Abstract
Purpose
Peritoneal mesothelial cysts (PMC) are a clinical dilemma because of their true pathogenic nature. Many definitions have been associated with PMC, including “benign multicystic mesothelioma”, “cystic mesothelioma”, “multilocular peritoneal inclusion cysts”, ‘‘inflammatory cysts of the peritoneum” or “postoperative peritoneal cyst”.
Methods
We herein performed a systematic review of the literature focusing on clinical and histopathological aspects of PMC, diagnosis, and therapies. Moreover, we described our experience with a case of PMC in a young female.
Results
Since there is often a history of prior surgery or inflammatory disease, most authors consider PMC of reactive origin. However, in some cases they occur without any documentable signs of disease or injury. A variety of clinical findings can complicate the preoperative assessment and a multitude of histological pictures may potentially lead to a misdiagnosis. The absence of a uniform treatment strategy and lack of long-term follow-up often hinder the accurate definition leading to unnecessary or unnecessarily aggressive therapy.
Conclusions
PMC are more common than had previously been thought. Most authors consider them non-neoplastic; thus the designation of “peritoneal inclusion cyst” is preferable. The term “mesothelioma” should be used only in cases of histological evidences of atypia. The high rates of recurrence suggest that the goal of treatment should not be necessarily complete eradication, but symptomatic relief through individualized treatment. This is a topic of particular importance, especially in young female where recurrence rates could be lower than those reported in adults and where an improperly aggressive treatment could have repercussions on fertility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peritoneal mesothelial cysts (PMC) are rare lesions, resulting from uncommon mesothelial proliferations that may primarily involve not only the pelvis, but also the upper abdomen, and retroperitoneum [1,2,3,4,5,6,7]. Since the first description in 1979 by Mennemeyer and Smith [8] approximately 200 cases have been reported in the literature [9]. Currently, it has not been definitively established whether these ones were due to a reactive response to injury or a neoplasia. For this reason, various terms were applied to designate them, including “benign multicystic mesothelioma”, “cystic mesothelioma”, “multilocular peritoneal inclusion cysts”, ‘‘inflammatory cysts of the peritoneum” or “postoperative peritoneal cyst”. Moreover, it created much disagreement regarding the most appropriate treatment [1, 10, 11], because these ones often tend to recur and their biological behavior still remains unclear [12]. Only a few cases have been reported in adolescent and young females in the past 30 years. They could show some particular features distinguishing them from those ones described in adult population [11].
These evidences prompt to clarify the nature of these lesions and to provide a well-defined denomination establishing the most appropriate management. This review describes the state of the art on PMC, according to recent literature findings. Our attention has been focused on the clinical presentation, diagnosis, natural history and treatment with particular attention on young females (age range 10–20 years). Particularly, the aim of this paper was to clarify the best management for PMC since there is no standard of care in the literature and different approaches have been proposed. Indeed, this review tries to suggest when it is advisable to manage a conservative treatment, analyzing clinical and histopathological aspects, symptoms, main complications and surgical outcomes. Finally, we described our experience with a case of PMC, involving the pouch of Douglas in a 19-year-old female.
Methods
In this systematic review, we performed a PubMed search comprising the terms: “benign multicystic mesothelioma” and “peritoneal inclusion cyst”. A total of 338 articles were found through this search, with publication dates from 1960 to 2017. A total of 181 papers were excluded for the following reasons: the abstract was not available, the study was not published in English, the subject was not related to the topic of our review or not provided any noteworthy information. Thus, a total of 157 references were selected. We sequentially reviewed all available articles on PMC describing clinical presentation, pathogenesis, macroscopic and histopathological aspects, natural history, diagnosis and treatment. Moreover, our attention has been focused on the young female group. Therefore, we selected all reported cases aged 10–20 years and analyzed the following aspects: clinical features, symptoms, associated diseases, previous surgery, macroscopic features, localization, cancer markers, intervention and follow-up. Finally, we described our experience with a case of PMC, involving the pouch of Douglas in a 19-year-old female.
Results
Our research highlighted that no uniform data about PMC exist. The controversial pathogenesis and consequently, the lack of consistent definitions, led to a lack of agreement on the therapeutic approach. Moreover, the shorter follow-up times made it difficult drawing any firm conclusions from published reports.
Current literature on PMC is mostly based on case reports or series of cases analyzing small subject groups. Cases described in the literature are updated and reported in Table 1. Clinical features, pathogenesis, macroscopic and histopathological aspects, natural history, diagnosis and treatment of PMC were described. Moreover, we found a total of 21 cases of PMC in young females (aged 10–20 years) that we analyzed. Data were summarized in Table 2. Of the reported cases, 7 patients were nulliparous [11, 13,14,15] and 1 [16], parous. Data regarding parity were not recorded in the other cases. The mean age at diagnosis was 16.7 years (range 11–20 years). The most typical localization was the pelvis; indeed almost all of the examined cases showed a pelvic localization of the lesions. In six cases the lesions were specifically localized at the level of the Douglas [11, 16, 17], in five cases they involved also the abdomen, including colon, omentum or appendix [14, 16, 18,19,20]. Pelvic pain was the most common presenting symptom, recorded in ten cases [11, 21,22,23,24]; other reported symptoms were abdominal pain or distension, constipation, amenorrhea, dysuria, recurrent urinary symptoms, fever and anorexia [1, 11, 13,14,15, 17,18,19,20]. In four cases the presenting symptom was acute abdominal pain [13,14,15, 20], in two of these [14, 20], this symptom was associated with fever, simulating the occurrence of acute appendicitis or a bowel inflammatory disease. Only one case [16] of reported series was completely asymptomatic. Only one had a medical history positive for PID [15]. One patient reported ulcerative colitis [21], two had congenital genitourinary anomaly [11, 24] and in another case, although medical history was negative for any noteworthy diseases, an unconfirmed suspicion of bowel inflammatory disease was done during the intervention [14].
Ten patients underwent previous surgery. Appendectomy (three cases) [11, 21] and oophorectomy (four cases) [11, 21,22,23] were the most reported prior surgery; others included pancreatectomy, splenectomy, colectomy, cystectomy and renal transplant [21, 22, 24]. The main macroscopic aspects observed during the interventions were multiloculated thin-walled cyst (six cases) [11, 14, 17, 18, 20, 24] or multiple cysts [14, 15, 21], sometimes with a grapelike appearance [15, 19] or cystic mass [1, 22, 23], occasionally free-floating [23, 24] or connected to the peritoneum by a pedicle [11]. In one case, ascites were also reported [19]. As regards the preoperative evaluation of tumor markers in our selected series, unfortunately, most studies did not pay attention to such aspect, except for one case [17] in which an increase of serum Ca 19.9 was observed. The treatment strategy adopted in most of the examined cases was the surgical removal by laparoscopy or laparotomy [11, 14, 15, 17, 19,20,21,22], while in three cases cyst drainage [18, 24] was performed. In two of the reported cases [22, 23], hormonal therapy with tamoxifen [23] or leuprolide acetate plus estrogen progestin [24] was carried out. Patients had a history of recurrence of PMC and they previously had undergone surgical procedures in the management of the disease. In another reported case [19], complete cytoreductive surgery (resection of the pelvic peritoneum en bloc with the internal female genitalia, and low anterior resection), greater and lesser omentectomy in combination with hyperthermic intraoperative intraperitoneal chemotherapy was performed. The mean follow-up period was 33.9 months (range 2–253 months). One patient was lost to follow-up [11] and in eight cases, recurrence rates were not reported [14,15,16, 18, 21]. Recurrences in only three cases occurred [1, 24]. In two of these cases the previous treatment was cyst drainage [24].
Discussion
History and pathogenesis
PMC were first described by Plaut in 1928 who incidentally observed ‘loose cysts of the pelvis’ during an operation for uterine leiomyoma [25, 26]. However, their mesothelial nature was confirmed later in 1979 by Menemeyer and Smith [8]. In 1989 Ross et al. [1] reported the clinical and pathological features of 25 cases of PMC, most of them derived from Dr. Scully’s consultation files. A history of abdominal surgery, pelvic inflammatory disease and/or endometriosis, was found in most of their cases, supporting a reactive nature opposing to the view, still supported by others, that PMC are well-differentiated cystic mesotheliomas [25]. To date the pathogenesis of PMC still remains controversial. According to some authors [1, 12] PMC are the result of particular proliferative reactions within the peritoneal tissue secondary to intra-abdominal inflammation and subsequent cyst formation [27, 28]. The normal peritoneum is able to easily transport the fluid produced by the ovaries, but when its integrity is compromised, as a result of injury, its absorption ability is impaired. In addition, postsurgical adhesions can trap ovarian fluid forming complex cystic masses. According to this theory, functional, active ovaries and adhesions are thus essential for the development of PMC [24].
In several reports, 30–87% of patients with PMC had a history of previous abdominal surgery [1, 21, 29, 30]. The time between the most recent surgery and detection of the PMC ranged from 6 months to 20 years [13].
Although PMC is often accompanied by endometriosis, histologic findings of the lesion have not been well documented [28, 31].
In one case the lesion consisted of multiple cysts having thin walls lined with single-layered cuboidal mesothelial, and inside the cystic walls, small foci of endometriosis were found. In another case the mesothelial lesion was next to the endometriotic cysts, in the pelvic cavity [28].
These histologic findings further support the hypothesis that endometriosis plays a role in the pathogenesis of PMC and that cystic lesions are the results of a reactive rather than a neoplastic process [31].
In contrast, the lack of previous surgery or inflammation observed in some cases, as well as the developmental behavior and the high recurrence rate, led other authors to theorize a neoplastic origin for these lesions. For this reason, it has been suggested that PMC may be placed on a spectrum between an adenomatoid tumor and a malignant mesothelioma [12, 25, 32, 33].
It is well known that there are some precipitating factors such as foreign fibers or dust, inflammatory mediators and mechanical injuries that may promote hyperplastic and neoplastic changes in mesothelial cells. Proliferation and metaplasia of underlying connective tissue cells, surface attachment and differentiation of mononuclear cells have all been postulated as mechanisms of mesothelial proliferation in pathological conditions [34].
Moreover, a possible genetic or familial predisposition for PMC has been proposed [35,36,37,38]. Specifically, a case report by Bernstein et al. [35] described the occurrence of PMC in two sisters. In addition, a third sister had also findings consistent with PMC. However, the discrete histological diagnosis was never confirmed. Another case report, published by Curgunlu et al. [36], describes a man with familial Mediterranean fever who also developed PMC.
Finally, the evidence that the great majority of patients are women of reproductive age has suggested a hormonal dependency and, as such, a potential role for hormonal manipulation as medical management and an alternative to surgery [39].
Some important epidemiological differences between peritoneal and pleural mesothelial proliferations have been reported in literature. Indeed, while peritoneal mesotheliomas are more frequent in women, peritoneal mesotheliomas more often occurs in men. Likewise, prognosis seems better in females [40, 41].
Asbestos exposure is strongly related with an increased risk of malignant pleural mesothelioma. On the contrary, the link between asbestos exposure and peritoneal mesotheliomas is less strong, and it is estimated that approximately 20–40% of all cases occur spontaneously without any evidence of previous asbestos exposure [40, 42].
The median age at diagnosis is earlier in peritoneal mesotheliomas, and the latency period between asbestos exposure and development of peritoneal mesotheliomas is shorter (20 years) compared with pleural mesotheliomas (30–40 years). The mechanism whereby asbestos fibers reach the peritoneum is unknown but they have been found in the omentum and in the mesentery of the gastrointestinal tract. It has been supposed that irritation of the peritoneum is able to induce a chronic inflammatory process with disruption of the mitotic process and chromosomal instability [40, 43, 44].
Although PMC are usually qualified as benign, their natural history has not been definitively established. There are several case reports of these cysts recurring despite successful operative removal with some patients requiring multiple operations to reduce the cystic load and relieve symptoms. Local recurrence is reported to be as high as 50%, even when all visible lesions have been removed [1]. Local relapse may occur decades after diagnosis and primary surgery [45]. Other concerns are reports of malignant transformation observed in some patients with a primary PMC [46,47,48]. The absence of a uniform treatment approach, lack of long-term follow-up in most patients, and the rarity of this entity seriously hinder an accurate assessment of the disease process [49].
Clinical features
PMC typically arise from the peritoneum of the pelvic region; exceptionally, they can develop on the serosal surfaces of the pelvic viscera including kidney, bladder, lymph nodes, liver and spleen [50,51,52,53,54,55,56,57,58,59,60,61,62]. Occasionally, PMC can also be accompanied by ascites [63,64,65].
They mostly occur in women of reproductive age [66], although cases involving men [67,68,69,70,71,72,73,74,75,76,77,78] and children [79,80,81,82,83,84,85,86,87] are documented. The average age at diagnosis is approximately 32 years [1].
A diagnosis of PMC during pregnancy has been reported [88,89,90,91]. In most cases, these cysts have been found incidentally at the time of full term Cesarean sections in asymptomatic patients with uneventful pregnancies. Indeed, the most typical presentation of PMC is characterized by the absence of specific symptoms and there could be an incidental finding at surgery for other abdomino-pelvic complaints [92,93,94,95,96]. In other cases, common presenting symptoms are vague lower abdominal pain or discomfort [97,98,99,100,101] and fullness [102, 103]. Other described features include abdominal distension [104], pelvic pain [105, 106], palpable mass [107,108,109,110,111,112,113,114], weight loss, nausea, vomiting [115], constipation or signs of bowel obstruction [116] and urinary retention or dysuria [117, 118]. Sometimes the presenting symptom can be acute abdominal pain [119] simulating the occurrence of acute appendicitis [20, 120,121,122,123]. Knowledge of PMC is important because especially in symptomatic patients undergoing evaluation for abdomino-pelvic complaints, PMC may be mistaken for borderline or malignant cystic tumors or tumor with degenerative cystic changes that prompt unnecessary or unnecessarily aggressive therapy [124,125,126,127,128,129,130,131,132].
Macroscopic aspects
Macroscopically, PMC can be unilocular or multilocular. They may be adherent to surrounding structures, including the ovaries, fallopian tubes, large bowel, appendix, omentum and uterus with a strong predilection for the pelvic peritoneum [133,134,135]. The cysts can also be free floating in the peritoneal cavity. The cystic formations can be of different sizes ranging from few millimeters to 20 cm [136]. Unilocular cysts can be single or multiple; they are usually small, thin-walled, translucent, attached or lie free in the peritoneal cavity. Multilocular cysts, typically consists of multiple grapelike clusters of mesothelium-lined cysts, confluent or discontinuous, studding the peritoneal surface. Unlike the smaller unilocular cysts, in multilocular cysts the septa and the walls may contain considerable amounts of fibrous tissue. The intracystic fluid varies from clear to blood tinged and may occasionally be mucinous or gelatinous [1, 9, 12, 137, 138].
Histopathologic characteristics
On microscopic examination PMC are typically lined by a single layer of flat to cuboidal mesothelial cells with generally bland nuclear features. The septa typically consist of a loose, fibrovascular connective tissue with a sparse inflammatory infiltrate accompanied by fibrin, granulation tissue, and recent and old hemorrhages in the cyst walls. However, many unusual morphological features may pose problems for differential diagnosis with malignant lesions. The lining cells may exhibit unusual reactive histological findings such as atypia with hyperchromatic enlarged nuclei that may have a hobnail appearance and exhibit complex architectural arrangements including intraluminal small papillae, gland-like structures or nests and cribriform patterns or they may resemble squamous metaplasia, mimicking a malignant primitive peritoneal mesothelioma [13, 16, 139, 140]. Furthermore, patterns resembling adenomatoid tumors may be encountered. Occasional vacuolated mesothelial cells in the stroma may simulate signet-ring cells [1]. Mesothelial cells are typically immunoreactive for calretinin, cytokeratin 5/6, CA125, Vimentin and Wilms’ tumor antigen and, in some cases, they are positive for estrogen (ER), progesterone receptors (PR), or both [21, 32, 34]. A multitude of definitions has been associated with PMC [1, 10, 12], creating misunderstandings between clinicians and pathologists. Since most authors agree to consider these lesions non-neoplastic [1, 12, 21], the designation of “peritoneal inclusion cyst” rather than “benign cystic mesothelioma” is preferable. However, no specific histopathological features predicting the risk of recurrences have been identified. The term “mesothelioma” should be used only in the presence of atypia, suggestive of malignancy such as proliferative mesothelial components, moderate to severe atypia or numerous mitoses. Improper use of this term could result in very aggressive and unjustified therapeutic attitudes.
New markers in mesothelial proliferations
The distinction of a benign mesothelial reaction from a malignant proliferation is crucial for patient care and prognosis, but it often results exceedingly difficult on biopsy. Indeed, while morphology is diagnostic in many instances, a significant proportion of cases show equivocal aspects, making it necessary to resort to ancillary tests.
Immunohistochemistry (IHC) has been instrumental in allowing the pathologist to distinguish malignant proliferations from other processes. Loss of specific genes expression is useful in supporting the diagnosis of malignant mesotheliomas (MM) in a subset of patients with atypical biopsy findings, but lack without traditional definitive morphologic features of MM (invasion or tumefactive growth). [141,142,143]. A variety of immunostains have been investigated, including p53 nuclear positivity, desmin, membranous staining for epithelial membrane antigen (EMA), GLUT-1 and IMP3 positivity; however, the lack of specificity has not made them suitable for extensive use in clinical practice [143,144,145,146]. Recently, loss of BRCA1-associated protein-1 (BAP1) expression and/or homozygous deletion of cyclin-dependent kinase inhibitor 2A (CDKN2A) were identified in some MM, but not in reactive mesothelial proliferations [147, 148]. BAP1 is a tumor suppressor gene that encodes a protein involved in the regulation of important target genes implied in transcription, cell cycle control, DNA damage repair, and cellular differentiation through its deubiquitinase activity. The inactivating mutations in the BAP1 gene have been identified in 23–63.6% of MM [149,150,151,152,153].
CDKN2A gene encodes p16INK4a, a protein that acts through inhibition of CDK4 and CDK6 as a negative regulator of cell cycle progression leading to uncontrolled tumor cell proliferation. The loss of p16 tumor suppressor results from homozygous deletion of the 9p21 region, and it is detectable by fluorescence in situ hybridization (FISH). Homozygous deletion of CDKN2A is highly specific for malignancy, but only demonstrable in 22–88% of MM [154, 155]. Although neither loss of BAP1 expression nor homozygous deletion of CDKN2A is entirely sensitive for MM, the combination of both tests has been shown to increase the sensitivity for MM to 58–92% with 100% specificity [156].
Diagnosis
Since cystic lesions in the pelvic cavity are common in post-pubertal women, their detection prompts a long list of differential diagnoses, including ovarian cancer [157]. However, in premenopausal women with peritoneal adhesions due to previous surgery, endometriosis or PID, PMC should be included in the differential diagnosis. This is even more important considering that these cysts are suggested to be more common than had been thought [158]. To improve the preoperative assessment of pelvic masses, assessing the risk of malignancy, several parameters are used such as grayscale sonographic parameters, color Doppler ultrasonography, gynecological examination, tumor markers, and patient characteristics, and most of these parameters have been combined in diagnostic models [159, 160]. Transabdominal and/or transvaginal (TV) ultrasound (US) are the first-line imaging techniques in detection of pelvic masses. At US, PMC typically appear as multiseptate, anechoic cystic structures lacking internal vascularity at color Doppler evaluation, and they have an intimate anatomical association with the uterus and ovaries [161,162,163].
Peritoneal adhesions may extend to the surface of the ovary and distort the ovarian contour but do not penetrate the ovarian parenchyma. As fluid accumulates from these adhesions, the ovary appears entrapped within the cystic lesion. This appearance has been described as a “spider’s web” where a morphologically normal ipsilateral ovary is identified within a “web” of adhesions. The position of the ovary is variable and it can be centrally or eccentrically placed [164,165,166]. In the presence of typical imaging features, the diagnosis of PMC is relatively straightforward, particularly when accompanied by an appropriate clinical history. In many cases, however, the lesion may show a non-classic appearance; thus PMC could be misdiagnosed. The differential diagnosis includes benign and malignant conditions such as ovarian cystadenoma or cystadenocarcinoma, endometriosis, cystic teratoma, Brenner tumors, mesenteric-omental cysts, pseudomyxoma peritonei, lymphangioma, cystic adenomatoid tumor, malignant mesothelioma and other serous tumors of the peritoneum [132, 166,167,168,169,170,171,172,173]. Computed tomography (CT) and magnetic resonance imaging (MRI) may be complementary in preoperative diagnosis [137, 174]. MRI is the most useful second-line technique in problematic cases thanks to its high soft-tissue resolution and multidimensional imaging capabilities [175]. CT can be useful in differentiating ovarian malignancy from PMC by enhancing solid components within the lesion. The presence of calcification, peritoneal deposits and ascites are further features that should raise the suspicion of a malignancy [165, 176, 177]. However, a tissue sample is required for definitive histologic diagnosis. Negative cytology on fine-needle aspiration can decrease suspicion for malignancy, but results are usually inconclusive because the aspirate commonly shows reactive mesothelial cells that are non-specific [178,179,180]. Therefore, especially in any suspicion of malignancy, a biopsy is recommended [12]. Laparoscopy remains the best diagnostic tool because it can perform biopsies and establish a definitive diagnosis [181]. Evaluation of complex pelvic masses usually includes a serum assessment of tumor markers. In most reported cases of the literature a significant increase in serum tumor markers has not been observed. However, PMC have occasionally been associated with an increased CA 19.9 serum concentration [17, 182, 183]. Some tumor cells express this antigen and it has a role in adhesion between tumor and endothelial cells. It was suggested that metaplastic changes in mesothelial cells were responsible for secretion of this marker [17]. A few cases of PMC were associated with a raised serum CA125 level, which makes the distinction with serous epithelial tumors, including papillary serous carcinoma and borderline serous tumors, extremely difficult. However, ovarian malignancies often showed a markedly elevated CA-125 level. Moreover, an elevated CA-125 level may be seen in peritoneal inclusion cysts with associated endometriosis [12]. It has also been reported that PMC have the worst behavior when CA125 levels are high [184].
Treatment
Current literature is mostly based on case reports and small series and a uniform treatment approach and long-term follow-up data are lacking. The treatment options for PMC, range from observation to complete resection [15]. Although the treatment of choice has not been firmly established, conservative management is preferable to surgery in asymptomatic patients [158, 185]. There are several reasons for conservative management. First, most authors consider PMC pathologically reactive, not neoplastic lesions and even in the presence of squamous metaplasia, they have no malignant potential [1, 12, 13, 24, 29]. Second, PMC can adhere to the surface of the ovary not involving the ovarian parenchyma, and most cases occur in patients of reproductive age; consequently, fertility preservation techniques are advisable [1, 165, 186]. Moreover, PMC tend to easily rupture, frequently just after the abdomen is opened, and resection is difficult because tissue planes are poorly defined [187]. Third, after surgical resection, the recurrence rate is 30–50% [1, 45]. Moreover, most patients are asymptomatic and had previously undergone several laparotomies; thus additional surgical intervention is undesirable [1, 16, 25]. Conservative treatment includes observation with serial imaging in asymptomatic patients, with more aggressive treatment if the disease develops. Hormonal treatment with oral contraceptives, gonadotropin-releasing hormone agonist and the anti-estrogenic agent, tamoxifen, can be administered to suppress the formation of ovarian fluid [22, 23, 39, 158, 188]. Minimally invasive/radiological treatment involves image-guided drainage. PMC are generally accessible to US-guided drainage or aspiration via a transabdominal route. Previous reports have suggested that aspiration is a safe and effective nonsurgical treatment for PMC; simple aspiration is, however, associated with a high recurrence rate; therefore, sclerotherapy following drainage can improve treatment success [158, 189,190,191]. Image-guided aspiration provides fluid for cytological evaluation and can lead to the resolution of symptoms with minimal intervention and few complications. However, in doubtful cases and especially when there is any suspicion of malignancy, conservative management has not been recommended and a tissue sample is required for making the histologic diagnosis [49, 181, 192,193,194]. Considering the high rate of recurrence, some authors have proposed surgery with complete removal. Indeed, complete surgical excision is considered the treatment of choice for relief of symptoms and for prevention of recurrence [181, 195]. The choice of a surgical approach can depend on gross appearance, organ involvement, and differential diagnosis at the time of surgery [12, 181]. While laparoscopy is the approach of choice for investigation of masses or pain in women, open surgery is safer when a malignant process is suspected owing to the possibility of cyst rupture and seeding [20, 196,197,198,199,200,201,202,203]. Finally, given the rare reported case of malignant transformation, some authors have advocated aggressive surgery (extended peritonectomy) followed by hyperthermic intraperitoneal chemotherapy (HIPEC). They have been performed in cases of recurrence or even in first-line treatment, as the optimal treatment to prevent transition to a truly aggressive tumor [18, 19, 204,205,206]. However, since PMC mainly affect women of reproductive age, these therapeutic strategies often result very aggressive, with important repercussions on fertility. For these reasons, in the choice of the most appropriate treatment, careful consideration should be given on above discussed aspects and, especially in the light of the knowledge of the biological behavior of such lesions, it would be advisable, when possible, to offer the woman a more conservative treatment.
PMC in young female
Although PMC represent a well-established entity in the adult population, they remain a poorly characterized phenomenon in the pediatric population. In the young age group, this condition is fairly rare and difficult to diagnose because the clinical presentation might mimic numerous diseases [11]. After Mennemeyer and Smith’s definition of this pathology, there are few reports of PMC occurring in adolescent female individually cited in case reports. The 21 cases of adolescent female (age range 11–20 years) included in this investigation represent the largest case collection of PMC analyzed in the young female adolescent population, although unfortunately, some features were partially reported by several authors.
Typical reported localization was the pelvis, while the cul de sac was the only anatomical location in five reports [11, 16, 17]. Pelvic pain was the most common presenting symptom, in accordance with the collective data from the literature deriving from adult population [1, 9, 12]. In four cases the presenting symptom was acute abdominal pain [13,14,15, 20]; in two of these [14, 20], it was associated with fever. Such acute presentations, occasionally simulating appendicitis, have also been described in adult females [1]. For all the patients with acute presentations, PMC were the major intraoperative finding. In the McFadden et al. report [13], a ruptured PMC was considered the source of hemoperitoneum and multiple cysts were adherent to the ovarian surfaces. Only in one case of our reported series [16], the condition was asymptomatic and lesions were occasionally discovered at surgery for a ruptured left tubal ectopic gestation. Among all the examined patients, medical history was negative for endometriosis and only one patient reported a history of PID [15]: a patient reported a previous diagnosis of ulcerative colitis [21], while in another case, although medical history was negative for any noteworthy diseases, an unconfirmed suspicion of bowel inflammatory disease was done during the intervention [14]. These data are in apparent contrast with the previous reports of adult females, where PMC usually occurred in a background of PID, endometriosis and/or bowel inflammatory diseases [1, 6]. No other relevant associated diseases were identified, with the exception of two cases: one patient reported congenital genitourinary anomaly [24], and another had a horseshoe kidney [11]. Other cases of PMC associated with various congenital renal abnormalities have been reported in literature, and all occurred in young patients [207, 208]. In this series, ten patients underwent previous surgery [11, 21,22,23]; a history of previous abdominal surgery correlates with similar reports of adult women, but in about 30% of the analyzed cases, patients gave a negative history of prior surgery or associated inflammatory diseases. This finding may be related to the small sample size; alternatively, this atypical occurrence could characterize some variants with particular features and different pathogenesis, which may distinguish them from those ones reported in adult patients with a positive history for the above discussed factors.
The main macroscopic aspects observed were similar to those described in adult population group: multiloculated thin-walled cyst (six cases) [11, 14, 17, 18, 20, 24] or multiple cysts [14, 15, 21], sometimes with a grapelike appearance [15, 19] or cystic mass [1, 22, 23] occasionally free-floating [23, 24] or connected to the peritoneum [11]. The presence of a well-formed peduncle was previously identified, though this has been rarely described. It is possible that peduncles are common components of PMC, but it has been widely overlooked [11]. As regards the preoperative evaluation of tumor markers in our selected series, unfortunately, most studies had not paid attention to such aspect, except for one case [17], so it was not possible to analyze this aspect. The treatment strategy adopted in most of the examined cases was surgical removal by laparoscopy or laparotomy [11, 14, 15, 17, 19,20,21,22], and in three cases cyst drainage [18, 24] was performed.
In Letterie et al. report [22], the reduction of tumor size secondary to the hypoestrogenism induced by the GnRH agonist suggests a sensitivity to manipulation of the hormonal milieu. Indeed, the immediate increase in size with the addition of add-back therapy of estrogen and progestin further confirmed these findings. Another report by Letterie et al. [23] described the role of the antiestrogen tamoxifen in the management of recurrent PMC after radical surgery. The initial reduction in tumor size was followed by a stabilization in size and disappearance of symptoms. The use of tamoxifen in these circumstances may offer conservative management for long-term therapy in cases of recurrences.
Finally, Tentes et al. reported [19] a demolitive treatment strategy, and despite the histopathological definition of PMC, they performed a complete cytoreductive surgery (resection of the pelvic peritoneum en bloc with the internal female genitalia, and low anterior resection) with greater and lesser omentectomy in combination with hyperthermic intraoperative intraperitoneal chemotherapy. Unfortunately, further histopathological features, useful to better characterize such case, have not been reported. Such aggressive therapy is based on the assumption of s neoplastic origin, in which microscopic residual disease can disseminate thought the peritoneal cavity [204,205,206].
Despite a full spectrum of management options, because of a lack of knowledge of pathogenesis and long-term outcomes, there is no clear standard of treatment. In addition, there are no guidelines for following patients and no standard definition of recurrent versus persistent disease in temporal relation to medical or surgical management. Nevertheless, data from our collection suggest that local recurrences of PMC could follow a less aggressive course in young female group. It may be less likely than in adult females, in whom recurrences approach is nearly 50% [1, 11, 12]. Thus, in these cases, and especially when PMC have been discovered incidentally with no concerning history and in the absence of pelvic pain or compressive symptoms, a conservative management may be advisable, following the patient over time using transvaginal and/or transabdominal ultrasound [209]. This is a topic of particular importance, especially in young patients, where the choice of demolitive strategy may be extremely aggressive and irrational, with important repercussions on fertility [209, 210]. However, it is still unknown whether the cysts, over time, can spontaneously develop to potentially compress nearby structures. Therefore, treatment strategies must be carefully evaluated and individualized for each case, until larger studies will be conducted and definitive management guidelines established.
Case presentation
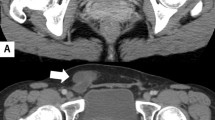
We herein described our experience with a case of PMC in a young female. A 19-year-old woman presented to our Department [omissis for blind review] for chronic pelvic pain. The patient was admitted to our hospital for a preliminary assessment that included: medical history, physical examination, complete blood biochemical assessment, including tumor biomarker (CA 125; CA 19.9; CA 15-3; carcinoembryonic antigen (CEA); Alpha fetoprotein (AFP); human chorionic gonadotropin (hCG)) and pelvic ultrasound (US). As standard protocol, the patient was informed and signed a consent allowing data collection for research purposes. This case report is in accordance with the Helsinki Declaration, conforms to the Consensus-based Clinical Case Reporting Guideline Development (http://www.equator-network.org/) the Committee on Publication Ethics (COPE) guidelines (http://publicationethics.org/) and was approved by the Institutional Review Board (IRB) of the university hospital in which it was performed. She had no relevant family history for main pathologies and cancers. She did not smoke or drink alcohol. She had no previous surgical history; she only reported an idiopathic thrombocytopenia during childhood that spontaneously resolved. Gastrointestinal and genitourinary symptoms, as well as sexual activity, were denied. Menstrual cycles were reported as regular. On physical examination, vital signs were normal; abdomen was treatable in all quadrants, with achiness to deep palpation of the left lower quadrant. Laboratory data were within normal ranges. Particularly, all cancer marker values were in the range of normality although there was a minimal increase in serum levels of CA 125 and CA 19.9, which, however, were a little below the threshold considered as significant. Pelvic US evaluation revealed a normal retroverted uterus, with a mild bilateral increment in ovarian volume, which also showed a micropolycystic appearance. A wide, well-organized multilocular anechoic mass of 57 mm × 36 mm × 32 mm was detected, floating in a free fluid effusion at the level of the pouch of Douglas. A total abdomen magnetic resonance imaging (MRI) was performed giving no more details concerning the pelvic mass, but just showing a collection of free fluid, partially septate, at the level of the pouch of Douglas. No other noteworthy lesions were documented and no bulky lymph-nodes were observed in the pelvic and retroperitoneal areas. The patient was evaluated through pelvic US 1 month later. Surprisingly, the free fluid effusion, previously noted, had disappeared and only a pelvic mass of unvaried size was detected. This was described as multilocular mass with regular walls, internal septa and low-level fluid content without any feature of blood supply at color and spectral Doppler. Given the ultrasound findings and the persistence of pelvic pain it was decided to perform a laparoscopic investigation. At laparoscopy, the uterus and the adnexa appeared regular for volume and morphology. We observed a single, thin-walled, cystic mass, with multiple septa inside rising from the bottom of the pouch of Douglas; it was of opaque appearance and was connected to peritoneum by a short peduncle. Cysts freely filled the pouch of Douglas because no adherence between cyst walls and peritoneal surface was seen. No other lesions in the upper and inferior abdomen or evidence of ascites were found. Because of its delicate constitution, a rupture occurred during resection and a yellowish liquid came out. The deflated cyst was detached from its peduncle and removed (Fig. 1). Microbiological examination of the contents of the cyst did not detect signs of bacteria proliferation, mycetes or other microorganisms. At the histological examination, the cyst was lined by a single, flattened layer of mesothelial cells, with bland ovoid to flattened nuclei and with a wall of fibrous connective tissue. No mitosis or metaplasia was observed and the cyst did not exhibit reactive features such as chronic or acute inflammatory components and degenerative cytological changes. Immunohistochemically, it showed a diffuse nuclear and cytoplasmic expression for calretinin and nuclear expression of WT-1 confirmed the mesothelial origin of the cyst (Fig. 2). The definitive diagnosis of PMC was made. The patient had an uneventful recovery. She was then enrolled to follow-up including trimestral cancer marker dosage and pelvic US for the first year. No evidence of pelvic mass at US and no positivity for cancer marker were reported after 3 months from the intervention.
Peritoneal inclusion cyst: cystic spaces are lined by a single layer of flat mesothelial cells and are separated by thin fibrous septa [haematoxylin and eosin] (a); immunohistochemical view of Mesothelial cells with diffuse nuclear and cytoplasmic expressions for calretinin (b) and nuclear expression of WT-1 (c)
Conclusions
To date, PMC have represented a clinical dilemma because their true pathogenic nature still remains controversial. This systematic review demonstrates the variety of information available regarding the etiology, clinical features, method of diagnosis and treatment of PMC. Lack of consistent definitions, uniform treatment approaches and mostly short follow-up times make it difficult to draw any firm conclusions from published reports. However, when the typical histological aspects are identified, most authors agree to consider these lesions as non-neoplastic, eschewing the term “cystic mesothelioma” and using the more appropriate term “peritoneal inclusion cyst”. This is even more important, considering that PMC are suggested to be more common than had previously been thought. Actually, there are no standard algorithms by which the patients are evaluated, treated, or followed up but it is apparent that PMC have a low mortality and the potential for high morbidity. Thus the goal of treatment should not be necessarily complete eradication, but symptomatic relief through individualized approach. This is a topic of particular importance, especially in young patients, where recurrence risk could be lower than those reported in adults and knowledge of the clinical context and typical features can avoid unnecessary, or unnecessarily aggressive, therapy.
References
Ross MJ, Welch WR, Scully RE (1989) Multilocular peritoneal inclusion cysts (so-called cystic mesotheliomas). Cancer 64:1336–1346. https://doi.org/10.1002/1097-0142(19890915)64:6<1336::aid-cncr2820640628>3.0.co;2-x
Bakhshi GD, Wankhede KR, Tayade MB, Bhandarwar AH, Gore ST, Choure DD (2013) Retroperitoneal approach for recurrent benign multicystic peritoneal mesothelioma. Clin Pract 3:e3. https://doi.org/10.4081/cp.2013.e3
Pitta X, Andreadis E, Ekonomou A, Papachristodoulou A, Tziouvaras C, Papapaulou L, Sapidis N, Chrisidis T (2010) Benign multicystic peritoneal mesothelioma: a case report. J Med Case Rep 4:385. https://doi.org/10.1186/1752-1947-4-385
Hollington P (2010) Benign multicystic mesothelioma. ANZ J Surg 80:186–187. https://doi.org/10.1111/j.1445-2197.2010.05222.x
Muscarella P, Cowgill S, Derenne LA, Ellison EC (2004) Retroperitoneal benign cystic peritoneal mesothelioma. Surgery 135:228–231. https://doi.org/10.1016/S0039
Durak E, Cokmez A, Orsel A, Tarcan E (2005) Multilocular peritoneal inclusion cyst. Surgery 137:580. https://doi.org/10.1016/j.surg.2003.12.005
Varma R, Wallace R (2004) Multicystic benign mesothelioma of the peritoneum presenting as postmenopausal bleeding and a solitary pelvic cyst—a case report. Gynecol Oncol 92:334–336. https://doi.org/10.1016/j.ygyno.2003.09.036
Mennemeyer R, Smith M (1979) Multicystic, peritoneal mesothelioma: a report with electron microscopy of a case mimicking intra-abdominal cystic hygroma (lymphangioma). Cancer 44:692–698. https://doi.org/10.1002/1097-0142(197908)44:2<692::aid-cncr2820440242>3.0.co;2-6
Momeni M, Pereira E, Grigoryan G, Zakashansky K (2014) Multicystic benign cystic mesothelioma presenting as a pelvic mass. Case Rep Obstet Gynecol 2014:852583. https://doi.org/10.1155/2014/852583
Ianieri MM, Buca DI, Falò E, Di Lorito A, Liberati M (2016) Incidental benign cystic mesothelioma of the peritoneum: a case report. J Obstet Gynaecol 36:135–136. https://doi.org/10.3109/01443615.2015.1033387
Amesse LS, Gibbs P, Hardy J, Jones KR, Pfaff-Amesse T (2009) Peritoneal inclusion cysts in adolescent females: a clinicopathological characterization of four cases. J Pediatr Adolesc Gynecol 22:41–48. https://doi.org/10.1016/j.jpag.2008.02.003
Vallerie AM, Lerner JP, Wright JD, Baxi LV (2009) Peritoneal inclusion cysts: a review. Obstet Gynecol Surv 64:321–334. https://doi.org/10.1097/OGX.0b013e31819f93d4
McFadden DE, Clement PB (1986) Peritoneal inclusion cysts with mural mesothelial proliferation. A clinicopathological analysis of six cases. Am J Surg Pathol 10:844–854. https://doi.org/10.1097/00000478-198612000-00003
Pollack CV Jr, Jorden RC (1991) Benign cystic mesothelioma presenting as acute abdominal pain in a young woman. J Emerg Med 9:21–25. https://doi.org/10.1016/0736-4679(91)90582-Z
Yaegashi N, Yajima A (1996) Multilocular peritoneal inclusion cysts (benign cystic mesothelioma): a case report. J Obstet Gynaecol Res 22:129–132. https://doi.org/10.1111/j.1447-0756.1996.tb00954.x
Sawh RN, Malpica A, Deavers MT, Liu J, Silva EG (2003) Benign cystic mesothelioma of the peritoneum: a clinicopathologic study of 17 cases and immunohistochemical analysis of estrogen and progesterone receptor status. Hum Pathol 34:369–374. https://doi.org/10.1053/hupa.2003.31
Pinto V, Rossi AC, Fiore MG, D’Addario V, Cicinelli E (2010) Laparoscopic diagnosis and treatment of pelvic benign multicystic mesothelioma associated with high CA19.9 serum concentration. J Minim Invasive Gynecol 17:252–254. https://doi.org/10.1016/j.jmig.2009.11.013
Ho-Fung V, Jaimes CE, Pollock AN (2011) Peritoneal inclusion cyst. Pediatr Emerg Care 27:430–431. https://doi.org/10.1097/PEC.0b013e3182184967
Tentes AA, Zorbas G, Pallas N, Fiska A (2012) Multicystic peritoneal mesothelioma. Am J Case Rep 13:262–264. https://doi.org/10.12659/AJCR.883523
O’Connor DB, Beddy D, Aremu MA (2010) Benign cystic mesothelioma of the appendix presenting in a woman: a case report. J Med Case Rep 4:394. https://doi.org/10.1186/1752-1947-4-394
Hoffer FA, Kozakewich H, Colodny A, Goldstein DP (1988) Peritoneal inclusion cysts: ovarian fluid in peritoneal adhesions. Radiology 169:189–191. https://doi.org/10.1148/radiology.169.1.3047785
Letterie GS, Yon JL (1995) Use of a long-acting GnRH agonist for benign cystic mesothelioma. Obstet Gynecol 85:901–903. https://doi.org/10.1016/0029-7844(94)00431-C
Letterie GS, Yon JL (1998) The antiestrogen tamoxifen in the treatment of recurrent benign cystic mesothelioma. Gynecol Oncol 70:131–133. https://doi.org/10.1006/gyno.1998.5031
Goldfisher R, Awal D, Amodio J (2014) Peritoneal inclusion cysts in female children: pathogenesis, treatment, and multimodality imaging review. Case Rep Radiol 2014:427427. https://doi.org/10.1155/2014/427427
Weiss SW, Tavassoli FA (1988) Multicystic mesothelioma. An analysis of pathologic findings and biologic behavior in 37 cases. Am J Surg Pathol 12:737–746. https://doi.org/10.1097/00000478-198810000-00001
Dzieniecka M, Kałużyński A (2011) Benign multicystic peritoneal mesothelioma (BMPM)—case report and review of the literature. Pol J Pathol 62:122–124
Mazziotti S, D’Angelo T, Racchiusa S, Salamone I, Blandino A, Ascenti G (2016) Peritoneal inclusion cysts in patients affected by Crohn’s disease: magnetic resonance enterography findings in a case series. Clin Imaging 40:152–155. https://doi.org/10.1016/j.clinimag.2015.09.010
Kurisu Y, Tsuji M, Shibayama Y, Yamada T, Ohmichi M (2011) Multicystic mesothelioma caused by endometriosis: 2 case reports and review of the literature. Int J Gynecol Pathol 30:163–166. https://doi.org/10.1097/PGP.0b013e3181f99def
Lees RF, Feldman PS, Brenbridge AN, Anderson WA, Buschi AJ (1978) Inflammatory cysts of the pelvic peritoneum. AJR Am J Roentgenol 131:633–636. https://doi.org/10.2214/ajr.131.4.633
Koninckx PR, Renaer M, Brosens IA (1980) Origin of peritoneal fluid in women: an ovarian exudation product. Br J Obstet Gynaecol 87:177–183. https://doi.org/10.1111/j.1471-0528.1980.tb04514.x
Groisman GM, Kerner H (1992) Multicystic mesothelioma with endometriosis. Acta Obstet Gynecol Scand 71:642–644. https://doi.org/10.3109/00016349209006236
Singh A, Chatterjee P, Pai MC, Chacko RT (2013) Multicystic peritoneal mesothelioma: not always a benign disease. Singap Med J 54:e76–e78. https://doi.org/10.11622/smedj.2013085
Snyder JA, Carman R Jr, Aggon AA, Cardinale JP (2011) Benign multicystic peritoneal mesothelioma: a rare case presenting as pneumoperitoneum and pneumotosis intestinalis. J Gastrointest Oncol 2:55–58. https://doi.org/10.3978/j.issn.2078-6891.2010.026
Pelosi G, Zannoni M, Caprioli F, Faccincani L, Battistoni MG, Balercia G, Bontempini L (1991) Benign multicystic mesothelial proliferation of the peritoneum: immunohistochemical and electron microscopical study of a case and review of the literature. Histol Histopathol 6:575–583
Bernstein EM, Tate A, Silasi DA, Rutherford T (2009) Benign multicystic mesothelioma: a case report of three sisters. Rare Tumors 1:e46. https://doi.org/10.4081/rt.2009.e46
Curgunlu A, Karter Y, Tüfekci IB, Tunckale A, Karahasanoglu T (2003) Benign cystic mesothelioma: a rare cause of ascites in a case with familial mediterranean fever. Clin Exp Rheumatol 21:S41–S43
Rougemont AL, Sartelet H, Oligny LL, Bensoussan A, Yazbeck S, Fournet JC (2007) Accessory liver lobe with mesothelial inclusion cysts in an omphalocele: a new malformative association. Pediatr Dev Pathol 10:224–228. https://doi.org/10.2350/06-08-0148.1
Tangjitgamol S, Erlichman J, Northrup H, Malpica A, Wang X, Lee E, Kavanagh JJ (2005) Benign multicystic peritoneal mesothelioma: cases reports in the family with diverticulosis and literature review. Int J Gynecol Cancer 15:1101–1107. https://doi.org/10.1111/j.1525-1438.2005.00198.x
Nozawa S, Iwata T, Yamashita H, Banno K, Kubushiro K, Aoki R, Tsukazaki K (2000) Gonadotropin-releasing hormone analogue therapy for peritoneal inclusion cysts after gynecological surgery. J Obstet Gynaecol Res 26:389–393. https://doi.org/10.1111/j.1447-0756.2000.tb01347.x
García-Fadrique A, Mehta A, Mohamed F et al (2017) Clinical presentation, diagnosis, classification and management of peritoneal mesothelioma: a review. J Gastrointest Oncol 8:915–924
Cao C, Yan TD, Deraco M et al (2012) Importance of gender in diffuse malignant peritoneal mesothelioma. Ann Oncol 23:1494–1498. https://doi.org/10.1093/annonc/mdr477
Spirtas R, Heineman EF, Bernstein L et al (1994) Malignant mesothelioma: attributable risk of asbestos exposure. Occup Environ Med 51:804–811. https://doi.org/10.1136/oem.51.12.804
Dodson RF, O’Sullivan MF, Huang J et al (2000) Asbestos in extrapulmonary sites: omentum and mesentery. Chest 117:486–493
Yang H, Bocchetta M, Kroczynska B et al (2006) TNF- inhibits asbestos-induced cytotoxicity via a NF-B-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc Natl Acad Sci 103:10397–10402. https://doi.org/10.1073/pnas.0604008103
Iversen OH, Hovig T, Brandtzaeg P (1988) Peritoneal, benign, cystic mesothelioma with free-floating cysts, re-examined by new methods. A case report. APMIS 96:123–127. https://doi.org/10.1111/j.1699-0463.1988.tb05278.x
DeStephano DB, Wesley JR, Heidelberger KP, Hutchinson RJ, Blane CE, Coran AG (1985) Primitive cystic hepatic neoplasm of infancy with mesothelial differentiation: report of a case. Pediatr Pathol 4:291–302. https://doi.org/10.3109/15513818509026902
González-Moreno S, Yan H, Alcorn KW, Sugarbaker PH (2002) Malignant transformation of “benign” cystic mesothelioma of the peritoneum. J Surg Oncol 79:243–251. https://doi.org/10.1002/jso.10081
Santangelo G, Accardo M, De Vita F, Del Giudice S, Gallucci F, Fabozzi A, De Falco M (2016) Malignant transformation in non-recurrent peritoneal cystic mesothelioma Our experience and review of the literature. Ann Ital Chir 87
Søreide JA, Søreide K, Korner H, Soiland H, Greve OJ, Gudlaugsson E (2006) Benign peritoneal cystic mesothelioma. World J Surg 30:560–566. https://doi.org/10.1007/s00268-005-0639-z
Campbell B, Mehanna D, Stone J (2017) Benign multicystic peritoneal mesothelioma: a rare cause of intra-abdominal cystic disease. ANZ J Surg 87:E15–E16. https://doi.org/10.1111/ans.12912
Somasundaram S, Khajanchi M, Vaja T, Jajoo B, Dey AK (2015) Benign multicystic peritoneal mesothelioma: a rare tumour of the abdomen. Case Rep Surg 2015:613148. https://doi.org/10.1155/2015/613148
Jouvin I, Dohan A, Gergi P, Pocard M (2014) Intra-abdominal benign multicystic peritoneal mesothelioma. J Visc Surg 151:155–157. https://doi.org/10.1016/j.jviscsurg.2013.12.014
Flemming P, Becker T, Klempnauer J, Högemann D, Kreft A, Kreipe HH (2002) Benign cystic mesothelioma of the liver. Am J Surg Pathol 26:1523–1527. https://doi.org/10.1097/00000478-200211000-00016
Urbańczyk K, Skotniczny K, Kuciński J, Friediger J (2005) Mesothelial inclusion cysts (so-called benign cystic mesothelioma)—a clinicopathological analysis of six cases. Pol J Pathol 56:81–87
Abdullahi H, Fawzi H (2003) Gynaecological presentation of benign multicystic mesothelioma. J Obstet Gynaecol 23:576. https://doi.org/10.1080/0144361031000153708
Vara-Thorbeck C, Toscano-Mendez R (2002) Peritoneal cystic mesothelioma. Surg Endosc 16:220. https://doi.org/10.1007/s00464-001-0052-0
Guzzo MH, Davis CA, Belzer GE, Virata RL (2001) Multiloculated peritoneal inclusion cysts with splenic involvement: a case report. Am Surg 67:619–621
Talib YA, Hefni H (2000) Benign cystic peritoneal mesothelioma. J Obstet Gynaecol 20:549–550. https://doi.org/10.1080/014436100434938
Rosen DM, Sutton CJ (1999) Use of the potassium titanyl phosphate (KTP) laser in the treatment of benign multicystic peritoneal mesothelioma. Br J Obstet Gynaecol 106:505–506. https://doi.org/10.1111/j.1471-0528.1999.tb08307.x
Di Blasi A, Boscaino A, De Dominicis G, Marsilia GM, D’Antonio A, Nappi O (2004) Multicystic mesothelioma of the liver with secondary involvement of peritoneum and inguinal region. Int J Surg Pathol 12:87–91. https://doi.org/10.1177/106689690401200116
Marrano D, Alampi G, Taffurelli M, Santini D, Galassi A, Grassigli A (1983) Cystic lesion of the kidney with ultrastructural evidence of mesothelial origin. Ital J Surg Sci 13:323–328
D’Antonio A, Baldi C, Addesso M, Napolitano C (2016) The first case of benign multicystic mesothelioma presenting as a splenic mass. Ecancermedicalscience 10:678. https://doi.org/10.3332/ecancer.2016.678
Shin HD, Benign Kim SB (2016) Cystic mesothelioma misdiagnosed as peritoneal carcinomatosis. Case Rep Gastroenterol 10:115–120. https://doi.org/10.1159/000444445
Firatligil FB, Dede M, Bodur S, Fidan U, Deveci S, Yagci G, Ozturk M, Yenen MC (2015) A case series of benign cystic mesothelioma as a rare etiology of ascites. J Minim Invasive Gynecol 22:S220. https://doi.org/10.1016/j.jmig.2015.08.781
Iacoponi S, Calleja J, Hernandez G, de la Cuesta RS (2015) Asymptomatic peritoneal carcinomatosis originating from benign cystic peritoneal mesothelioma. Ecancermedicalscience 9:605. https://doi.org/10.3332/ecancer.2015.605
Safioleas MC, Constantinos K, Michael S, Konstantinos G, Constantinos S, Alkiviadis K (2006) Benign multicystic peritoneal mesothelioma: a case report and review of the literature. World J Gastroenterol 12:5739–5742. https://doi.org/10.3748/wjg.v12.i35.5739
Sienkowski IK, Russell AJ, Dilly SA, Djazaeri B (1986) Peritoneal cystic mesothelioma: an electron microscopic and immunohistochemical study of two male patients. J Clin Pathol 39:440–445. https://doi.org/10.1136/jcp.39.4.440
Khurram MS, Shaikh H, Khan U, Edens J, Ibrar W, Hamza A, Zaka A, Bano R, Hadid T (2017) Benign multicystic peritoneal mesothelioma: a rare condition in an uncommon gender. Case Rep Pathol 2017:9752908. https://doi.org/10.1155/2017/9752908
Tuncer AA, Narcı A, Dilek FH, Embleton DB, Çetinkurşun S (2016) Benign cystic mesothelioma in a child: case report and review of the literature. Balkan Med J 33:232–234. https://doi.org/10.5152/balkanmedj.2015.15886
Stallone G, Infante B, Cormio L, Macarini L, Grandaliano G (2017) Rapamycin treatment for benign multicystic peritoneal mesothelioma: a rare disease with a difficult management. Am J Case Rep 18:632–636. https://doi.org/10.12659/AJCR.903548
Wang TB, Dai WG, Liu DW, Shi HP, Dong WG (2013) Diagnosis and treatment of benign multicystic peritoneal mesothelioma. World J Gastroenterol 19:6689–6692. https://doi.org/10.3748/wjg.v19.i39.6689
Aber A, Tahir A, Arumuham V, Smith G, Almpanis S (2012) Benign cystic mesothelioma: a rare cause for scrotal swelling. Case Rep Med 2012:572186. https://doi.org/10.1155/2012/572186
van Ruth S, Bronkhorst MW, van Coevorden F, Zoetmulder FA (2002) Peritoneal benign cystic mesothelioma: a case report and review of the literature. Eur J Surg Oncol 28:192–195. https://doi.org/10.1053/ejso.2000.1215
Häfner M, Novacek G, Herbst F, Ullrich R, Gangl A (2002) Giant benign cystic mesothelioma: a case report and review of literature. Eur J Gastroenterol Hepatol 14:77–80. https://doi.org/10.1097/00042737-200201000-00013
Lane TM, Wilde M, Schofield J, Trotter GA (1999) Benign cystic mesothelioma of the tunica vaginalis. BJU Int 84:533–534. https://doi.org/10.1046/j.1464-410x.1999.00239.x
Ozgen A, Akata D, Akhan O, Tez M, Gedikoglu G, Ozmen MN (1998) Giant benign cystic peritoneal mesothelioma: US, CT, and MRI findings. Abdom Imaging 23:502–504. https://doi.org/10.1007/s002619900387
Takenouchi Y, Oda K, Takahara O, Niinomi N, Ichikawa M, Yokoi S, Kanda H, Suzuki M, Horisawa M, Hayakawa S et al (1995) Report of a case of benign cystic mesothelioma. Am J Gastroenterol 90:1165–1167
Bhandarkar DS, Smith VJ, Evans DA, Taylor TV (1993) Benign cystic peritoneal mesothelioma. J Clin Pathol 46:867–868. https://doi.org/10.1136/jcp.46.9.867
Durell J, Dagash H, Eradi B, Nour S (2016) Pediatric benign cystic peritoneal mesothelioma. J Pediatr Adolesc Gynecol 29:e33–e34. https://doi.org/10.1016/j.jpag.2015.10.018
Hinsch N, Rauofi R, Stauch G (2015) Benign cystic mesothelioma of the peritoneum in a 12-year-old boy, diagnosed via telepathology. BMJ Case Rep 2015. https://doi.org/10.1136/bcr-2015-211419
Fernandez Eire P, Lemos Bouzas MX, Ortiz Rey JA, Herreros Villaraviz M, Prada Arias M (2015) Benign cystic mesothelioma in a boy: an uncommon case mimicking a lymphangioma. J Paediatr Child Health 51:841–842. https://doi.org/10.1111/jpc.12961
Shakya VC, Agrawal CS, Karki S, Sah PL, Poudel P, Adhikary S (2011) Benign cystic mesothelioma of the peritoneum in a child-case report and review of the literature. J Pediatr Surg 46:e23–e26. https://doi.org/10.1016/j.jpedsurg.2011.01.00
Saxena AK, Castellani C, Zaupa P, Höllwarth ME (2011) Pre-pubertal presentation of peritoneal inclusion cyst associated with congenital lower extremity venous valve agenesis. JSLS 15:264–267. https://doi.org/10.4293/108680811X13071180406835
Terry NE, Fowler CL (2009) Benign cystic mesothelioma in a child. J Pediatr Surg 44:e9–e11. https://doi.org/10.1016/j.jpedsurg.2009.01.073
McCullagh M, Keen C, Dykes E (1994) Cystic mesothelioma of the peritoneum: a rare cause of ‘ascites’ in children. J Pediatr Surg 29:1205–1207. https://doi.org/10.1016/0022-3468(94)90801-X
Hanukoglu A, Gewurtz G, Zaidel L, Krispin M, Fried D (1992) Benign cystic mesothelioma of the peritoneum: the occurrence of an adult entity in a child. Med Pediatr Oncol 20:169–171. https://doi.org/10.1002/mpo.2950200215
Raafat F, Egan M (1988) Benign cystic mesothelioma of the peritoneum: immunohistochemical and ultrastructural features in a child. Pediatr Pathol 8:321–329. https://doi.org/10.3109/15513818809042975
Nayak S, Parate RC, Bobhate S (2005) Multilocular peritoneal inclusion cyst—a case report. Indian J Pathol Microbiol 48:247–249
Akbayir O, Gedikbasi A, Akyol A, Numanoglu C, Koroglu N, Gulkilik A (2011) Benign cystic mesothelioma: a case series with one case complicated by pregnancy. J Obstet Gynaecol Res 37:1126–1131. https://doi.org/10.1111/j.1447-0756.2010.01474.x
Tamhankar VA (2015) Multicystic benign mesothelioma complicating pregnancy. Case Rep Obstet Gynecol 2015:687183. https://doi.org/10.1155/2015/687183
Hitzerd E, Jeurgens-Borst AJ, Pijnenborg JM (2014) Peritoneal inclusion cysts in pregnancy, a diagnostic challenge. BMJ Case Rep 2014. https://doi.org/10.1136/bcr-2014-204963
Macedo FI, Race AJ, Hoesel LM (2016) Ruptured cystic mesothelioma diagnosed after blunt trauma; case report and literature review. Bull Emerg Trauma 4:244–247
Lehwald N, Cupisti K, Baldus SE, Kröpil P, Schulte Am Esch J 2nd, Eisenberger CF, Knoefel WT (2010) Unusual histological findings after partial pancreaticoduodenectomy including benign multicystic mesothelioma, adenomyoma of the ampulla of Vater, and undifferentiated carcinoma, sarcomatoid variant: a case series. J Med Case Rep 4:402. https://doi.org/10.1186/1752-1947-4-402
Cuartas JE, Maheshwari AV, Qadir R, Cooper AJ, Robinson PG, Pitcher JD Jr (2008) Benign multicystic peritoneal mesothelioma in a cesarean-section scar presenting as a fungating mass. Int J Clin Oncol 13:275–278. https://doi.org/10.1007/s10147-007-0732-4
de Keizer B, Arsos G, Smit JW, Lam MG, Rinkes IH, Goldschmeding R, van Isselt JW (2008) I-131 accumulation in a benign cystic mesothelioma in a patient with follicular thyroid cancer. Thyroid 18:369–371. https://doi.org/10.1089/thy.2007.0155
Bansal A, Zakhour HD (2006) Benign mesothelioma of the appendix: an incidental finding in a case of sigmoid diverticular disease. J Clin Pathol 59:108–110. https://doi.org/10.1136/jcp.2005.026674
Marien T, Zhou M, Brucker B (2014) Benign multicystic mesothelioma masquerading as a urachal cyst. Can J Urol 21:7586–7588
Elbouhaddouti H, Bouassria A, Mouaqit O, el Benjelloun B, Ousadden A, Mazaz K, Taleb KA (2013) Benign cystic mesothelioma of the peritoneum: a case report and literature review. World J Emerg Surg 8:43. https://doi.org/10.1186/1749-7922-8-43.3
Uzüm N, Ozçay N, Ataoğlu O (2009) Benign multicystic peritoneal mesothelioma. Turk J Gastroenterol 20:138–141
Adolph AJ, Smith TE, Adolph J (2002) Benign multicystic mesothelioma: a case report. J Obstet Gynaecol Can 24:246–247. https://doi.org/10.1016/S1701-2163(16)30225-0
Schneider V, Partridge JR, Gutierrez F, Hurt WG, Maizels MS, Demay RM (1983) Benign cystic mesothelioma involving the female genital tract: report of four cases. Am J Obstet Gynecol 145:355–359. https://doi.org/10.1016/0002-9378(83)90724-X
Cotter TG, Van Arnam JS, Schaffner JA (2016) A case of abdominal discomfort caused by benign multicystic peritoneal mesothelioma. Clin Gastroenterol Hepatol 14:e147–e148. https://doi.org/10.1016/j.cgh.2016.05.029
Chen YC, Chang SP, Huang TW (1990) Benign cystic mesothelioma of the peritoneum: report of a case. J Formos Med Assoc 89:479–483
Sethna K, Mohamed F, Marchettini P, Elias D, Sugarbaker PH (2003) Peritoneal cystic mesothelioma: a case series. Tumori 89:31–35
Mishra A, Malik S, Agarwal K, Yadav A, Gautam A (2016) Benign cystic mesothelioma of uterus: an unusual cause of pelvic pain. J Obstet Gynaecol India 66:720–722. https://doi.org/10.1007/s13224-016-0917-8
Petrou G, Macindoe R, Deane S (2001) Benign cystic mesothelioma in a 60-year-old woman after cholecystectomy. ANZ J Surg 71:615–618. https://doi.org/10.1046/j.1445-2197.2001.02218.x
Gupta A, Rao HK, Pande R, Gupta S (2013) A rare case of benign multicystic peritoneal mesothelioma: a clinical dilemma. Indian J Surg 75:27–29. https://doi.org/10.1007/s12262-011-0314-6
Canbay E, Ishibashi H, Sako S, Kitai T, Nishino E, Yonemura Y (2013) Late recurrence of benign multicystic peritoneal mesothelioma complicated with an incisional hernia. Case Rep Surg 2013:903795. https://doi.org/10.1155/2013/903795
Dellaportas D, Polymeneas G, Dastamani C, Kairi-Vasilatou E, Papaconstantinou I (2012) Strangulated femoral hernia turned to be peritoneal cyst. Case Rep Surg 2012:528780. https://doi.org/10.1155/2012/528780
Asghar S, Qureshi N, Awan A (2008) Benign mesothelioma of peritoneum presenting as a pelvic mass. J Coll Physicians Surg Pak 18:723–725. https://doi.org/11.2008/JCPSP.723725
Coskun A, Guven MA, Ozdemir O, Cirakli H, Karakus S (2006) Benign cystic mesothelioma presenting as a huge pelvic mass—a case report. Eur J Gynaecol Oncol 27:621–622
Cavallaro A, Berretta M, Lo Menzo E, Cavallaro V, Zanghì A, Di Vita M, Cappellani A (2011) Cystic peritoneal mesothelioma: report of a case. Surg Today 41:141–146. https://doi.org/10.1007/s00595-010-4301-5
Kampschöer PH, Ubachs HM, Theunissen PH (1992) Benign abdominal multicystic mesothelioma. Acta Obstet Gynecol Scand 71:555–557. https://doi.org/10.3109/00016349209041451
Suh YL, Choi WJ (1989) Benign cystic mesothelioma of the peritoneum—a case report. J Korean Med Sci 4:111–115. https://doi.org/10.3346/jkms.1989.4.2.111
McCaffrey JC, Foo FJ, Dalal N, Siddiqui KH (2009) Benign multicystic peritoneal mesothelioma associated with hydronephrosis and colovesical fistula formation: report of a case. Tumori 95:808–810
Bray Madoué K, Boniface M, Annick Laure E, Pierre H (2016) Benign cystic peritoneal mesothelioma revealed by small bowel obstruction. Case Rep Radiol 2016:6728160. https://doi.org/10.1155/2016/6728160
Advincula AP, Hernandez JC (2006) Acute urinary retention caused by a large peritoneal inclusion cyst: a case report. J Reprod Med 51:202–204
Katsube Y, Mukai K, Silverberg SG (1982) Cystic mesothelioma of the peritoneum: a report of five cases and review of the literature. Cancer 50:1615–1622. https://doi.org/10.1002/1097-0142(19821015)50:8<1615::aid-cncr2820500826>3.0.co;2-k
Hong JH, Jeon S, Lee JH, Nam KH, Bae DH (2013) Multicystic benign mesothelioma of the pelvic peritoneum presenting as acute abdominal pain in a young woman. Obstet Gynecol Sci 56:126–129. https://doi.org/10.5468/OGS.2013.56.2.126
Occhionorelli S, Tartarini D, Pascale G, Maccatrozzo S, Stano R, Vasquez G (2016) Benign multicystic mesothelioma of peritoneum complicating acute appendicitis in a man: a case report. J Med Case Rep 10:44. https://doi.org/10.1186/s13256-016-0826-6
Cavallaro A, Murazio M, Modugno P, Vona A, Revelli L, Potenza AE, Colli R (2002) Benign multicystic mesothelioma of the peritoneum: a case report. Chir Ital 54:569–572
Yeom S, Son T, Hong YO (2015) Complicated benign cystic mesothelioma of mesoappendix misdiagnosed as an appendiceal abscess in a postpartum period woman. Ann Surg Treat Res 88:170–173. https://doi.org/10.4174/astr.2015.88.3.170
Moore JH Jr, Crum CP, Chandler JG, Feldman PS (1980) Benign cystic mesothelioma. Cancer 45:2395–2399. https://doi.org/10.1002/1097-0142(19800501)45:9<2395::aid-cncr2820450926>3.0.co;2-5
Singh A, Sehgal A, Mohan H (2015) Multilocular peritoneal inclusion cyst mimicking an ovarian tumor: a case report. J Midlife Health 6:39–40. https://doi.org/10.4103/0976-7800.153648
Takemoto S, Kawano R, Honda K, Nakazono A, Shimamatsu K (2012) Benign multicystic peritoneal mesothelioma mimicking recurrence of an ovarian borderline tumor: a case report. J Med Case Rep 6:126. https://doi.org/10.1186/1752-1947-6-126
Dellaportas D, Kairi-Vassilatou E, Lykoudis P, Mavrigiannaki P, Mellou S, Kleanthis CK, Kondi-Pafiti A (2012) Peritoneal mesotheliomas mimicking adnexal tumors. Clinicopathological characteristics of four cases and a short literature review. Eur J Gynaecol Oncol 33:101–104
Husain A, Ozdemirli M (2012) Benign multicystic mesothelioma with concurrent colonic adenocarcinoma: a report of two cases. Surg Today 42:978–982. https://doi.org/10.1007/s00595-011-0099-z
Testa AC, Zannoni GF, Ferrari S, Lecca A, Marana E, Marana R (2011) Benign cystic peritoneal mesothelioma incorrectly diagnosed as an ovarian borderline mucinous tumor of intestinal type at transvaginal preoperative ultrasound evaluation. Ultrasound Obstet Gynecol 37:248–250. https://doi.org/10.1002/uog.8865
Kanasugi T, Kikuchi A, Omi H, Ikeda M, Fukushima A, Sugiyama T (2001) Appendiceal mucocele and peritoneal inclusion cyst mimicking right adnexal masses: a diagnostic challenge in gynecologic practice. J Med Ultrason 40:51–55. https://doi.org/10.1007/s10396-012-0379-2
Brustmann H (2000) Multilocular peritoneal inclusion cyst with extensive xanthogranulomatous stromal changes: a differential diagnosis of cystic pelvic tumors in women. Ann Diagn Pathol 4:308–310. https://doi.org/10.1053/adpa.2000.17889
Gungor T, Cetinkaya N, Yalcin H, Ozdal B, Ozgu E, Baser E, Uygur D, Caglar M, Sirvan L, Erkaya S (2015) Retrospective evaluation of borderline ovarian tumors: single center experience of 183 cases. Arch Gynecol Obstet 291:123–130. https://doi.org/10.1007/s00404-014-3381-7
Veldhuis WB, Akin O, Goldman D, Mironov S, Mironov O, Soslow RA, Barakat RR, Hricak H (2013) Peritoneal inclusion cysts: clinical characteristics and imaging features. Eur Radiol 23:1167–1174. https://doi.org/10.1007/s00330-012-2695-8
Koo PJ, Wills JS (2009) Case 146: benign multicystic mesothelioma. Radiology 251:944–946. https://doi.org/10.1148/radiol.2513071235
Dillman JR, DiPietro MA (2009) Hemorrhagic ‘spider-in-web’: atypical appearance of a peritoneal inclusion cyst. Pediatr Radiol 39:1252. https://doi.org/10.1007/s00247-009-1295-5
Ng JW, Lau RL, Ng WF (2006) Benign multicystic peritoneal mesothelioma: a diagnostic challenge. ANZ J Surg 76:421–422. https://doi.org/10.1111/j.1445-2197.2006.03735.x
Jerraya H, Ghariani W, Blel A, Gaja A, Dziri C (2016) Benign multicystic peritoneal mesothelioma presenting as a ghost abdominal mass. Diagn Interv Imaging 97:361–363. https://doi.org/10.1016/j.diii.2015.08.005
Hove Kanstrup M, Joergensen A, Grove A (2002) Benign multicystic peritoneal mesothelioma. Acta Obstet Gynecol Scand 81:1083–1085. https://doi.org/10.1034/j.1600-0412.2002.811116.x
Jerbi M, Hidar S, Ziadi S, Khairi H (2006) Benign multicystic peritoneal mesothelioma. Int J Gynaecol Obstet 93:267–268. https://doi.org/10.1016/j.ijgo.2006.03.013
Baker PM, Clement PB, Young RH (2014) Selected topics in peritoneal pathology. Int J Gynecol Pathol 33:393–401. https://doi.org/10.1097/PGP.0000000000000146
Omeroglu A, Husain A (2001) Multilocular peritoneal inclusion cyst (benign cystic mesothelioma). Arch Pathol Lab Med 125:1123–1124. https://doi.org/10.1043/0003-9985(2001)125<1123:mpicbc>2.0.co;2
Sheffield BS, Hwang HC, Lee AF et al (2015) BAP1 immunohistochemistry and p16 FISH to separate benign from malignant mesothelial proliferations. Am J Surg Pathol 39:977–982. https://doi.org/10.1097/PAS.0000000000000394
Churg A, Galateau-Salle F (2012) The separation of benign and malignant mesothelial proliferations. Arch Pathol Lab Med 136:1217–1226. https://doi.org/10.5858/arpa.2012-0112-RA
Kato Y, Tsuta K, Seki K et al (2007) Immunohistochemical detection of GLUT-1 can discriminate between reactive mesothelium and malignant mesothelioma. Mod Pathol 20:215–220. https://doi.org/10.1038/modpathol.3800732
Shi M, Fraire AE, Chu P et al (2011) Oncofetal protein IMP3, a new diagnostic biomarker to distinguish malignant mesothelioma from reactive mesothelial proliferation. Am J Surg Pathol 35:878–882. https://doi.org/10.1097/PAS.0b013e318218985b
Cagle PT, Brown RW, Lebovitz RM (1994) p53 immunostaining in the differentiation of reactive processes from malignancy in pleural biopsy specimens. Hum Pathol 25:443–448. https://doi.org/10.1016/0046-8177(94)90115-5
Lee AF, Gown AM, Churg A (2013) IMP3 and GLUT-1 immunohistochemistry for distinguishing benign from malignant mesothelial proliferations. Am J Surg Pathol 37:421–426. https://doi.org/10.1097/PAS.0b013e31826ab1c0
Illei PB, Ladanyi M, Rusch VW, Zakowski MF (2003) The use of CDKN2A deletion as a diagnostic marker for malignant mesothelioma in body cavity effusions. Cancer 99:51–56. https://doi.org/10.1002/cncr.10923
Ladanyi M (2005) Implications of P16/CDKN2A deletion in pleural mesotheliomas. Lung Cancer 49:S95–S98. https://doi.org/10.1016/j.lungcan.2005.03.017
Murali R, Wiesner T, Scolyer RA (2013) Tumours associated with BAP1 mutations. Pathology 45:116–126. https://doi.org/10.1097/PAT.0b013e32835d0efb
Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, Wang L, Creaney J, Lake RA, Zakowski MF, Reva B, Sander C, Delsite R, Powell S, Zhou Q, Shen R, Olshen A, Rusch V, Ladanyi M (2011) The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet 43:668–672. https://doi.org/10.1038/ng.855
White AE, Harper JW (2012) Cancer. Emerging anatomy of the BAP1 tumor suppressor system. Science 337:1463–1464. https://doi.org/10.1126/science.1228463
Nasu M, Emi M, Pastorino S, Tanji M, Powers A, Luk H, Baumann F, Zhang YA, Gazdar A, Kanodia S, Tiirikainen M, Flores E, Gaudino G, Becich MJ, Pass HI, Yang H, Carbone M (2015) High Incidence of Somatic BAP1 alterations in sporadic malignant mesothelioma. J Thorac Oncol 10:565–576. https://doi.org/10.1097/JTO.0000000000000471
Cigognetti M, Lonardi S, Fisogni S, Balzarini P, Pellegrini V, Tironi A, Bercich L, Bugatti M, Rossi G, Murer B, Barbareschi M, Giuliani S, Cavazza A, Marchetti G, Vermi W, Facchetti F (2015) BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod Pathol 28:1043–1057. https://doi.org/10.1038/modpathol.2015.65
Chung CT, Santos Gda C, Hwang DM, Ludkovski O, Pintilie M, Squire JA, Tsao MS (2010) FISH assay development for the detection of p16/CDKN2A deletion in malignant pleural mesothelioma. J Clin Pathol 63:630–634. https://doi.org/10.1136/jcp.2010.076794
Pillappa R, Maleszewski JJ, Sukov WR, Bedroske PP, Greipp PT, Boland JM, Yi ES, Peikert T, Aubry MC, Roden AC (2018) Loss of BAP1 expression in atypical mesothelial proliferations helps to predict malignant mesothelioma. Am J Surg Pathol 42:256–263. https://doi.org/10.1097/PAS.0000000000000976
Hida T, Hamasaki M, Matsumoto S, Sato A, Tsujimura T, Kawahara K, Iwasaki A, Okamoto T, Oda Y, Honda H, Nabeshima K (2016) BAP1 immunohistochemistry and p16 FISH results in combination provide higher confidence in malignant pleural mesothelioma diagnosis: ROC analysis of the two tests. Pathol Int 66:563–570. https://doi.org/10.1111/pin.12453
Buhling KJ, Lezon S, Eulenburg C, Schmalfeldt B (2017) The role of transvaginal ultrasonography for detecting ovarian cancer in an asymptomatic screening population: a systematic review. Arch Gynecol Obstet 295:1259–1268. https://doi.org/10.1007/s00404-017-4346-4
Jeong JY, Kim SH (2001) Sclerotherapy of peritoneal inclusion cysts: preliminary results in seven patients. Korean J Radiol 2:164–170. https://doi.org/10.3348/kjr.2001.2.3.164
Jacobs I, Oram D, Fairbanks J, Turner J, Frost C, Grudzinskas JG (1990) A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br J Obstet Gynaecol 97:922–929. https://doi.org/10.1111/j.1471-0528.1990.tb02448.x
Jiang ZH, Li KT, Tian JW, Ren M (2016) An overview of the development and application of the sonographic scoring system: differentiation of malignant from benign ovarian tumors. Arch Gynecol Obstet 293:303–310. https://doi.org/10.1007/s00404-015-3957-x
Lee R, Tong A, Kurtis B, Gilet AG (2016) Benign multicystic peritoneal mesothelioma: AIRP best cases in radiologic-pathologic correlation. Radiographics 36:407–411. https://doi.org/10.1148/rg.2016150157
Mehta V, Chowdhary V, Sharma R, Golia Pernicka JS (2017) Imaging appearance of benign multicystic peritoneal mesothelioma: a case report and review of the literature. Clin Imaging 42:133–137. https://doi.org/10.1016/j.clinimag.2016.10.008
Guerriero S, Ajossa S, Mais V, Angiolucci M, Paoletti AM, Melis GB (2004) Role of transvaginal sonography in the diagnosis of peritoneal inclusion cysts. J Ultrasound Med 23:1193–1200. https://doi.org/10.7863/jum.2004.23.9.1193
Sohaey R, Gardner TL, Woodward PJ, Peterson CM (1995) Sonographic diagnosis of peritoneal inclusion cysts. J Ultrasound Med 14:913–917. https://doi.org/10.7863/jum.1995.14.12.913
Bharwani N, Crofton ME (2013) Peritoneal pseudocysts: aetiology, imaging appearances, and natural history. Clin Radiol 68:828–836. https://doi.org/10.1016/j.crad.2013.03.006
Kim JS, Lee HJ, Woo SK, Lee TS (1997) Peritoneal inclusion cysts and their relationship to the ovaries: evaluation with sonography. Radiology 204:481–484. https://doi.org/10.1148/radiology.204.2.9240539
Kiseli M, Caglar GS, Cengiz SD, Karadag D, Yılmaz MB (2012) Clinical diagnosis and complications of paratubal cysts: review of the literature and report of uncommon presentations. Arch Gynecol Obstet 285:1563–1569. https://doi.org/10.1007/s00404-012-2304-8
Heller DS (2016) Lesions of the pouch of Douglas: a review. J Minim Invasive Gynecol 23:28–33. https://doi.org/10.1016/j.jmig.2015.08.878
Vetvicka V, Laganà AS, Salmeri FM, Triolo O, Palmara VI, Vitale SG, Sofo V, Králíčková M (2016) Regulation of apoptotic pathways during endometriosis: from the molecular basis to the future perspectives. Arch Gynecol Obstet 294:897–904. https://doi.org/10.1007/s00404-016-4195-6
Liaci AL, Boesmueller H, Huebner M, Brucker SY, Reisenauer C (2017) Perivaginal benign masses: diagnosis and therapy in a series of 66 women. Arch Gynecol Obstet 295:367–374. https://doi.org/10.1007/s00404-016-4234-3
Meinhold-Heerlein I, Fotopoulou C, Harter P, Kurzeder C, Mustea A, Wimberger P, Hauptmann S, Sehouli J (2016) The new WHO classification of ovarian, fallopian tube, and primary peritoneal cancer and its clinical implications. Arch Gynecol Obstet 293:695–700. https://doi.org/10.1007/s00404-016-4035-8
Rosenberg P, Nappi L, Santoro A, Bufo P, Greco P (2011) Pelvic mass-like florid cystic endosalpingiosis of the uterus: a case report and a review of literature. Arch Gynecol Obstet 283:519–523. https://doi.org/10.1007/s00404-010-1700-1
Hidvégi J, Schneider F, Rohonyi B, Flautner L, Szlávik L (1991) Peritoneal benign cystic mesothelioma. Pathol Res Pract 187:103–106. https://doi.org/10.1016/s0344-0338(11)81052-6 (discussion 106–108)
Kagalwala DZ, Shankar S, Zota V, Sandor A, Litwin DE (2005) Preoperative computed tomography-guided hookwire needle localization of a peritoneal multilocular inclusion cyst. J Comput Assist Tomogr 29:602–603. https://doi.org/10.1097/01.rct.0000174377.56134.8b
Romero JA, Kim EE, Kudelka AP, Edwards CL, Kavanagh JJ (1994) MRI of recurrent cystic mesothelioma: differential diagnosis of cystic pelvic masses. Gynecol Oncol 54:377–380. https://doi.org/10.1006/gyno.1994.1227
O’Neil JD, Ros PR, Storm BL, Buck JL, Wilkinson EJ (1989) Cystic mesothelioma of the peritoneum. Radiology 170:333–337. https://doi.org/10.1148/radiology.170.2.2643136
Hasan AK, Sinclair DJ (1993) Case report: calcification in benign cystic peritoneal mesothelioma. Clin Radiol 48:66–67. https://doi.org/10.1016/S0009-9260(05)80113-8
Assaly M, Bongiovanni M, Kumar N, Egger JF, Pelte MF, Genevay M, Finci V, Tschanz E, Pache JC (2008) Cytology of benign multicystic peritoneal mesothelioma in peritoneal washings. Cytopathology 19:224–228. https://doi.org/10.1111/j.1365-2303.2007.00489.x
Samson P, Cacala S (2005) Rare case of benign multicystic peritoneal mesothelioma. ANZ J Surg 75:619–620. https://doi.org/10.1111/j.1445-2197.2005.03448.x
Baddoura FK, Varma VA (1990) Cytologic findings in multicystic peritoneal mesothelioma. Acta Cytol 34:524–528
Lee SW, Lee SJ, Jang DG, Yoon JH, Kim JH (2012) Comparison of laparoscopic and laparotomic surgery for the treatment of peritoneal inclusion cyst. Int J Med Sci 9:14–19. https://doi.org/10.7150/ijms.9.14
Holtzman RN, Heymann AD, Bordone F, Marinoni G, Barillari P, Wahl SJ (2001) Carbohydrate antigen 19-9 and carcinoembryonic antigen immunostaining in benign multicystic mesothelioma of the peritoneum. Arch Pathol Lab Med 125:944–947. https://doi.org/10.1043/0003-9985(2001)125<0944:caacai>2.0.co;2
Walz MK, Metz KA, Sastry M, Eigler FW, Leder LD (1994) Benign mesothelial splenic cyst may cause high serum concentration of CA 19-9. Eur J Surg 160:389–391
Almudévar Bercero E, García-Rostán Pérez GM, García Bragado F, Jiménez C (1997) Prognostic value of high serum levels of CA-125 in malignant secretory peritoneal mesotheliomas affecting young women. A case report with differential diagnosis and review of the literature. Histopathology 31:267–273. https://doi.org/10.1046/j.1365-2559.1997.2510855.x
Yokoyama N, Yasuda R, Ichida K, Murakoshi H, Okada J, Yoshida S, Motoyama S (2014) Recurrent peritoneal inclusion cysts successfully treated with oral contraceptives: a report of two cases. Clin Exp Obstet Gynecol 41:83–86
Vitale SG, La Rosa VL, Rapisarda AM, Laganà AS (2017) Comment on: “Anxiety and depression in patients with advanced ovarian cancer: a prospective study”. J Psychosom Obstet Gynaecol 38:83–84. https://doi.org/10.1080/0167482X.2016.1244182
Gussman D, Thickman D, Wheeler JE (1986) Postoperative peritoneal cysts. Obstet Gynecol 68:53S–55S
Kurachi H, Murakami T, Maeda T, Tsuda K, Higashiguchi O, Nakamura H, Miyake A (1996) Value of gonadotropin-releasing hormone agonist in diagnosing peritoneal pseudocysts. Acta Obstet Gynecol Scand 75:294–297. https://doi.org/10.3109/00016349609047105
Lipitz S, Seidman DS, Schiff E, Achiron R, Menczer J (1995) Treatment of pelvic peritoneal cysts by drainage and ethanol instillation. Obstet Gynecol 86:297–299. https://doi.org/10.1016/0029-7844(95)00157-M
Kairaluoma MI, Leinonen A, Ståhlberg M, Päivänsalo M, Kiviniemi H, Siniluoto T (1989) Percutaneous aspiration and alcohol sclerotherapy for symptomatic hepatic cysts. An alternative to surgical intervention. Ann Surg 210:208–215. https://doi.org/10.1097/00000658-198908000-00012
Lim HK, Cho JY, Kim SH (2010) Sclerotherapy of peritoneal inclusion cysts: a long-term evaluation study. Abdom Imaging 35:431–436. https://doi.org/10.1007/s00261-009-9538-3
Kemp AM, Nayar R, De Frias D, Lin X (2010) Cytomorphologic characteristics of fine needle core biopsy of multicystic peritoneal mesothelioma: a case report and review of the literature. Diagn Cytopathol 38:192–197. https://doi.org/10.1002/dc.2119
Inman DS, Lambert AW, Wilkins DC (2000) Multicystic peritoneal inclusion cysts: the use of CT guided drainage for symptom control. Ann R Coll Surg Engl 82:196–197
van der Klooster JM, Lambers MD, van Bommel EF, Scholten PC (1997) Successful catheter drainage of recurrent benign multicystic mesothelioma of the peritoneum. Neth J Med 50:246–249. https://doi.org/10.1016/S0300-2977(97)00007-7
Nezhat FR, DeNoble SM, Brown DN, Shamshirsaz A, Hoehn D (2010) Laparoscopic management of peritoneal mesothelioma associated with pelvic endometriosis. J Minim Invasive Gynecol 17:646–650. https://doi.org/10.1016/j.jmig.2010.03.025
Bellia A, Vitale SG, Laganà AS, Cannone F, Houvenaeghel G, Rua S, Ladaique A, Jauffret C, Ettore G, Lambaudie E (2016) Feasibility and surgical outcomes of conventional and robot-assisted laparoscopy for early-stage ovarian cancer: a retrospective, multicenter analysis. Arch Gynecol Obstet 294:615–622. https://doi.org/10.1007/s00404-016-4087-9
Ricci F, Borzellino G, Ghimenton C, Cordiano C (1995) Benign cystic mesothelioma in a male patient: surgical treatment by the laparoscopic route. Surg Laparosc Endosc 5:157–160
Birch DW, Park A, Chen V (1998) Laparoscopic resection of an intra-abdominal cystic mass: a cystic mesothelioma. Can J Surg 41:161–164
Saad S, Brockmann M, Maegele M (2007) Benign peritoneal multicystic mesothelioma diagnosed and treated by laparoscopic surgery. J Laparoendosc Adv Surg Tech A 17:649–652. https://doi.org/10.1089/lap.2006.0230
Limone A, Maier J, Chiantera V, Elezkurtaj S, Foss HD, Schneider A (2010) Laparoscopic excision of a benign peritoneal cystic mesothelioma. Arch Gynecol Obstet 281:577–578. https://doi.org/10.1007/s00404-009-1203-0
Sizzi O, Rosetti A, Torcia F, Lo Cane F, Loddo A (2011) Laparoscopic treatment of benign multicystic mesothelioma. J Minim Invasive Gynecol 18:287. https://doi.org/10.1016/j.jmig.2010.03.023
Trehan A, Trehan AK (2014) Peritoneal inclusion cyst with an unusual presentation treated by laparoscopic peritonectomy. J Obstet Gynaecol 34:112–113. https://doi.org/10.3109/01443615.2013.835306
Witek TD, Marchese JW, Farrell TJ (2014) A recurrence of benign multicystic peritoneal mesothelioma treated through laparoscopic excision: a case report and review of the literature. Surg Laparosc Endosc Percutan Tech 24:e70–e73. https://doi.org/10.1097/SLE.0b013e31828f7269
Vitale SG, Marilli I, Lodato M, Tropea A, Cianci A (2013) The role of cytoreductive surgery in advanced-stage ovarian cancer: a systematic review. Updates Surg 65:265–270. https://doi.org/10.1007/s13304-013-0213-4
Khuri S, Gilshtein H, Abboud W, Assalia A, Kluger Y (2012) Benign cystic mesothelioma of the peritoneum: a rare case and review of the literature. Case Rep Oncol 5:667–670. https://doi.org/10.1159/000346187
Baratti D, Kusamura S, Sironi A, Cabras A, Fumagalli L, Laterza B, Deraco M (2008) Multicystic peritoneal mesothelioma treated by surgical cytoreduction and hyperthermic intra-peritoneal chemotherapy (HIPEC). In Vivo 22:153–157
Murro D, Harbhajanka A, Mahon B, Deziel D (2014) Benign cystic mesothelioma associated with ipsilateral renal agenesis: a case report and review of literature. Pediatr Dev Pathol 17:487–490. https://doi.org/10.2350/14-06-1510-CR.1
Stojsic Z, Jankovic R, Jovanovic B, Vujovic D, Vucinic B, Bacetic D (2012) Benign cystic mesothelioma of the peritoneum in a male child. J Pediatr Surg 47:e45–e49. https://doi.org/10.1016/j.jpedsurg.2012.06.029
Al-Safi ZA, Edil BH, Post MD, Pearlman NW, Alvero R (2014) Fertility preservation in a patient with benign multicystic peritoneal mesothelioma. J Surg Oncol 110:372–374. https://doi.org/10.1002/jso.23653
Vallerie AM, Hsieh T, Baxi LV (2008) Peritoneal inclusion cyst: effects on fertility and antepartum course. Obstet Gynecol 112:498–500. https://doi.org/10.1097/AOG.0b013e3181809e5c
Acknowledgements
A thoughtful thanks to Mr. Antony Bridgewood for his support in the linguistic review process.
Funding
This study was not supported by any Grant/fund.
Author information
Authors and Affiliations
Contributions
AMCR: Project administration, Writing—original draft. AC: Writing—review and editing. SC: Writing—review and editing. SGV: Project administration, Writing—original draft. GV: Data curation. EP: Data curation. SC: Writing—original draft, Supervision.
Corresponding author
Ethics declarations
Research involving animals
This article does not contain any studies with animals performed by any of the Authors.
Conflict of interest
The authors have no proprietary, financial, professional or other personal interest of any nature in any product, service or company mentioned in this study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the subject included in the study.
Rights and permissions
About this article
Cite this article
Rapisarda, A.M.C., Cianci, A., Caruso, S. et al. Benign multicystic mesothelioma and peritoneal inclusion cysts: are they the same clinical and histopathological entities? A systematic review to find an evidence-based management. Arch Gynecol Obstet 297, 1353–1375 (2018). https://doi.org/10.1007/s00404-018-4728-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-018-4728-2