Abstract
Background
Previous studies showed that the association of gestational weight gain (GWG) with fetal birthweight and offspring developmental growth was unclear. The aim of this study is to investigate the respective effect of 1 kg of GWG during three trimesters on birthweight and offspring growth from birth to 3 years of age.
Methods
We extracted the decoded information from the Maternal and Child Health Information Management System of Zhoushan Maternal and Child Health Hospital in Zhejiang, China from October 2001 to March 2015, and used multiple linear and logistic regression models.
Results
This study included 20,232 women with a full-term singleton birth and 15,557 newborns who took regular health check-ups. Compared to that in the 2nd and 3rd trimester, 1 kg GWG increasing in the 1st trimester had the strongest positive association with higher birthweight, body weight, and height from 1 to 36 months. Their associations with BMI after birth were similar among the three trimesters. In addition, some positive dose–response effects found between quartiles of GWG in the 1st trimester and offspring body weight, as well as BMI. The 1 kg GWG in 1st trimester played the strongest role in contributing to birth weight and benefiting to body growth among children aged up to 3 years.
Conclusion
The 1 kg GWG in 1st trimester contributed more to birth weight and body development from birth to 3 years compared to the 2nd and 3rd trimesters. The possible beneficial effects of GWG in the 1st trimester on birthweight and offspring development in under/normal weight mothers are found.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nearly half of the Chinese women exceed the recommended gestational weight gain (GWG) [1], which also exists in developed countries. Approximately 50% of pregnant women in Australia [2] and over 50% [3, 4] of pregnant women in the United States reported exceeding GWG recommended by the Institute of Medicine (IOM) guidelines in 2009 [5]. Women with excessive GWG are associated with risk for many maternal and fetal short-term and long-term outcomes, such as gestational hypertension [6], preeclampsia, and gestational diabetes mellitus [7], large for gestational age (LGA) infant [8], child, and adult obesity. Meanwhile, women with inadequate GWG are also at increased risk for neonatal adverse outcomes, such as preterm birth [9], low birth weight (LBW) [10] and metabolism relevant diseases.
Weight at birth is a reflection of intrauterine growth and development [11]. Many factors can influence birth weight, including maternal age, pre-pregnancy body mass index (BMI), GWG, environmental and socioeconomic factors, as well as illnesses encountered in pregnancy. Previous studies have revealed that birth weight was highly associated with GWG [12, 13]. A study suggested that the association of each kg of GWG with birth weight may be stronger in the 2nd trimester than that in the 1st or 3rd trimester [14]. However, there is an inconsistency among the reported results. A population-based, prospective study in Minnesota found that GWG in the 1st and 2nd trimesters predicted birthweight, but did not in the 3rd trimester [15]. Furthermore, the association between trimester-specific GWG and birthweight depended on maternal pre-pregnancy BMI [16]. In terms of fetal growth, studies about the long-term effects of the perinatal maternal size on offspring health still need. Some studies have shown an independent effect of GWG on children’s BMI. One study in the US found that 1 kg of the 1st trimester GWG was significantly associated with increased child BMI z-score in low and normal pre-pregnancy BMI pregnancies. Their results also showed that only GWG in the 1st trimester associated with modest increases in child BMI z-score and overweight at age 5 years [14]. A study in Denmark showed that GWG in the first and second period (g/week) of pregnancy positively associated with the offspring’s BMI at 7 years of age [17]. Furthermore, sex-specific differences in fetal development and growth trajectories were also recognized [18]. Trimester-specific GWG may vary in terms of male and female infants.
The effect of GWG on weight at birth and offspring growth may depend on the trimester of pregnancy. It is unclear whether there is a critical time window which GWG most strongly associated with birthweight and fetal growth. There are limited studies on the relationship between 1 kg of trimester-specific GWG with fetal birth weight, and postnatal growth. This study aims to investigate the associations of trimester-specific GWG with fetal birth weight, postnatal growth among the Chinese population.
Methods
Dataset and study population
Zhoushan city locates in the east coast island of Zhejiang province, China, which has about one million inhabitants. Zhoushan Maternal and Child Care Hospital is a tertiary care hospital, where some 3000 pregnant women obtain the prenatal medical service and delivery per year, almost all children after birth receive conventional health examination. The institutional review board of Zhejiang University School of Medicine and Zhoushan Maternal and Child Care Hospital approved the study protocol.
We extracted data from decoded datasets, which was the Electronic medical record system (EMRS) from Zhoushan Maternal and Child Care Hospital constructed in 1999 and formally run from October 2001. The EMRS originally utilized by Zhoushan Maternal and Child Care Hospital from 2001 to 2010 and then was introduced to all hospitals in Zhoushan city since January 2011. The EMRS used between 2001 and 2015 contained two datasets: prenatal health dataset and conventional child health examination dataset. For prenatal health dataset, it covered the information of prenatal health care of pregnant women and fetal delivery records; there were 65,218 pregnant women took the prenatal health care and delivered babies. For the conventional child health examination, it covered the medical records of 52,711 children. In the present study, pregnant women should meet the following inclusion and exclusion criteria. Inclusion criteria were as follows: (1) singleton pregnancy; (2) maternal age between 18 and 45 years; (3) term birth (37–42 gestational weeks); (4) pre-pregnant body mass index (BMI) ≤ 40. The exclusion criteria include: (1) missing neonate birth weight; (2) failing to predict the given GWG.
Data extraction
From the decoded prenatal health datasets, we extracted the maternal and fetal information, including maternal ID, calendar year, maternal age, pre-pregnancy weight and height, body weight at each gestational visit, visiting date, gestational week, cigarette smoking and alcohol drinking, date of birth, birth weight, fetal gender, etc. From the postnatal health datasets, the offspring information contained offspring ID, body weight and height, visiting date, feeding type, etc.
Assessment of GWG
Pre-pregnancy weight and height were self-reported by mothers, and we extracted body weight at each prenatal visit. The time points that some pregnant women visited hospital were not the exact targeted time (at 6th, 12th, 18th, 24th, 28th, 32th and the last gestational week) that we expected. If pre-pregnancy weight or body weight at a given gestational age were not available, the available weight of the closest gestational week was used to predict using the linear regression model (the least adjusted R2 in all predicted models was 0.81). BMI was calculated as weight in kilograms divided by the square of height in meters. According to the Chinese maternal pre-pregnancy BMI status [19], pre-pregnancy BMI categories were defined as follows: “underweight” (pre-pregnancy BMI < 18.5 kg/m2), “normal weight” (BMI 18.5–23.9 kg/m2), “overweight” (BMI 24.0–27.9 kg/m2) and “obesity” (BMI ≥ 28 kg/m2).
The difference between weight at delivery and pre-pregnancy was total GWG in kg. We categorized GWG into two forms, by trimester and by stage. WgainT1, WgainT2, and WgainT3 were used to indicate GWG during the gestational interval from week 1 to 12 (the 1st trimester), 13 to 28 (the 2nd trimester) and week 29 to delivery (the 3rd trimester), respectively. Wgain6, Wgain12, Wgain16, Wgain20, Wgain24, Wgain28, Wgain32, and WgainF were used to indicated that GWG during the gestational interval from week 1 ~ , 7 ~ , 13 ~ , 17 ~ , 21 ~ , 25 ~ , 29 ~ and from week 32 to delivery, respectively.
Assessment of birthweight and offspring growth
We obtained birth weight from the hospital delivery logs, and postnatal height and weight from the medical records. Standardized birth weight below the 10th percentile and above the 90th percentile was used to define the infant small for gestational age (SGA) and large for gestational age (LGA). Low birth weight (LBW) and macrosomia were defined as birth weight < 2500 g and ≥ 4000 g, respectively.
Generally, babies after birth must, respectively, receive the regular health examinations at the age of 1 month, 3 months, 6 months, 9 months, 12 months, 18 months, 24 months, 30 months and 36 months, according to Chinese children health management procedure. However, the timing that children visited the hospital was not always punctually follow the scheduled time. Hence, the weight and height were replaced by the measurement at the given point in 2 weeks if the measurement was not at the exact scheduled time. Weight gain after birth in g was calculated as the difference between weight at a given time point and birthweight.
Outcomes
The primary outcomes of pregnancy included birth weight, SGA, LGA, LBW, and macrosomia. During postnatal periods, offspring developmental variables included continuous variables (weight, weight gain, height and BMI at the age of 1 month, 3 months, 6 months, 9 months, 12 months, 18 months, 24 months, 30 months, and 36 months) and categorical variables (low, normal and high level of weight, height and BMI) according to Chinese Children Development Norm under the age of 7 years [20].
Statistical analysis
The general information of continuous variables and categorical variables were described as mean ± SD and frequencies and percentages (%), respectively. Multiple linear regression models were used to analyze the association of GWG by trimester and by stage in kg with birth weight and offspring developmental outcomes. We also used multiple logistic regression models to assess the association of WgainT1, WgainT2, and WgainT3 with categorical outcomes. Considering the sample size and the time of the visiting, we selected 6th month, 12th month and 24th month to explore the association of GWG of mothers with weight, height, and BMI after birth stratified by the categorical variable of birthweight (LBW, normal and macrosomia). To test the hypothesis that trimester-specific GWG might vary for male and female infants, we performed sex-stratified analyses. We also fitted linear regression models to evaluate the statistical significance of sex-GWG interactions with an interaction term (WgainT1* infant gender, WgainT2* infant gender, WgainT3* infant gender).
All models were adjusted for the following potential confounders: maternal age, gestation age, pre-pregnancy BMI, fetal gender and calendar year; the models about offspring developmental outcomes after birth included another two potential confounders: feeding type and offspring’s age. Due to only several females had cigarette smoking and alcohol drinking during the pregnancy, both of them were not included in the models. While the relevant variables were standardized, this community-based population was taken as the standard population.
To explore the dose–response effect of trimester-specific GWG on offspring’s weight and BMI, each trimester GWG was quartered as Q1, Q2, Q3, and Q4. For intuitively presenting the effects of 1 kg WgainT1, WgainT2 and WgainT3 on birthweight, postnatal development variables, slop values (β and se) of WgainT1, WgainT2 and WgainT3 in the linear models were plotted against offspring’s age months. WgainT1 was divided into ten groups by decile to present the dose–response effect on LGA and SGA. Then the figure was plotted by the decile of WgainT1 against the odds ratio of LGA and SGA, respectively. Quartiles of WgainT1 and WgainT2 (Q1, Q2, Q3, and Q4) were, respectively, plotted against offspring’s weight and BMI aged from 1 to 36 months. All analyses were performed using the statistical software R version 3.3.2 with significance defined as a P value of < 0.05.
Results
Participant characteristics
There were 20,232 mothers included in this study, of whom 100 mothers were minorities. Range maternal age was from 18 to 45 years with the mean of 25.77 years (SD 3.05), and maternal weight was 51.62 kg (SD 4.06 kg) with the range from 34.29 to 94.93 kg, and maternal height ranged from 130 to 179 cm with the mean of 160.2 cm (SD 4.55 cm). Mean (SD) maternal GWG was 16.63 kg (SD 6.19) and ranged from − 2.02 to 37.65 kg. 10,768 male newborns and 9464 female newborns were included. 15,557 infants took regularly health check-ups, including 8303 male infants and 7254 female infants. The birth weight ranged from 1075 to 5850 g, the offspring height ranged from 41 to 120 cm, and weight ranged from 2.40 to 29.50 kg.
Table 1 showed the general characteristics of pregnant women and children by pre-pregnancy BMI categories. 23.0% of mothers were underweight, and 71.8% were normal weight. The distribution of infant gender in four groups was comparable (P = 0.1443), and the gestational week and feeding type had significant differences (P < 0.05). But both maternal age and birth weight were positively associated with pre-pregnancy BMI (P < 0.0001). The most feeding type was mixed feeding. The association of pre-pregnancy BMI level with WgainT1 was positive, but their relationship was reverse with WgainT2, and the distribution of WgainT3 was various. Table 2 presented the distribution of weight, height, and BMI of offspring at age 1, 3, 6, 9, 12, 18, 24, 30, 36 months.
Association of 1 kg of trimester-specific GWG with birth weight
Table 3 presented the association of trimester-specific GWG with fetal birth weight stratified by pre-pregnancy BMI category. WgainT1, WgainT2, and WgainT3 were independently and positively associated with birthweight after adjustment for the potential confounders. WgainT1 had the strongest association with fetal birth weight in total population, and the strength of association successively followed by WgainT2 and WgainT3. Similar patterns observed across BMI groups (Table 3). When pregnancy duration divided into more stages, similar associations, which 1 kg of GWG from early pregnancy to late pregnancy (except GWG from week 1 to 6—WgainT6) gradually decreased the effect on birthweight, were observed (Supplemental Table S1).
Table 4 shows the association of GWG with SGA/LGA and LBW/macrosomia after adjustment for each other of WgainT1, WgainT2 and WgainT3, and other potential confounders. GWG in each trimester was independently associated with an increased risk of LGA and macrosomia, a decreased risk of SGA and LBW, respectively. The strongest risk of WgainT1 on LGA and macrosomia and strongest protective effects of WgainT1 on SGA and LBW were observed. The lowest effect of WgainT3 on LGA, macrosomia, SGA, and LBW was found. Meanwhile, the dose–response effect of WgainT1 on LGA and SGA is presented in Fig. 1. With WgainT1 increased, the risk of LGA increased and the risk of SGA decreased.
Association of 1 kg of trimester-specific GWG with offspring growth
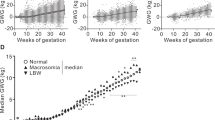
The association of trimester-specific GWG with body weight, height and BMI from 1 month to 36 months was analyzed, respectively. GWG in three trimesters were independently and positively associated with body weight (Table 5 and Fig. 2), weight gain (Supplemental Table S2, Supplemental Figure S1), and height (Fig. 3). Furthermore, the effect gradually increased with age growth. But the effect was the strongest in WgainT1, and 1 kg WgainT3 was the lowest. However, it was very interesting that GWG by trimester or by stage had similar effects on offspring BMI at each age time point, except at the age of 6-months. Even, we found that 1 kg WgainT1 had lower effects on BMI at some time-points than WgainT2 and WgainT3 (Table 6, Fig. 4 and Supplemental Table S3).
The association of trimester-specific GWG with birthweight, weight gain and BMI aged at 6th, 12th and 24th week showed in Supplemental Table S4. The results showed that 1 kg WgainT1 still had the highest effect on body weight at three time points and 1 kg WgainT3 also had the lowest effects, despite birthweight levels. The associations of GWG at each trimester with weight gain offspring aged at 6th, 12th, and 24th week were similar to those of body weight. In three subgroups of birthweight, WgainT1, WgainT2, and WgainT3 similarly contributed BMI of offspring after birth aged at 6th, 12th, and 24th week, respectively (Supplemental Table S4). The association of GWG with the categorical variable of offspring height was similar to that of GWG with offspring weight. WgainT1, WgainT2, and WgainT3 were independently associated with an increased risk of high weight and a decreased risk of low weight, regardless of the age time points. Furthermore, the effect of 1 kg WgainT1 on the risk of low and high weight was stronger than that of WgainT2 and WgainT3 (Supplemental Table S5). However, WgainT1, WgainT2, and WgainT3 independently increased the risk of high BMI and independently decreased the risk of low BMI in offspring aged 6th, 12th, and 24th week. But 1 kg GWG during the 1st, 2nd, and 3rd trimester had similar effects on the risk of low and high BMI (Supplemental Table S5).
The quartile of GWG during the 1st and 2nd trimester was plotted against weight and BMI of offspring. The lines of offspring’s weight and BMI from 1 to 36 months kept clear space between four quartiles of WgainT1 (Supplemental Figure S2 and Figure S3), but not found in the quartiles of WgainT2 (Supplemental Figure S4 and Figure S5). The dose–response effect of WgainT1 on postnatal body weight and BMI was observed, but the effect of WgainT2 was not clear. It indirectly indicated that WgainT1 contributed more to the offspring growth than that WgainT2.
When stratified by fetal gender, the patterns of the association of trimester-specific with weight, weight gain, and BMI were similar (Supplemental Figure S6-11). Terms for the 1st, 2nd and 3rd trimester GWG interactions with birth weight (P values 0.742, 0.990, 0.704, respectively), fetal weight (P values 0.168, 0.955, 0.005, respectively), height (P values 0.187, 0.313, 0.199, respectively), BMI (P values 0.682, 0.144, 0.379, respectively) were not significant (data not shown).
Discussion
In this community-based population, 1 kg of GWG in three trimesters was independently and significantly associated with birth weight, SGA, LGA, macrosomia, and LBW. Meanwhile, 1 kg of GWG in three trimesters was associated with birthweight and body growth. Moreover, every increase in 1 kg of GWG in the 1st trimester had the strongest association with birthweight and body growth during early life.
The prevalence of overweight and obesity in our study were 4.7% and 0.5%, respectively, which were lower than that in other studies of Chinese women. A study in Beijing showed that 12.0% of the subjects were overweight and 2.6% were obese [1] and the number was, respectively, 18.3% and 6.8% in a study from Shenyang, China [21]. As with studies in Italy and New York [22, 23], the risk of macrosomia and LGA increased with GWG increasing. We also observed a positive association of excessive GWG with macrosomia and LGA. The results of LBW and SGA in our study were also similar to previous studies [24,25,26].
There were previous studies that in comparison to the association of GWG in different trimesters with birth weight. The study in Washington showed that 1 kg increase in early or late GWG was associated with an increased birth weight of 14.1 g (95% CI 10.3–18.0) and 21.0 g (95% CI 16.7–25.4), respectively [27]. Margerison-Zilco et al. found that GWG in three trimesters were associated with birthweight, and the association was stronger in the 2nd trimester [14]. Sekiya et al. [28] found significant correlations between weight gain rate with birth weight in the 2nd trimester. Our data showed 1 kg of GWG during the 1st, 2nd, 3rd trimester independently increased birth weight of 42.60 g, 37.52 g and 14.25 g. Our study also showed that pre-pregnancy BMI modified the effect of GWG on birth weight, that is, the strength of association of GWG with birthweight decreased with BMI level increased in the 1st and 2nd trimesters.
Pregnancy is a unique time in women’s lives. Weight gain across specific intervals of pregnancy has differential contributions to fetal growth. GWG includes the growing fetus, enlarging maternal fluid and soft tissue throughout pregnancy [29]. Furthermore, placenta grows in intrauterine during the early pregnancy, while the fetus does not accumulate fat or much lean tissue [15]. For placental volume, the rate of placental growth and expansion of fat tissue may influence fetal size and birthweight. These effects are evident in the first half of pregnancy [30]. These findings are similar to our results: the effect of GWG during the 1st trimester on birthweight was the strongest. Besides, early GWG may act to predispose the fetus to gain different levels of fat and lean tissue later in gestation [15].
Compared with that in the 2nd and 3rd trimesters, 1 kg of GWG in the 1st trimester contributed more to the postnatal height and weight, but the effect on BMI was similar across trimesters. When quartered GWG in the 1st trimester, there was a clear dose–response effect on offspring’s weight and BMI. Furthermore, with age growth, this dose–response effect increased. However, the association of 1 kg of GWG in the 2nd trimester with child BMI did not represent similar trend as height and weight, that is, 1 kg of GWG in early pregnancy was advantageous to postnatal healthy growth and development. We found all associations of trimester-specific GWG with birthweight and fetal growth outcomes were similar between two genders. It is very similar with Wander, et al. [27], who revealed that the association of trimester-specific GWG with infant birth size does not differ for early (< 20 weeks gestation) or late (≥ 20 weeks gestation) GWG.
The potential biological mechanisms for the impact of GWG on fetal growth may via epigenetic mechanisms [31, 32]. Evidence from animal models showed an effect of greater maternal pregnancy adiposity and overfeeding on offspring epigenome [33]. The study from the Avon Longitudinal Study of Parents and Children cohort suggested that greater GWG in early pregnancy was related to higher DNA methylation in offspring cord blood [34].
Strengths of the present study include the large sample size in a community-based population. To our knowledge, this is the first study to examine the relationship between trimester-specific GWG with birthweight and offspring growth in a Chinese population. The eligible criterion can reduce possible confounders. Nevertheless, there are limitations. Firstly, information bias may exist with partly self-reported pre-pregnancy weight, since directly measuring pre-pregnancy weight is not possible. Pregnant women come to antenatal visit for the first time is around 8–12 weeks. Secondly, the prediction of missing weight during pregnancy may introduce bias. However, all adjusted R2 of prediction models were greater than 0.81. Thirdly, the data of pregnancy complications are incomplete, as women with inadequate GWG are more likely to experience pregnancy complications compared to women with adequate GWG [35].
Conclusion
The 1 kg GWG in 1st trimester has the strongest positive association with birthweight and body development up to 3 years compared to the association found in 2nd and 3rd. More GWG in the 1st trimester may benefit birth weight and offspring growth in under/normal weight mothers. These results are needed to be confirmed by future research.
References
Zhang CH, Liu XY, Zhan YW, Zhang L, Huang YJ, Zhou H (2015) Effects of prepregnancy body mass index and gestational weight gain on pregnancy outcomes. Asia Pac J Public Health 27(6):620–630
Hill B, Skouteris H, McCabe M, Fuller-Tyszkiewicz M (2013) Body image and gestational weight gain: a prospective study. J Midwifery Women’s Health 58(2):189–194
Bodnar LM, Hutcheon JA, Platt RW, Himes KP, Simhan HN, Abrams B (2011) Should gestational weight gain recommendations be tailored by maternal characteristics? Am J Epidemiol 174(2):136–146
Wrotniak BH, Shults J, Butts S, Stettler N (2008) Gestational weight gain and risk of overweight in the offspring at age 7 y in a multicenter, multiethnic cohort study. Am J Clin Nutr 87(6):1818–1824
Pregnancy Weight Gain During (2009) Reexamining the guidelines. National Academy of Sciences, Washington DC
Fortner RT, Pekow P, Solomon CG, Markenson G, Chasan-Taber L (2009) Prepregnancy body mass index, gestational weight gain, and risk of hypertensive pregnancy among Latina women. Am J Obstet Gynecol. 200(2):167 e1–7
Carreno CA, Clifton RG, Hauth JC et al (2012) Excessive early gestational weight gain and risk of gestational diabetes mellitus in nulliparous women. Obstet Gynecol 119(6):1227–1233
Li N, Liu E, Guo J et al (2013) Maternal prepregnancy body mass index and gestational weight gain on pregnancy outcomes. PLoS One 8(12):e82310
Woolfolk CL, Harper LM, Flick L, Mathews K, Chang JJ (2016) Gestational weight gain and preterm birth: disparities in adolescent pregnancies. J Perinatol 36(12):1055–1060
Han Z, Lutsiv O, Mulla S, Rosen A, Beyene J, McDonald SD (2011) Low gestational weight gain and the risk of preterm birth and low birthweight: a systematic review and meta-analyses. Acta Obstet Gynecol Scand 90(9):935–954
Villar J, Puglia FA, Fenton TR et al (2017) Body composition at birth and its relationship with neonatal anthropometric ratios: the newborn body composition study of the INTERGROWTH-21(st) project. Pediatr Res 82(2):305–316
Lof M, Hilakivi-Clarke L, Sandin S, Weiderpass E (2008) Effects of pre-pregnancy physical activity and maternal BMI on gestational weight gain and birth weight. Acta Obstet Gynecol Scand 87(5):524–530
Ludwig DS, Currie J (2010) The association between pregnancy weight gain and birthweight: a within-family comparison. Lancet 376(9745):984–990
Margerison-Zilko CE, Shrimali BP, Eskenazi B, Lahiff M, Lindquist AR, Abrams BF (2012) Trimester of maternal gestational weight gain and offspring body weight at birth and age five. Matern Child Health J 16(6):1215–1223
Brown JE, Murtaugh MA, Jacobs DR Jr, Margellos HC (2002) Variation in newborn size according to pregnancy weight change by trimester. Am J Clin Nutr 76(1):205–209
Lantz ME, Chez RA, Rodriguez A, Porter KB (1996) Maternal weight gain patterns and birth weight outcome in twin gestation. Obstet Gynecol 87(4):551–556
Andersen CS, Gamborg M, Sorensen TI, Nohr EA (2011) Weight gain in different periods of pregnancy and offspring’s body mass index at 7 years of age. Int J Pediatric Obesity 6(2–2):e179–e186
Lampl M, Gotsch F, Kusanovic JP et al (2010) Sex differences in fetal growth responses to maternal height and weight. Am J Human Biol 22(4):431–443
Chen C, Lu FC (2004) The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci 17(Suppl):1–36
Chinese Children Development Norm under the age of 7. http://www.nhfpc.gov.cn/zwgk/wtwj/201304/b64543eaaee1463992e8ce97441c59bb.shtml
Liu X, Du J, Wang G, Chen Z, Wang W, Xi Q (2011) Effect of pre-pregnancy body mass index on adverse pregnancy outcome in north of China. Arch Gynecol Obstet 283(1):65–70
Alberico S, Montico M, Barresi V et al (2014) The role of gestational diabetes, pre-pregnancy body mass index and gestational weight gain on the risk of newborn macrosomia: results from a prospective multicentre study. BMC Pregnancy Childbirth 14:23
Savitz DA, Stein CR, Siega-Riz AM, Herring AH (2011) Gestational weight gain and birth outcome in relation to prepregnancy body mass index and ethnicity. Ann Epidemiol 21(2):78–85
Baugh N, Harris DE, Aboueissa AM, Sarton C, Lichter E (2016) The impact of maternal obesity and excessive gestational weight gain on maternal and infant outcomes in Maine: analysis of pregnancy risk assessment monitoring system results from 2000 to 2010. J Pregnancy 2016:5871313
Xu Z, Wen Z, Zhou Y, Li D, Luo Z (2017) Inadequate weight gain in obese women and the risk of small for gestational age (SGA): a systematic review and meta-analysis. J Matern Fetal Neonatal Med 30(3):357–367
Shinohara S, Uchida Y, Hirai M, Hirata S, Suzuki K (2016) Relationship between maternal hypoglycaemia and small-for-gestational-age infants according to maternal weight status: a retrospective cohort study in two hospitals. BMJ Open 6(12):e013749
Wander PL, Sitlani CM, Badon SE, Siscovick DS, Williams MA, Enquobahrie DA (2015) Associations of early and late gestational weight gain with infant birth size. Matern Child Health J 19(11):2462–2469
Sekiya N, Anai T, Matsubara M, Miyazaki F (2007) Maternal weight gain rate in the second trimester are associated with birth weight and length of gestation. Gynecol Obstet Invest 63(1):45–48
Rauff EL, Downs DS (2011) Mediating effects of body image satisfaction on exercise behavior, depressive symptoms, and gestational weight gain in pregnancy. Ann Behav Med 42(3):381–390
Thame M, Osmond C, Bennett F, Wilks R, Forrester T (2004) Fetal growth is directly related to maternal anthropometry and placental volume. Eur J Clin Nutr 58(6):894–900
Lawlor DA (2013) The Society for Social Medicine John Pemberton Lecture 2011. Developmental overnutrition–an old hypothesis with new importance? Int J Epidemiol. 42(1):7–29
Lawlor DA, Relton C, Sattar N, Nelson SM (2012) Maternal adiposity–a determinant of perinatal and offspring outcomes? Nat Rev Endocrinol 8(11):679–688
Yang QY, Liang JF, Rogers CJ, Zhao JX, Zhu MJ, Du M (2013) Maternal obesity induces epigenetic modifications to facilitate Zfp423 expression and enhance adipogenic differentiation in fetal mice. Diabetes 62(11):3727–3735
Morales E, Groom A, Lawlor DA, Relton CL (2014) DNA methylation signatures in cord blood associated with maternal gestational weight gain: results from the ALSPAC cohort. BMC Res Notes 7:278
Li C, Liu Y, Zhang W (2015) Joint and independent associations of gestational weight gain and pre-pregnancy body mass index with outcomes of pregnancy in Chinese Women: a retrospective cohort study. PLoS One 10(8):e0136850
Funding
This work was supported by the National Key Research and Development Programme of China (2016YFC1305301), Projects of Science and Technology Plan of Zhejiang Province (2014C33243), Medical Health Science and Technology Project of Zhejiang Province (2013KYB273), Science and Technology Project of Zhoushan (2013C31066), Medical Health Science and Technology Key Project of Zhoushan (2013G02), Medical Health Science and Technology Project of Zhejiang Province (2014KYA277), Medical Health Science and Technology Key Project of Zhoushan (2014A03), Science and Technology Project of Zhoushan (2015C31038).
Author information
Authors and Affiliations
Contributions
YM: Obtaining data and review. SW: Manuscript writing, data curation. ML: Data analysis. MH: Investigation. MM: Methodology. LG: Project administration, resources. XM: Validation and review. HL: Validation, visualization. SJ: Visualization, writing. ZW: Writing and review. BS: Review and editing. LP: Revise the paper. YY: Project development
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study protocol was approved by the institutional review board of Zhejiang University School of Medicine and Zhoushan Maternal and Child Care Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mao, Y., Wang, S., Li, M. et al. Association of trimester-specific gestational weight gain with birth weight and fetal growth in a large community-based population. Arch Gynecol Obstet 300, 313–322 (2019). https://doi.org/10.1007/s00404-019-05188-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-019-05188-8