Abstract
Background
Despite a trend for less radical surgical approaches in breast cancer due to better understanding of tumour biology and new treatment options such as neoadjuvant chemotherapy (NAC) and intra-operative radiotherapy (IORT), seroma production remains one of the main surgical side effects that can result in prolonged recovery, delay of radiotherapy and patient discomfort. The aim of this study is to provide an update on risk factors for seroma production after breast cancer surgery considering the latest treatment options.
Methods
A retrospective analysis of seroma production in primary breast cancer patients treated between 01.01.2010 and 31.12.2014 at the Breast Cancer Centre, University Hospital Ulm, was performed. Patients with previous breast/axillary surgery or more than one intervention were excluded. Seroma formation was measured using wound drains placed in breast and axilla.
Results
In total, 581 patients met the inclusion criteria. Median age at diagnosis was 60 years, and median BMI 25.6 kg/m2. 60 (10.3%) patients had a mastectomy, 175 (30.1%) patients received IORT, and 72 (12.4%) patients received NAC. Median amount of seroma production was 82.5 ml (range 0–3012.5 ml). Multivariate analysis revealed that most of the observed variation in seroma production was due to type of surgery (mastectomy vs. breast conserving), length of surgery and number of removed lymph nodes. Both NAC and IORT explained a significant but very small amount of the observed variation in seroma production.
Conclusion
The most important factors for seroma production are extent and duration of breast surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last decades, the surgical approach in the treatment of breast cancer has become less radical. Surgically ‘no ink on tumour’ is considered oncological ‘save’ and the axillary dissection is now in most cases a ‘sentinel only’ procedure. More and more breast cancer patients are not treated in the traditional order with surgery and radiotherapy followed by systemic treatment, but instead receive neoadjuvant chemotherapy (NAC) and/or intra-operative radiotherapy (IORT). Due to improvements in NAC and extension of patient selection criteria, breast conserving surgery (BCS) is now the surgical standard for the majority of patients. The main advantages of BCS over mastectomy are less morbidity, better aesthetic outcome and greater patient satisfaction [1]. Though it has been shown that the wounds of BCS do not need routine drainage, still some surgeons use NAC as a reason to insert them [2].
BCS should always be followed by radiotherapy to obtain a comparable oncological outcome to mastectomy. Traditionally this radiotherapy is a whole breast radiation. In order to improve the therapy in effectiveness and application length, IORT was developed for BCS [3]. IORT distributes a radiation dose directly on the tumour bed just shortly after the removal of the tumour and before skin closure [4]. This reduces the length or possibly completely substitutes the post-operative radiation course with equal or improved local tumour control [5].
However, despite these new developments and the trend towards less radical surgical approaches, seroma production continues to remain an important clinical issue for breast surgeons. Seroma still represents one of the main surgical side effects that can result in prolonged recovery, delay of radiotherapy and patient discomfort. Post-surgical wound seroma is very common in all patients, but not all seromas are clinically relevant and only a minority requires an intervention. Seroma production is not yet fully understood and the influence of various treatment combinations on seroma formation is also largely unknown [6]. The aim of this paper is to re-evaluate the risk factors for seroma production including the treatment options of NAC and IORT.
Materials and methods
The database of the certified breast centre of the University Hospital Ulm contains records on all treated breast cancer patients with data on tumour characteristics and treatment. The data were filtered for primary patients presenting between 01.01.2010 and 31.12.2014. From the regular patient chart of the first breast cancer surgery the number of drains, amount of seroma on day 1/3/5 and the total seroma production per drain was noted. In addition, information on body mass index (BMI), total surgical time, volume of removed tissue, ASA, diabetes, and smoking habits were extracted from the database or patient sheets to be included in the analysis. Patient data on the second or later surgery was excluded. Alongside cases that had a breast cancer surgery outside our department were excluded too. The last cycle of NAC was minimum 3 weeks, maximum 6 weeks prior to the scheduled surgery. Surgery was performed with bipolar scissors or a mono-polar knife. All patients received a single shot antibiotic dose with cefuroxime or in case of intolerance clindamycin during surgery. IORT was offered to patients with tumours smaller than 3 cm according to national guidelines [7]. A boost irradiation using the mobile Intra beam applicator was applied once the tumour had been removed. The tumour bed boost with 50-kV X-rays delivered 9 Gy at the applicator surface in selected patients. Slit drains (Redon) were placed in each wound cavity and were connected to a negative pressure collection bottle. Two drains were placed in the larger cavities (i.e. large mastectomies or intramammarial reconstruction) to ensure complete drainage. Drains were removed if the collected volume in 24 h was less than 50 ml, the drain dislocated accidentally or the patient requested the drain removal.
Data analysis
Categorical data are described using absolute and relative frequencies, and metric variables were described using median and ranges as they differed significantly from a normal distribution (Shapiro–Wilk test, all p < 0.05). Comparisons of total seroma production (ml) between groups were performed using the non-parametric Mann–Whitney U test (in case of two groups) or Kruskal–Wallis test (in case of more than two groups) and illustrated using box-and-whisker plots, where the horizontal line inside the box indicates the median and the box represents the interquartile range (IQR; the middle 50% of scores). The lower and upper end of the box represent the lower and upper quartile, respectively, and the ends of the whiskers denote the lowest and highest values still within 1.5 IQR of the lower and upper quartile. If there are no values more than 1.5 IQR below the lower or above the upper quartile (i.e. outliers), the ends of the whiskers denote minimum and maximum of the data. Outliers that are more than 1.5 IQR but less than 3 IQR below the lower or above the upper quartile are indicated by open circles, and extreme outliers more than 3 IQR below the lower or above the upper quartile are indicated by stars. Correlations between total seroma production and metric variables were analysed based on the non-parametric Spearman rank correlation coefficient rs and illustrated using scatterplots.
To evaluate which parameters had a significant independent effect on total seroma production, we applied a general linear model analysis with total amount of seroma production as the dependent variable. The following independent variables initially entered the model: year of diagnosis (2010, 2011, 2012, 2013, 2014), age at the time of surgery (years), body mass index (BMI) at the time of surgery (kg/m2), smoking (yes, no), diabetes type II (yes, no), type of surgery (breast conserving, mastectomy), intra-operative radiotherapy (IORT, yes, no), neoadjuvant chemotherapy (NAC, yes, no), duration of surgery (min), volume of tissue removed during surgery (cm3), hormone receptor status (negative, positive), HER2 status (negative, positive), tumour stage (pT0/Tis, pT1, pT2, pT3, pT4), nodal stage (pN0, pN1, pN2, pN3), ASA score (1, 2, 3, 4), Ki 67 (%), number of lymph nodes removed and number of positive lymph nodes. After stepwise removal of non-significant parameters (p > 0.05) from the full model, we obtained the final model that included only variables that were significantly associated with total seroma production.
All statistical analyses were performed with the software IBM® SPSS® Statistics version 22 (IBM Corp., Armonk, NY, USA); all tests were two-sided and p values below 0.05 were considered statistically significant, i.e. there was no adjustment of significance levels for multiple testing.
Results
Patient and treatment characteristics
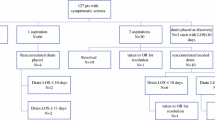
1691 primary breast cancer patients were treated between 01.01.2010 and 31.12.2014 in our unit. Overall, 581 patients were eligible for this analysis. The main reasons for exclusion were primary surgeries in combination with reconstructive surgery, omission of drains and incomplete documentation of seroma volume. The median age at the time of breast surgery was 60 years (range 27–95 years), and median BMI was 25.6 kg/m2 (range 16.7–53.8 kg/m2); 84.7% were non-smokers and 8.1% were diabetic. About two-thirds (66.3%, n = 385) of the patients were classified as ASA 1 or 2, 54.6% (n = 317) were classified as pT1, 38.2% (n = 222) as pT2, and 4.0% (n = 23) as pT3/pT4. The nodal status was pN0 in 82.4% (n = 479), while 11.2% (n = 65) patients had positive nodes (37 patients with missing data). The cancers were mostly hormone receptor positive (83.8%) and HER2 negative (90.2%). BCS was performed in 89.7% (n = 521), while the remaining 10.3% (n = 60) patients received a mastectomy. Median duration of surgery was 120 min (range 35–350 min) and the median volume of tissue removed was 76 cm3 (range 7–5956 cm3). Median number of lymph nodes removed was 2 (range 1–29), and the 65 patients with positive nodes had a median number of 2 infested lymph nodes (range 1–18). 12.4% of patients (n = 72) received NAC, 30.1% of patients (n = 175) received IORT and the remaining 57.5% of patients (n = 334) had none of these two treatments. Further details regarding patients, tumours and surgery are provided in Table 1.
Drain numbers, duration and amounts of seroma production
Seroma was collected using one drain only in 48.4% of patients (n = 281), while 45.3% of patients (n = 263) had two drains, and 6.4% of patients (n = 37) had even three drains laid to collect wound seroma after breast surgery. Drain one (placed in the breast) collected a median of 50 ml (range 0–2970 ml) wound seroma and was removed after a median of 2 days (range 1–13 days), drain two (placed in the axilla) collected a median of 52.5 ml (range 0–1460 ml) and was removed after a median of 3 days (range 1–18 days), and drain three (second breast drain) collected a median of 30 ml (range 3–938 ml) and was also removed after a median of 3 days (range 2–11 days). The total amount of seroma production was 82.5 ml (median) and ranged from 0 ml to 3012.5 ml. Interquartile range for total seroma production was 40–170 ml, and 5.2% of the patients (n = 30) showed a total seroma production of 10 ml or less, while 2.4% of the patients (n = 14) had a total seroma production of 1000 ml or higher.
Factors affecting total seroma production
Univariate analyses showed significant correlations between the total amount of seroma production and duration of surgery (rs = 0.340, p < 0.0001, Fig. 1a), volume of tissue removed during surgery (rs = 0.525, p < 0.0001, Fig. 1b), number of lymph nodes removed (rs = 0.471, p < 0.0001, Fig. 1c) and number of positive lymph nodes (rs = 0.309, p < 0.0001, Fig. 1d). In contrast, there were no significant correlations between the total amount of seroma production and age at the time of surgery, BMI, or Ki 67 (all p > 0.05).
In addition, total amount of seroma production differed significantly among the years of diagnosis (p < 0.0001), and according to tumour stage (p < 0.0001, Fig. 2a), nodal stage (p < 0.0001, Fig. 2b) and ASA score (p = 0.005, Fig. 2c). Furthermore, total amount of seroma production was significantly higher after mastectomy than after BCS (median 540 vs. 74 ml, p < 0.0001, Fig. 3a), without IORT than with IORT (median 91 vs. 70 ml, p < 0.0001, Fig. 3b), with NAC than without NAC (median 234 vs. 80 ml, p < 0.0001, Fig. 3c), and in HER2-positive tumours than in HER2-negative tumours (median 119 vs. 82 ml, p = 0.014, Fig. 3d). No significant differences with regard to the total amount of seroma was found between smokers and non-smokers, between patients with and without diabetes type II, and between hormone receptor-positive and hormone receptor-negative tumours (all p > 0.05).
To assess which factors are significantly and independently from other variables associated with the total amount of seroma production, we performed a multivariate general linear model analysis with all factors being entered into the model in a first step, followed by stepwise removal of non-significant parameters (see “Materials and methods”). The final model with the remaining significant parameters is shown in Table 2 and explains two-thirds of the observed variation (R2 = 0.667). According to the partial Eta squared values, type of surgery (mastectomy vs. BCS) is the most important factor explaining the highest amount of the observed variability in seroma production, followed by the parameters duration of surgery and number of lymph nodes removed. The remaining factors shown in Table 2, age at the time of surgery, tumour stage, nodal stage, IORT and NAC are still significant independent predictors of the total amount of seroma production, but explain only small amounts of the observed variability in seroma production.
Discussion
The surgical approach to breast cancer treatment has become less radical and difficile. The most common ‘complication’ of the surgery is wound seroma that may delay further treatment and/or impair the patients’ quality of live. It has been known for some time that the extent of the surgery influences the amount of seroma production [8]. The rate of BCS as ‘smaller’ surgery increased considerably due to the use of NAC and the recommendation to excise within the ‘new’ borders [7]. Sentinel node biopsy replaced the axillary dissection in early breast cancer. The sentinel node biopsy technique is known to reduce the rate of axillary seroma [9, 10], but still seroma production is inevitably associated with surgical treatment especially for larger tumours (i.e. resulting in larger wound cavities). To solve this clinical issue, patients need either to have a drainage inserted or return after discharge for seroma aspiration. The need for a drain insertion has been critically evaluated by several authors [11,12,13]. In case of an axillary lymph node dissection advantages and disadvantages of drainage insertion have been described [14]. However, based on the Z0011 study and others the need for an axillary dissection even in patients with positive sentinel nodes has been questioned [15,16,17].
Our data confirm the strong associations between the extent of surgery and seroma production with regard to both surgery of the primary tumour (mastectomy vs. BCS) and the axilla (number of lymph nodes removed). This has not changed compared to previous publications, and only ‘no surgery’ [6] might be a fundamental improvement regarding the prevention of seroma production. The ‘no surgery in the axilla’ question is evaluated for early breast cancer patients in the ongoing INSEMA study [18]. For pN + breast cancer patients there is no conclusive evidence showing a benefit of an axillary dissection in terms of improved survival prior or after systemic treatment. In addition, a retrospective study questioned the current practice of axillary dissection [19]. In patients with advanced breast cancer (i.e. distant metastasis) an axillary staging has not been recommended for several years [7]. With regard to avoiding surgery, there are some case series indicating that in elderly patients unfit or unwilling to undergo surgery with hormone receptor-positive breast cancer primary endocrine therapy may be an option [20,21,22]. However, similar studies for younger patients are yet missing and surgery of the primary tumour remains standard of care [7]. If after NAC imaging suggests a complete response this can currently only be confirmed by surgical excision of the clip marked area/prior tumour bed. At the moment there is a clinical trial evaluating vacuum biopsy of the tumour bed which might lead to the omission of surgery in future. In the metastatic setting removal of the primary tumour might be beneficial for the patient [23].
According to our results, the second most important parameter associated with seroma production was the duration of the surgery. There are only few studies that investigated the association between duration of breast surgery and seroma production. In a review on risk factors for seroma formation after breast cancer surgery, Kuroi et al. cited one prospective study that reported a positive association between operation time and seroma production and one retrospective study that showed no such association [9]. Both cited studies were published over 20 years ago and therefore might not reflect current surgical management. It seems reasonable that a larger tumour or more extended lymph node dissection requires a longer surgery time; however, our finding that the duration of surgery was significantly associated with seroma production independently from the extent of surgery was surprising. Our results may indicate that experienced surgeons who are able to perform the surgery in less time may add an additional benefit for their patients beyond the surgical procedure. The underlying cause might perhaps be due to pathophysiological mechanisms that are initiated by extended wound opening [8].
Other factors possibly associated with seroma formation after breast surgery include type of surgical device [24,25,26], use of sclerosants, fibrin glue and sealants, timing of shoulder physiotherapy or patient factors like BMI but the results are conflicting and consistent evidence is scarce [8, 9].
Only limited data are available regarding the possible effect of modern treatment options such as NAC and/or IORT on seroma production following breast cancer surgery. A prospective randomized study evaluating complications after breast surgery with or without peri-operative adjuvant chemotherapy found no difference in seroma production [27]. One of the largest studies including 3700 NAC patients and 74,000 patients without NAC showed that NAC was associated with decreased odds of systemic morbidity in the first 30 days, but these results are based on more extended surgery as all patients received mastectomy with or without immediate breast reconstruction, and seroma formation was not specifically investigated [2]. Our data show that NAC significantly increases the amount of seroma production, but the effect size is small compared to the more important factors extent and duration of surgery and indicates limited clinical relevance.
IORT is another current treatment development that is becoming more and more a routine for patients with smaller tumours and BCS. With two good quality trials showing a non-inferiority regarding local recurrence for the IORT compared to whole breast radiotherapy the question on hand is whether this intrasurgical treatment does not only prolong the surgical procedure, but also influences the seroma production. The rates of seroma treatment in a case–control series of 157 patients ± IORT did not differ [28]. Another clinical case series with 208 patients found palpable seromas in breast and axilla in 17%. But 55% of those patients underwent an axillary lymph node dissection increasing the risk for axillary seroma [29]. In our previous publication with pN0 (sn) patients no significant difference in the seroma production until drain removal was found [30].
However, looking at the overall post-operative complication rate (including liponecrosis, inflammation, seroma, hematoma and infection/dehiscence,) IORT patients have a significantly higher complication rate [31].
With the increased diversity of breast cancer treatment our study re-evaluates the risk factors on seroma production for the time period between surgery and discharge.
As the underlying causes for seroma production following breast cancer surgery are still not well understood this retrospective study is an addition on factors associated with seroma production and current guideline adherent treatment. Our multivariate analysis identified the factors with the most influence (i.e. largest effect size) on seroma production, but to provide strong evidence for a causal relationship a prospectively randomized study would be needed. However, besides the difficulties for a randomization in a surgical study according to the extent of the surgery as a main parameter it would be unethical to withhold current treatment options (i.e. IORT or NAC) for suitable patients. Thus, it seems that large retrospective studies involving multivariate analysis and meta-analytical approaches are the best available options to disentangle the impact of various factors on the amount of seroma production after breast surgery.
Conclusions
The amount of seroma formation after breast conserving surgery in early breast cancer is significantly and clinically relevant associated with type of surgery (mastectomy vs. BCS), number of removed lymph nodes, and length of surgery. Significant but not clinically relevant associations were found for age, tumour stage, IORT and NAC. Our results indicate that seroma in breast surgery is mainly caused by the surgeon.
References
Veronesi U, Luini A, Galimberti V, Zurrida S (1991) Conservation approaches for the management of stage I/II carcinoma of the breast: Milan Cancer Institute trials. World J Surg 18(1):70–75
Abt NB, Flores JM, Baltodano PA, Sarhane KA, Abreu FM, Cooney CM et al (2014) Neoadjuvant chemotherapy and short-term morbidity in patients undergoing mastectomy with and without breast reconstruction. JAMA Surg 149(10):1068–1076
Sedlmayer F, Sautter-Bihl M-L, Budach W, Dunst J, Fastner G, Feyer P et al (2013) DEGRO practical guidelines: radiotherapy of breast cancer I: radiotherapy following breast conserving therapy for invasive breast cancer. Strahlenther Onkol 189(10):825–833
Boostrom SY, Throckmorton AD, Boughey JC, Holifield AC, Zakaria S, Hoskin TL et al (2009) Incidence of clinically significant seroma after breast and axillary surgery. J Am Coll Surg 208(1):148–150
Silverstein MJ, Fastner G, Maluta S, Reitsamer R, Goer DA, Vicini F et al (2014) Intraoperative radiation therapy: a critical analysis of the ELIOT and TARGIT trials. Part 2–TARGIT. Ann Surg Oncol 21(12):3793–3799
Buchholz TA, Somerfield MR, Griggs JJ, El-Eid S, Hammond MEH, Lyman GH et al (2014) Margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer: American Society of Clinical Oncology endorsement of the Society of Surgical Oncology/American Society for Radiation Oncology consensus guideline. J Clin Oncol 32(14):1502–1506
Kreienberg R, Kopp I, Albert U-S, Bartsch HH, Beckmann MW, Berg D et al (2012) Interdisziplinäre S3-Leitlinie und Nachsorge des Leitlinie. Ger Cancer Soc 2012(7):32–45
Srivastava V, Basu S, Shukla VK (2012) Seroma formation after breast cancer surgery: what we have learned in the last two decades. J Breast Cancer 15(4):373–380
Kuroi K, Shimozuma K, Taguchi T, Imai H, Yamashiro H, Ohsumi S et al (2006) Evidence-based risk factors for seroma formation in breast surgery. Jpn J Clin Oncol 36(4):197–206
Purushotham AD, Upponi S, Klevesath MB, Bobrow L, Millar K, Myles JP et al (2005) Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol 23(19):4312–4321
Ebner FK, Friedl TWP, Reich A, Janni W, Rempen A, Degregorio N et al (2013) Does non-placement of a drain in breast surgery increase the rate of complications and revisions? Geburtshilfe Frauenheilkd 73(11):1128–1134
Garbay J-R, Thoury A, Moinon E, Cavalcanti A, Di Palma M, Karsenti G et al (2012) Axillary padding without drainage after axillary lymphadenectomy—a prospective study of 299 patients with early breast cancer. Breast Care (Basel) 7(3):231–235
Taylor JC, Rai S, Hoar F, Brown H, Vishwanath L (2013) Breast cancer surgery without suction drainage: the impact of adopting a “no drains” policy on symptomatic seroma formation rates. Eur J Surg Oncol 39:334–338
Thomson DR, Sadideen H, Furniss D (2013) Drainage tube placement after lymph gland removal from the armpit for breast cancer. Wiley, New York
Glechner A, Wöckel A, Gartlehner G, Thaler K, Strobelberger M, Griebler U et al (2013) Sentinel lymph node dissection only versus complete axillary lymph node dissection in early invasive breast cancer: a systematic review and meta-analysis. Eur J Cancer 49:812–825
Joyce DP, Manning A, Carter M, Hill ADK, Kell MR, Barry M (2015) Meta-analysis to determine the clinical impact of axillary lymph node dissection in the treatment of invasive breast cancer. Breast Cancer Res Treat 153:235–240
Shah-Khan M, Boughey JC (2012) Evolution of axillary nodal staging in breast cancer: clinical implications of the ACOSOG Z0011 trial. Cancer Control 19(4):267–276
Janni W, Kühn T, Schwentner L, Kreienberg R, Fehm T, Wöckel A (2014) Sentinel node biopsy and axillary dissection in breast cancer: the evidence and its limits. Dtsch Arztebl Int 111(14):244–249
Ebner F, Wöckel A, Janni W, Kreienberg R, Schwentner L, Wischnewsky M (2017) Personalized axillary dissection: the number of excised lymph nodes of nodal-positive breast cancer patients has no significant impact on relapse-free and overall survival. J Cancer Res Clin Oncol 143:1823–1831
Balakrishnan A, Ravichandran D (2011) Early operable breast cancer in elderly women treated with an aromatase inhibitor letrozole as sole therapy. Br J Cancer 105(12):1825–1829
Hille U, Soergel P, Länger F, Schippert C, Makowski L, Hillemanns P (2002) Aromatase inhibitors as solely treatment in postmenopausal breast cancer patients. Breast J 18(2):145–150
Hind D, Wyld L, Reed MW (2007) Surgery, with or without tamoxifen, vs tamoxifen alone for older women with operable breast cancer: Cochrane review. Br J Cancer 96(7):1025–1029
Headon H, Wazir U, Kasem A, Mokbel K (2016) Surgical treatment of the primary tumour improves the overall survival in patients with metastatic breast cancer: a systematic review and meta-analysis. Mol Clin Oncol 4(5):863–867
Cortadellas T, Córdoba O, Espinosa-Bravo M, Mendoza-Santin C, Rodríguez-Fernández J, Esgueva A et al (2011) Electrothermal bipolar vessel sealing system in axillary dissection: a prospective randomized clinical study. Int J Surg 9(8):636–640
Manouras A, Markogiannakis H, Genetzakis M, Filippakis GM, Lagoudianakis EE, Kafiri G et al (2008) Modified radical mastectomy with axillary dissection using the electrothermal bipolar vessel sealing system. Arch Surg. 143(6):575–580 (discussion 581)
Nespoli L, Antolini L, Stucchi C, Nespoli A, Valsecchi MG, Gianotti L (2012) Axillary lymphadenectomy for breast cancer. A randomized controlled trial comparing a bipolar vessel sealing system to the conventional technique. Breast 21(6):739–745
Canavese G, Catturich A, Vecchio C, Gipponi M, Tomei D, Sertoli MR et al (1997) Surgical complications related to peri-operative adjuvant chemotherapy in breast cancer. Results of a prospective, controlled, randomized clinical trial. Eur J Surg Oncol 23(1):10–12
Kraus-Tiefenbacher U, Welzel G, Brade J, Hermann B, Siebenlist K, Wasser KS et al (2010) Postoperative seroma formation after intraoperative radiotherapy using low-kilovoltage X-rays given during breast-conserving surgery. Int J Radiat Oncol Biol Phys 77(4):1140–1145
Tuschy B, Berlit S, Romero S, Sperk E, Wenz F, Kehl S et al (2013) Clinical aspects of intraoperative radiotherapy in early breast cancer: short-term complications after IORT in women treated with low energy X-rays. Radiat Oncol 8:95
Ebner F, Schramm A, Bottke D, Friedl TW, Wiegel T, Fink V et al (2016) Comparison of seroma production in breast conserving surgery with or without intraoperative radiotherapy as tumour bed boost. Arch Gynecol Obstet 294:861–866
Cracco S, Semprini G, Cattin F, Gregoraci G, Zeppieri M, Isola M et al (2015) Impact of intraoperative radiotherapy on cosmetic outcome and complications after oncoplastic breast surgery. Breast J 21(3):285–290
Funding
No external or additional funding was used for this study.
Author information
Authors and Affiliations
Contributions
FE: conceptualization, writing original draft, review and editing. TWPF: data curation, formal analysis. AG: resources. KL: supporting. IB: validation. WJ: supervision. NG: conceptualization, review and editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no potential conflict of interest.
Ethical approval
This study was approved by the local ethic committee.
Rights and permissions
About this article
Cite this article
Ebner, F., Friedl, T.W.P., de Gregorio, A. et al. Seroma in breast surgery: all the surgeons fault?. Arch Gynecol Obstet 298, 951–959 (2018). https://doi.org/10.1007/s00404-018-4880-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-018-4880-8