Abstract
Purpose
To diagnose polycystic ovary syndrome (PCOS) in young infertile women using different diagnostic criteria. To define serum anti-Müllerian hormone (AMH) cutoff values for PCOS definition. To investigate the correlation between AMH and body mass index (BMI).
Methods
Retrospective case–control study. A total of 140 infertile women (age 21–35 years) were enrolled. PCOS was defined according to the National Institutes of Health (NIH) criteria, the Rotterdam consensus criteria and the Androgen Excess and PCOS Society (AE-PCOS) criteria. ROC curve analysis was performed to define AMH thresholds for PCOS definition according to the three different diagnostic criteria. Correlation between AMH and BMI was investigated.
Results
The prevalence of PCOS under the NIH criteria, the Rotterdam criteria and the AE-PCOS criteria was 27.1, 40 and 29.3%, respectively. The optimal thresholds of AMH to distinguish NIH PCOS from infertile controls was 5.20 ng/ml (AUC = 0.86, sensitivity 79%, specificity 80%); the best cutoff to detect Rotterdam PCOS was 4.57 ng/ml (AUC = 0.85, sensitivity 78%, specificity 81%); a cutoff of 4.85 ng/ml (AUC = 0.85, sensitivity 80%, specificity 78%) defined PCOS women according to AE-PCOS criteria. The prevalence of the syndrome became 37.1, 44.3 and 39.2% according to the three criteria, respectively, using AMH threshold between 4.57 and 5.20 ng/ml as an alternative to antral follicle count and/or hyperandrogenism.
Conclusion
Anti-Müllerian hormone may reconcile the three diagnostic criteria and allow the PCOS diagnosis in women with mild symptoms. No significant correlation was found between AMH and BMI in PCOS women and controls.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorder in reproductive age women. It has important clinical features such as chronic anovulation and infertility [1], hyperandrogenism (HA) [2], pregnancy complications, long-term metabolic, cardiovascular and neoplastic risks [3,4,5,6]. The prevalence of PCOS differs according to the diagnostic criteria used [2, 7, 8]. Polycystic ovarian morphology (PCO), oligo-anovulation (OA), and HA are accepted diagnostic criteria for PCOS. The definition of polycystic ovarian morphology and clinical hyperandrogenism may be difficult because of technical characteristics of ultrasound devices, ultrasound operator dependence and interobserver variability of Ferriman–Gallwey hirsutism scoring system [9]. Several studies showed evidence about the essential role of anti-Müllerian hormone (AMH) in PCOS diagnosis [10]. AMH synthetized by the granulosa cells of small antral and pre-antral ovarian follicles is higher in PCOS women than in controls. The excessive number of follicles on ultrasound and/or the high AMH serum levels have been proposed as surrogate markers of HA in PCOS women [11]. Sahmay et al. [12] underlined that the combination of AMH level with OA and/or HA increased accuracy in PCOS diagnosis but it is not clear if AMH should be used alone or in combination with clinical symptoms. However, due to the variability in AMH assays, it is difficult to define universally agreed thresholds for PCOS diagnosis. Moreover, age may play a confounding effect on the relationship between AMH and hormonal parameters, and some authors suggest avoiding diagnosing PCOS before 18 years of age [13]. Also BMI may act as a confounding factor; both in PCOS women and in controls the relationship between AMH and BMI is controversial [14,15,16,17,18,19]. In Kriseman’s study conducted on infertile women, the average AMH levels in the PCOS group were significantly higher in lean women (BMI ≤ 25), than in overweight subjects [14] and in a retrospective study conducted on a large cohort of young infertile women, serum AMH levels were slightly but significantly lower in overweight women with PCOS [15]. It has not been observed any change of AMH level after body weight reduction in PCOS women [20].
The aim of the present study is to investigate the role of AMH in PCOS diagnosis according to the NIH, the Rotterdam and the AE-PCOS criteria, and to evaluate the correlation between AMH and BMI.
Materials and methods
Subjects
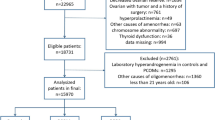
Patients included in this study were selected from a database of 1349 infertile women referred to the Infertility Center of Tor Vergata University Hospital, Section of Gynecology and Obstetrics between October 2007 and December 2014. The Institutional Review Board of Tor Vergata University Hospital in accordance with Helsinki Declaration approved the present study for Medical Research involving Human Subjects. All patients gave their written informed consent. The exclusion criteria were congenital adrenal hyperplasia, androgen-secreting tumors, Cushing’s syndrome, galactorrhea, hyperprolactinemia, thyroid dysfunctions and other endocrinological disorders, autoimmune diseases, hypothalamic amenorrhea, premature ovarian failure, age < 18 or > 35 years, serum FSH levels > 12 mIU/ml, use of hormonal contraceptive, pregnancy, puerperium, ovarian cysts or ovarian tumors, endometriosis, previous ovarian and uterine surgery or chemotherapy. The inclusion criteria were the presence of both ovaries and their adequate visualization on transvaginal ultrasonography. The diagnosis of PCOS was made according to three criteria; the National Institutes of Health (NIH) criteria: oligo-anovulation (OA) and clinical and/or biochemical signs of HA in the absence of other endocrinopathies [7], the Rotterdam criteria, i.e. presence of at least two of the following criteria: OA, clinical and/or biochemical signs of HA and polycystic ovarian morphology (PCO) in the absence of other etiologies [8], and the AE-PCOS criteria (the mandatory presence of hyperandrogenism associated with ovarian dysfunction, defined by OA and/or PCO) [2]. PCO was defined using a threshold of 12 follicles (2–9 mm in diameter) per whole ovary and/or increased ovarian volume (> 10 ml). These cutoffs are explained by the use of an ultrasound probe frequency of 6.5 MHz.
Investigations
Menstrual irregularity was assessed as the presence of chronic amenorrhea, or a cycle length of less than 21 days or more than 35 days. Anovulation was attested by a serial transvaginal ultrasound monitoring. Clinical definition of hyperandrogenism was determined by the presence of hirsutism (modified Ferriman and Gallwey score > 6) or severe and diffuse acne/seborrhea. Biochemical HA was established by TT serum level > 0.76 ng/ml (normal range 0.14–0.76 ng/ml), and/or A serum level > 2.2 ng/ml (normal range 0.21–2.2 ng/ml) and/or free testosterone (FT) serum level > 4.1 pg/ml (normal range 0.3–3.18 pg/ml). Women underwent AMH, FSH, LH, 17βestradiol (E2), inhibin B, TT, FT, A blood test and a pelvic ultrasound examination after a spontaneous or progesterone-induced bleeding within the fifth day of cycle. From 2007 to 2013, AMH blood levels were measured using the enzyme immunoassay AMH-EIA (reference A11893, Immunotech, Beckman Coulter company from Marseille, France) while from 2013 onwards AMH Gen II ELISA kit was applied (reference A79765 Beckman Coulter). Serum E2, FSH and LH, and TT levels were determined by immunoassay systems (ADVIA Centaur, Siemens Healthcare Diagnostic Inc.); Δ4 androstenedione was measured by automatic immunoassay systems (Immulite/Immulite 1000, Siemens Medical Solutions Diagnostics, Malvern, PA). Serum FT levels were tested by RIA (DIA source Immunoassay SA, Nivelles, Belgium), inhibin B was determined by Inhibin Gen II enzyme-linked immunosorbent assay kit (reference A81303, Beckman Coulter, Inc., Brea, CA). The ultrasound device used was Hitachi H21 HI Vision (probe frequency was 6.5 MHz). Through a slow and careful scanning from one margin of the ovary to the other, all follicles between 2 and 9 mm were considered to define antral follicle count (AFC). Ovarian volume was calculated by the formula: V = 0.526 × length × height × width. Metabolic syndrome was defined by the National Cholesterol Education Program Adult Treatment Panel III of 2001 (NCEP ATP III) criteria by the presence of at least three of the following five criteria: abdominal obesity (waist circumference—WC) > 88 cm, triglycerides ≥ 150 mg/dl, high-density lipoprotein cholesterol (HDL-C) < 50 mg/dl, blood pressure ≥ 130/≥ 85 mmHg, fasting glucose > 110 mg/dl [21]. Total cholesterol, HDL-C, triglycerides and glucose were measured with the use of standard enzymatic methods (Dimension Vista System, Siemens Healthcare Diagnostic Inc.); LDL-C was mathematically derived.
Statistical analysis
Statistical analysis was performed by SPSS software (v. 13.0 SPSS, Inc., Chicago, IL). Comparisons between the groups were performed with multivariate general linear model-based one-way analysis of variance (ANOVA), and Bonferroni’s post hoc test was applied whenever appropriated. Categorical data were analyzed by chi-square test. Univariate analysis was performed using the Spearman’s correlation test. Receiver operating characteristic (ROC) curves were constructed to examine the diagnostic test performance. Statistical significance was defined as p < 0.05.
Results
A group of 140 women meeting the inclusion criteria completed the diagnostic work-up and was included in the present study. The prevalence of PCOS under the NIH criteria, the Rotterdam criteria and the AE-PCOS criteria was 27.1, 40 and 29.3%, respectively (Tables 1, 2, 3). The distribution of the four phenotypes according to the NIH 2012 extension of the Rotterdam classification [22] was phenotype a (HA + PCO + OA) 34 women (24.3%), phenotype b (HA + OA) 4 women, phenotype c (HA + PCO) 5 women, and phenotype d (PCO + OA) 13 women (Table 4). The prevalence of metabolic syndrome in PCOS women was 21, 17.8 and 17.1% under the three criteria, respectively. Significant differences were found between PCOS and controls regardless of the criterion used (Tables 1, 2 and 3) and between phenotype a and controls (Table 4). A ROC curve analysis was performed to evaluate the diagnostic potency of AMH serum levels for the diagnosis of PCOS, HA and OA (Fig. 1). The optimal thresholds of AMH to distinguish NIH PCOS from infertile controls was 5.20 ng/ml (AUC = 0.86, sensitivity 79%, specificity 80%); the best cutoff to detect Rotterdam PCOS was 4.57 ng/ml (AUC = 0.85, sensitivity 78%, specificity 81%); a cutoff of 4.85 ng/ml (AUC = 0.85, sensitivity 80%, specificity 78%) defined PCOS women according to AE-PCOS criteria. ROC curve analysis showed a good diagnostic potency of AMH serum levels for the prediction of biochemical HA and OA (AUC was 0.84 and 0.80, respectively). For OA the best compromise between specificity and sensitivity was obtained with a threshold of 4.25 ng/ml (0.73–0.85, 95% CI; sensitivity 75%, specificity 76%); for biochemical HA the cutoff was 5.57 ng/ml (0.73–0.95, 95% CI; sensitivity 86%, specificity 76%). AMH positively correlated with AFC and ovarian volume in both women with PCOS and controls, regardless of the criterion used to define the syndrome (Table 5). AMH positively correlated with FT in PCOS group defined according to the Rotterdam criteria (r 0.47, p = 0.001) and in control group defined by the AE-PCOS criteria (r 0.32, p = 0.01). No significant correlation was found between AMH and BMI in PCOS women and in controls, regardless of the criterion used. The prevalence of the syndrome became 37.1, 44.3 and 39.2% according to the three criteria, respectively, using AMH threshold between 4.57 and 5.20 ng/ml as an alternative to antral follicle count and/or hyperandrogenism.
Discussion
The present study confirms that PCOS prevalence differs depending on the diagnostic criteria used for its definition [23] and that there is a significant AMH level increase in women with PCOS compared with controls, supporting our previous work on this topic [24]. ROC curve analyses showed that the AMH threshold for the definition of PCOS does not change significantly among the three criteria used: 5.20, 4.57 and 4.85 ng/ml, respectively. These AMH cutoff are close to the one found by Iliodromiti et al. [25] in a meta-analysis on ten studies (AMH = 4.7 ng/ml, 83% sensitivity and 79% specificity). Our study showed a positive correlation between AMH and AFC in both PCOS and controls, regardless of the criterion used; these data validate the appropriate use of high AMH levels as an objective marker of PCO [26, 27]. To define universally agreed AMH threshold, Pigny et al. [28] compared five commercial assays for AMH measurements and they showed that performance is comparable in PCO and PCOS diagnosis (4.2 ng/ml with the automatic assays and 5.6 ng/ml with the manual assays). In our study, even if there was a statistically significant difference in FT and Δ4 androstenedione between PCOS and controls, regardless of the criterion used, the correlation between AMH and FT was significant only when the syndrome was defined by the Rotterdam criteria. These controversial results may be due to the heterogeneous hyperandrogenic phenotype of PCOS subjects in our sample: more than 70% of PCOS women had clinical hyperandrogenism, but only 20–30% of them showed biochemical hyperandrogenism. A group of women with suspected PCOS was identified into the NIH control group by the presence of either HA or OA; AMH threshold of 5.20 ng/ml allowed us to distinguish, within the control group, women affected by a mild form of PCOS from true controls (Table 1). We observed that women affected by mild form of PCOS could be detected using AMH as a surrogate of AFC or HA, regardless of the criterion used to define the syndrome. Including these women into the PCOS group, the prevalence of the syndrome became 37.1, 44.3 and 39.2%, respectively. This method may reconcile Rotterdam criteria with the other definitions of the syndrome [11]. Pellat et al. [29] have shown that the mean AMH concentration in granulosa cells was 75 times higher in anovulatory women with PCO and 4 times higher in ovulatory women with PCO than the one observed in women with normal ovaries. This observation allows us to consider AMH as one of the main criteria for the diagnosis of PCOS.
The correlation between AMH and BMI is controversial in literature; in our study we could not find any significant correlation between AMH and BMI both in PCOS women and in controls. Literature heterogeneity may be explained by the different age range used in various studies: age may play a confounding effect in the relationship between AMH and BMI. Skalba et al. [16] in a study conducted on 87 PCOS women and 50 normal controls aged 18–35 years did not find any correlation between BMI and AMH in all study groups. In contrast Cui et al. [17] in a large cohort study on 304 PCOS women and 1896 infertile control women aged 20–47 years reported a slight negative correlation between AMH and BMI both in PCOS group (r = − 0.148; p = 0.01) and in infertile controls (r = − 0.064; p = 0.006). According to these data Feldman et al. [18], in a study conducted on 252 PCOS women aged 18–46 years, described a significant negative correlation between AMH and BMI (r = − 0.33, p < 0.0001) and a metabolic syndrome prevalence of 23.8% was reported. However, in these latter studies a high age range was considered. Our data suggest that AMH seems independent of BMI and demonstrated decreased fecundity in overweight women [30] may not be related to AMH. The relationship between obesity and reproductive health is complicated and the influence of BMI on fertility could depend on dysfunction of hypothalamic–pituitary–ovarian axis, anovulation or endometrial receptivity rather than on AMH, marker of ovarian reserve [31]. Anovulation is more frequent in obese women and, particularly in PCOS women, weight loss improves ovulatory dysfunction and fertility [30]; however, it does not change AMH level [20]. The relationship between AMH, BMI and metabolic syndrome needs to be further clarified in longitudinal multicenter studies characterized by properly defined age range.
A limitation of the present study is the small number of patients and its retrospective design. The strength of our study is the use and comparison of the three main classification criteria for the diagnosis of PCOS, the age range (21–35 years) of our sample and the case–control study design. Analyzing the four phenotypes proposed by the NIH 2012 extension of the Rotterdam criteria, we found that AMH was significantly higher in phenotypes characterized by the contemporary presence of OA and impaired ovarian morphology (phenotype a 24.3% and phenotype d 9.3%) than in controls. In conclusion, our findings indicate that AMH is an excellent marker of ovarian reserve and PCOS in young infertile women, and it does not seem to be influenced by BMI. AMH may reconcile all the criteria proposed for the classification of the syndrome, and it may help in PCOS diagnosis especially in women with mild symptoms. Further studies are needed to confirm universally agreed AMH thresholds to define PCOS. Prospective longitudinal studies are needed to investigate the follow-up of young women with PCOS to clarify their metabolic and cardiovascular risk in peri- and postmenopausal period.
References
The Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2008) Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril 89:505–522
Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W et al (2009) The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 91:456–488
Boomsma CM, Eijkemans MJC, Hughes EG, Visser GHA, Fauser BCJM, Macklon NS (2006) A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update 12:673–683
Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W et al (2010) Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab 95:2038–2049
Fauser BCJM, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R et al (2012) Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Hum Reprod 27:14–24
Barry JA, Azizia MM, Hardiman PJ (2014) Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 20:748–758
Zawadzki JK, Dunaif A (1992) Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GE (eds) Polycystic ovary syndrome. Blackwell Scientific Publications, Boston, pp 377–384
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81:19–25
Ape M, Badoglu B, Akca A, Api O, Gorgen H, Cetin A (2009) Interobserver variability of modified Ferriman–Gallwey hirsutism score in a Turkish population. Arch Gynecol Obstet 279:473–479
Singh AK, Singh R (2015) Can anti-Mullerian hormone replace ultrasonographic evaluation in polycystic ovary syndrome? A review of current progress. Indian J Endocr Metab 19:731–743
Dewailly D, Pigny P, Soudan B, Catteau-Jonard S, Decanter C, Poncelet E et al (2010) Reconciling the definitions of polycystic ovary syndrome: the ovarian follicle number and serum anti-Müllerian hormone concentrations aggregate with the markers of hyperandrogenism. J Clin Endocrinol Metab 95:4399–4405
Sahmay S, Aydin Y, Oncul M, Senturk L (2014) Diagnosis of Polycystic Ovary Syndrome: AMH in combination with clinical symptoms. J Assist Reprod Genet 31:213–220
Shayya R, Chang RJ (2010) Reproductive endocrinology of adolescent polycystic ovary syndrome. BJOG 117:150–155
Kriseman M, Mills C, Kovanci E, Sangi-Haghpeykar H, Gibbons W (2015) Antimullerian hormone levels are inversely associated with body mass index (BMI) in women with polycystic ovary syndrome. J Assist Reprod Genet 32:1313–1316
Lefebvre T, Dumont A, Pigny P, Dewailly D (2017) Effect of obesity and its related metabolic factors on serum anti-Müllerian hormone concentrations in women with and without polycystic ovaries. Reprod Biomed Online 35:325–330
Skałba P, Cygal A, Madej P, Dąbkowska-Huć A, Sikora J, Martirosian G et al (2011) Is the plasma anti-Müllerian hormone (AMH) level associated with body weight and metabolic, and hormonal disturbances in women with and without polycystic ovary syndrome? Eur J Obstet Gynecol Reprod Biol 158:254–259
Cui Y, Shi Y, Cui L, Han T, Gao X, Chen ZJ (2014) Age-specific serum antimüllerian hormone levels in women with and without polycystic ovary syndrome. Fertil Steril 102(230–236):e2
Feldman RA, O’Neill K, Butts SF, Dokras A (2017) Antimüllerian hormone levels and cardiometabolic risk in young women with polycystic ovary syndrome. Fertil Steril 107:276–281
Piouka A, Farmakiotis D, Katsikis I, Macut D, Gerou S, Panidis D (2009) Anti-Müllerian hormone levels reflect severity of PCOS but are negatively influenced by obesity: relationship with increased luteinizing hormone levels. Am J Physiol Endocrinol Metab 296:E238–E243
Thomson RL, Buckley JD, Moran LJ, Noakes M, Clifton PM, Norman RJ et al (2009) The effect of weight loss on anti-Müllerian hormone levels in overweight and obese women with polycystic ovary syndrome and reproductive impairment. Hum Reprod 24:1976–1981
Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (2001) Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 285:2486–2497
National Institutes of Health (2012) Evidence-based methodology workshop on polycystic ovary syndrome. National Institutes of Health, Washington. https://prevention.nih.gov/p2p/pcos/resources.aspx. Accessed 3–5 Dec 2012
Yildiz BO, Bozdag G, Yapici Z, Esinler I, Yarali H (2012) Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod 27:3067–3073
Casadei L, Madrigale A, Puca F, Manicuti C, Emidi E, Piccione E et al (2013) The role of serum anti-Müllerian hormone (AMH) in the hormonal diagnosis of polycystic ovary syndrome. Gynecol Endocrinol 29:545–550
Iliodromiti S, Kelsey TW, Anderson RA, Nelson SM (2013) Can anti-Müllerian hormone predict the diagnosis of polycystic ovary syndrome? A systematic review and meta-analysis of extracted data. J Clin Endocrinol Metab 98:3332–3340
Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P et al (2011) Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod 26:3123–3129
Fraissinet A, Robin G, Pigny P, Lefebvre T, Catteau-Jonard S, Dewailly D (2017) Use of the serum anti-Müllerian hormone assay as a surrogate for polycystic ovarian morphology: impact on diagnosis and phenotypic classification of polycystic ovary syndrome. Hum Reprod 32:1716–1722
Pigny P, Gorisse E, Ghulam A, Robin G, Catteau-Jonard S, Duhamel A et al (2016) Comparative assessment of five serum antimüllerian hormone assays for the diagnosis of polycystic ovary syndrome. Fertil Steril 105(1063–69):e3
Pellat L, Hanna L, Brincat M, Galea R, Brain H, Whitehead S et al (2007) Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab 92:240–245
Kominiarek MA, Jungheim ES, Hoeger KM, Rogers AM, Kahan S, Kim JJ (2017) American Society for Metabolic and Bariatric Surgery position statement on the impact of obesity and obesity treatment on fertility and fertility therapy Endorsed by the American College of Obstetricians and Gynecologists and the Obesity Society. Surg Obes Relat Dis 13:750–757
Simões-Pereira J, Nunes J, Aguiar A, Sousa S, Rodrigues C, Matias Sampaio et al (2018) Influence of body mass index in anti-Müllerian hormone levels in 951 non-polycystic ovarian syndrome women followed at a reproductive medicine unit. Endocrine. https://doi.org/10.1007/s12020-018-1555-y
Funding
This retrospective study was not funded.
Author information
Authors and Affiliations
Contributions
L Casadei: Protocol/project development, data collection and management, data analysis, manuscript writing/editing. F Fanisio: Protocol/project development, data collection, data analysis, manuscript writing/editing. R P Sorge: Data analysis. M Collamarini: Data collection, manuscript writing/editing. E Piccolo: Data collection, manuscript writing/editing. E Piccione: Protocol/project development
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Institutional Review Board of Tor Vergata University Hospital in accordance with Helsinki Declaration, approved the present study.
Informed consent
Informed consent was obtained from all individual participants included in the study for the processing of the sensitive data.
Rights and permissions
About this article
Cite this article
Casadei, L., Fanisio, F., Sorge, R.P. et al. The diagnosis of PCOS in young infertile women according to different diagnostic criteria: the role of serum anti-Müllerian hormone. Arch Gynecol Obstet 298, 207–215 (2018). https://doi.org/10.1007/s00404-018-4803-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-018-4803-8