1%) of intra-partum maternal fever was lower than that (11.2%, 14 in 125) of our previous study [1]. The large sample in this trial minimized selection bias than small sample study, which maybe explain the difference in the incidence of intra-partum maternal fever between the two studies.
Except for noninfectious inflammation, sympathetic nerve block is also a critical reason for maternal fever during epidural labor analgesia [17, 27]. Sympathetic nerve blockade resulting from epidural labor analgesia decreases thermoregulatory sweating. Therefore, sweating threshold raises and evaporative heat loss diminishes in the blockade part of the body, reactive vasoconstriction in the non-blocked upper body decreases heat loss. Together, all these factors lead to increased core temperature in parturients undergoing epidural labor analgesia.
The different epidural drug-delivering methods might account for the difference of the incidence of intra-partum maternal fever between the two groups. Although the epidural sympathetic nerve blockade levels were significantly wider beginning at 2 h post-analgesia and continuing until delivery in the PIEB group compared with those in the CEI group in our study, the constant management of epidural analgesics continuously blocked the narrow section of the lower body in the CEI group. In contrast, the blockage intensity became weaker between PIEBs in the PIEB group, which allowing sweating and subsequent evaporative heat loss partly recovered in the blockade part of the body, and decreasing the core temperature and the incidence of fever of mothers [12].
Bolus injection made the drug solution spread more evenly and had a greater number of blocked dermatomal segments than a continuous infusion in epidural apace [28, 29], these advantages suggested that bolus injection provides better analgesia and decreases the number and proportion of PCEA demand for labor analgesia than continuous infusion. In our current study, the consumptions of ropivacaine and sufentanil and mothers’ satisfaction score in the PIEB group decreased significantly compared with the CEI group, which was in accordance with earlier observation [10]. The optimal method of epidural labor analgesia remains undetermined. We employed a PIEB dose of 10 ml of 0.08% ropivacaine with 0.4 μg/ml sufentanil per hour, which was proved to can decrease the consumption of local analgesics [30]. We found no difference in both maternal GBS colonization, number of vaginal examinations, oxytocin augmentation, duration from rupture of the membranes to delivery, mode of membranes ruptured, the durations of labor courses, duration of analgesia, labor mode, placental inflammation, and neonatal weight, Apgar scores, and umbilical cord acid–base status between the two groups, without severe maternal and neonatal side effects. These findings showed that group PIEB did not increase the risks of maternal and neonatal harmful outcome.
There were some deficiencies in our trial. First, IL-6 was a critical marker for maternal intra-partum fever, we measured maternal serum IL-6 concentrations in our previous study with small sample size, and found that maternal IL-6 increase might be associated with maternal temperature elevation [1]. However, in the present study, we did not detect maternal and neonatal IL-6 concentrations because of insufficient funds. We also did not detect leukocytes and C-reactive protein levels because the cost was expensive and epidural-associated noninfectious inflammation was our main concern. Secondly, the liquid intake, which was not recorded, may be one of the critical factors for the regulation of maternal body temperature. Thirdly, sweating volumes were not been measured in our study for technical reason, which may play an important role in intra-partum fever. Finally, although different PIEB doses and time intervals might impact the ratio of maternal intra-partum fever, we had no sufficient human resource to divide more groups with more large sample subjects.
In conclusion, programmed intermittent epidural bolus with 10 ml of 0.08% ropivacaine and 0.4 μg/ml sufentanil hourly was a better method for epidural labor analgesia with less ratio of intra-partum fever compared with continuous infusion, without severe maternal and neonatal side effects.
References
Feng SW, Xu SQ, Ma L, Li CJ, Wang X, Yuan HM, Wang FZ, Shen XF, Ding ZN (2014) Regular intermittent bolus provides similar incidence of maternal fever compared with continuous infusion during epidural labor analgesia. Saudi Med J 35:1237–1242
Curtin WM, Katzman PJ, Florescue H, Metlay LA, Ural SH (2015) Intrapartum fever, epidural analgesia and histologic chorioamnionitis. J Perinatol 35:396–400
Douma MR, Stienstra R, Middeldorp JM, Arbous MS, Dahan A (2015) Differences in maternal temperature during labour with remifentanil patient-controlled analgesiaor epidural analgesia: a randomised controlled trial. Int J Obstet Anesth 24:313–322
Sharpe EE, Arendt KW (2017) Epidural labor analgesia and maternal fever. Clin Obstet Gynecol 60:365–374
Wassen MM, Winkens B, Dorssers EM, Marcus MA, Moonen RM, Roumen FJ (2014) Neonatal sepsis is mediated by maternal fever in labour epidural analgesia. J Obstet Gynaecol 34:679–683
Burgess APH, Katz JE, Moretti M, Lakhi N (2017) Risk Factors for intrapartum fever in term gestations and associated maternal and neonatal sequelae. Gynecol Obstet Invest 82:508–516
Petrova A, Demissie K, Rhoads GG, Smulian JC, Marcella S, Ananth CV (2001) Association of maternal fever during labor with neonatal and infant morbidity and mortality. Obstet Gynecol 98:20–27
Sultan P, David AL, Fernando R, Ackland GL (2016) Inflammation and epidural-related maternal fever: proposed mechanisms. Anesth Analg 122:1546–1553
Segal S, Pancaro C, Bonney I, Marchand JE (2017) Noninfectious fever in the near-term pregnant rat induces fetal brain inflammation: a model for the consequences of epidural-associated maternal fever. Anesth Analg 125:2134–2140
Wong CA, Ratliff JT, Sullivan JT, Scavone BM, Toledo P, Mccarthy RJ (2006) A randomized comparison of programmed intermittent epidural bolus with continuous epidural infusion for labor analgesia. Anesth Analg 102:904–909
George RB, Allen TK (2013) Habib AS (2013) Intermittent epidural bolus compared with continuous epidural infusions for labor analgesia: a systematic review and meta-analysis. Anesth Analg 116:133–144
Mantha VR, Vallejo MC, Ramesh V, Phelps AL, Ramanathan S (2008) The incidence of maternal fever during labor is less with intermittent than with continuous epidural analgesia: a randomized controlled trial. Int J Obstet Anesth 17:123–129
Salafia CM, Weigl C, Silberman L (1989) The prevalence and distribution of acute placental inflammation in uncomplicated term pregnancies. Obstet Gynecol 73:383–389
Lieberman E, Lang JM, Frigoletto F Jr, Richardson DK, Ringer SA, Cohen A (1997) Epidural analgesia, intrapartum fever, and neonatal sepsis evaluation. Pediatrics 99:415–419
Yancey MK, Zhang J, Schwarz J, Dietrich CS 3rd, Klebanoff M (2001) Labor epidural analgesia and intrapartum maternal hyperthermia. Obstet Gynecol 98:763–770
Vinson DC, Thomas R, Kiser T (1993) Association between epidural analgesia during labor and fever. J Fam Pract 36:617–622
Philip J, Alexander JM, Sharma SK, Leveno KJ, McIntire DD, Wiley J (1999) Epidural analgesia during labor and maternal fever. Anesthesiology 90:1271–1275
Herbst A, Wølner-Hanssen P, Ingemarsson I (1995) Risk factors for fever in labor. Obstet Gynecol 86:790–794
Dashe JS, Rogers BB, McIntire DD, Leveno KJ (1999) Epidural analgesia and intrapartum fever: placental findings. Obstet Gynecol 93:341–344
Ploeckinger B, Ulm MR, Chalubinski K, Gruber W (1995) Epidural anaesthesia in labour: influence on surgical delivery rates, intrapartum fever and blood loss. Gynecol Obstet Invest 39:24–27
Mayer DC, Chescheir NC, Spielman FJ (1997) Increased intrapartum antibiotic administration associated with epidural analgesia in labor. Am J Perinatol 14:83–86
Lucas MJ, Sharma SK, McIntire DD, Wiley J, Elaine Sidawi J, Ramin SM, Leveno KJ, Cunningham GC (2001) A randomized trial of labor analgesia in women with pregnancy-induced hypertension. Am J Obstet Gynecol 185:970–975
Ramin SM, Gambling DR, Lucas MJ, Sharma SK, Sidawi JE, Leveno KJ (1995) Randomized trial of epidural versus intravenous analgesia during labor. Obstet Gynecol 86:783–789
Sharma SK, Alexander JM, Messick G, Bloom SL, McIntire DD, Wiley J, Leveno KJ (2002) Cesarean delivery: a randomized trial of epidural analgesia versus intravenous meperidine analgesia during labor in nulliparous women. Anesthesiology 96:546–551
Sharma SK, Sidawi JE, Ramin SM, Lucas MJ, Leveno KJ, Cunningham FG (1997) Cesarean delivery: a randomized trial of epidural versus patient-controlled meperidine analgesia during labor. Anesthesiology 87:487–494
Kaul B, Vallejo M, Ramanathan S, Mandell G (2001) Epidural labor analgesia and neonatal sepsis evaluation rate: a quality improvement study. Anesth Analg 93:986–990
Glosten B, Savage M, Rooke GA, Brengelmann GL (1998) Epidural anesthesia and the thermoregulatory responses to hyperthermia-preliminary observations in volunteer subjects. Acta Anaesthesiol Scand 42:442–446
Hogan Q (2002) Distribution of solution in the epidural space: examination by cryomicrotome section. Reg Anesth Pain Med 27:150–156
Ueda K, Ueda W, Manabe M (2005) A comparative study of sequential epidural bolus technique and continuous epidural infusion. Anesthesiology 103:126–129
Wong CA, Mccarthy RJ, Hewlett B (2011) The Effect of manipulation of the programmed intermittent bolus time interval and injection volume on total drug use for labor epidural analgesia: a randomized controlled trial. Anesth Analg 112:904–911
Acknowledgements
The study was supported by grant 81971045 of National Natural Science Foundation of China.
Author information
Authors and Affiliations
Contributions
YRF Project development, data collection, WWH manuscript writing, data management, SF manuscript writing, PYM data collection, XW manuscript writing, JNJ data management, HMY data analysis, XFS project development, SWF project development, manuscript writing, PL project development, manuscript writing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fan, Y., Hou, W., Feng, S. et al. Programmed intermittent epidural bolus decreases the incidence of intra-partum fever for labor analgesia in primiparous women: a randomized controlled study. Arch Gynecol Obstet 300, 1551–1557 (2019). https://doi.org/10.1007/s00404-019-05354-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-019-05354-y