Abstract
Purpose
To evaluate published evidence in the literature on compartment syndrome (CS) in association with gynecologic surgery and to establish postoperative normal values for serum creatine kinase (CK) and myoglobin.
Methods
The present study consists of a case report of a patient with CS, a systematic review including 37 studies and 86 patients with CS, and a retrospective cohort study of 300 patients undergoing various types of laparoscopy for benign or malignant diseases in order to establish postoperative normal values.
Results
We report on a patient with early-stage ovarian cancer, who developed CS after laparoscopic surgery with massively elevated serum CK and myoglobin levels, i.e., 1109 U/L and 18151 µg/L, respectively. In our systematic review, median serum CK and myoglobin levels among women with CS were 19,223 (177–27,412) U/L and 1248 (285–1360) µg/L, respectively. In our cohort study, the median postoperative serum CK and myoglobin levels were 68 (14–1576) U/L and 45 (14–1040) µg/L, respectively. The 95th and 99th percentile of serum CK and myoglobin levels were 158 and 391.5 U/L, and 152.3 and 298.9 µg/L, respectively.
Conclusion
Markedly elevated postoperative serum levels of CK and myoglobin levels might raise the suspicion for CS and could therefore aid in the rapid diagnosis of CS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The term compartment syndrome (CS) describes a state of substantial tissue damage to muscles and nerves in response to a pathologic pressure increase within a confined inelastic space [1]. CS may develop in various anatomical locations and is mostly an acute event, progressing rapidly in response to an injury or a prolonged state of constant pressure [2]. Events typically associated with CS are skeletal trauma, muscular trauma, and fluid accumulation within a confined space such as hemoperitoneum [1, 2]. However, CS can also occur after prolonged surgical procedures mostly in patients aligned in a lithotomy position. While some cases of abdominal CS in the field of obstetrics and gynecology have been reported [3], most cases after gynecologic surgeries occur in otherwise healthy legs, also termed, well leg CS [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21]. CS can lead to a loss of function of the affected leg, amputation, or even death by subsequent multi-organ failure [1, 2].
CS is a rare but devastating event in gynecologic surgery. Due to its rarity, the incidence of CS can only be estimated on the base of case reports, reviews, and questionnaires. It has been reported to vary between 0.028% for gynecologic surgeries in general and 0.067–0.38% for surgeries lasting more than 180 min, in particular [2]. Surgeons have to be aware of CS as a potentially lethal complication of gynecologic surgery, especially after complex and long procedures. Prior to such surgery, patients therefore need to be counseled accordingly. In addition, prevention strategies such as compression stockings and time limits of surgical procedures may be applied, although robust scientific evidence for their effectiveness is lacking [1, 2].
A number of risk factors for the development of CS have been identified including lithotomy position, prolonged duration of surgery, intraoperative hypotension and hypovolemia, cardiovascular disease, and diabetes mellitus [1, 2, 4,5,6,7,8]. Regarding the duration of surgery, it has been shown that while some cases of CS might occur after short-lasting surgical procedures, most cases of CS have been observed in surgical procedures >180 min, suggesting that this is a reasonable threshold for defining an elevated risk of CS [1, 2].
Intraoperative and postoperative monitoring for early signs of CS including measurement of intracompartmental pressure (ICP) has been suggested, although based on the invasiveness of the intervention and the rarity of CS, the number needed to treat is enormously high. Thus, measurement of ICP has not been adopted into clinical practice. Suspicion for CS is mostly raised clinically, i.e., when typical signs and symptoms occur such as severe and otherwise unexplainable leg pain. Other symptoms are pain on passive stretch in the involved compartment, paresthesia and, in rare cases and at late stages of CS, pallor and paresis of extremities [22].
Measurement of serum parameters would be an ideal tool for both the risk evaluation and the early diagnosis of CS, especially in sedated patients. Creatine kinase (CK) is a marker of damage of CK-rich muscle tissues such as in myocardial infarction, rhabdomyolysis, muscular dystrophy, or autoimmune myositis [23]. Myoglobin is an iron- and oxygen-binding protein found in the muscle tissue and is the primary oxygen-carrying pigment. Elevated serum levels of myoglobin are exclusively found in the bloodstream after muscle injury [24]. Preliminary data in the literature suggest that both CK and myoglobin are elevated in patients with CS [5, 10, 20].
In our department, we observed a case of CS in September 2015 in a patient with early-stage ovarian cancer. After laparoscopic staging with omentectomy, appendectomy, peritoneal biopsies, pelvic and paraaortic lymphadenectomy, CS was suspected based on severe pain in the lower extremities. ICP, serum CK, and myoglobin were elevated, and prompt fasciotomy was initiated. Based on this index event, we discussed the measurement of serum CK and myoglobin as potential early detection markers of CS and included the measurement of both serum parameters after laparoscopy into our daily clinical practice.
We became aware of the fact that there are no established postoperative CK and myoglobin normal values in order to reliably discriminate between normal and elevated levels indicating an increased risk of CS. Therefore, we performed a systematic review of the literature on CS after gynecologic surgeries focusing on serum CK and myoglobin levels and analyzed CK and myoglobin serum levels in our series of patients undergoing gynecologic laparoscopy.
Materials and methods
We performed a systematic literature search of the databases PubMed and Cochrane Central Register of Controlled Trials (search date 19-01-2017) using search terms related to CS and gynecologic surgery. All abstracts of the studies identified by this search strategy were screened for the inclusion criteria of this literature review, i.e., quantitative reports of women undergoing gynecologic surgery and occurrence of CS in the described patients or in a population of described patients. Studies not reporting on CS or studies reporting on CS after non-gynecologic surgeries were excluded. Subsequently, we retrieved the full-text versions of all studies fulfilling the inclusion criteria. These studies were then analyzed in full. Studies were categorized into case reports, retrospective cases series, retrospective cohort studies, and prospective cohort studies. Authors were not contacted to acquire further information. We included a case report of a patient diagnosed with CS in our department.
In a monocentric cohort study, we included a series of 300 consecutive patients undergoing gynecologic laparoscopy. There were no inclusion or exclusion criteria other than undergoing a gynecologic laparoscopy. The institutional review of the Ordensklinikum Linz approved the present study [Institutional review board (IRB) number 3/2017]. All 300 patients were treated at the Ordensklinikum Linz, Linz, Austria, between November 2015 and January 2017. Patients undergoing laparoscopy were operated upon in general anesthesia in dorsal lithotomy position. The time of the beginning of surgery and the time of the end of surgery were documented according to our department’s standard operating procedures.
Serum CK and myoglobin levels were measured after surgery using commercially available test kits (G65502, Abbott Laboratories Diagnostics Division, Abbott Park, IL, and G59014, Abbott Laboratories Diagnostics Division, Abbott Park, IL, USA). Assays were run on the ARCHITECT c System™. The manufacturer claims normal values of 1–145 U/L and 20–106 µg/L for serum CK and myoglobin levels, respectively.
Statistics
After testing for normality using the Kolmogorov–Smirnov test, values are given as medians (range). The 95th and 99th percentile with the respective 95% confidence intervals are provided. Parameters between two groups were compared using Mann–Whitney U tests. Parameters between more than two groups were compared using one-way ANOVA on ranks. In a univariate analysis, continuous parameters were compared using Pearson’s correlation coefficient. In a multivariate analysis, continuous parameters were compared using Cox regression analysis. p values of <0.05 were considered statistically significant. For statistical analysis, we used SPSS statistical software system (SPSS 23.0; SPSS Inc., Chicago, IL, USA).
Results
Case report
A 45-year-old patient with a unilateral adnexal tumor underwent unilateral adnexectomy demonstrating serous ovarian cancer FIGO stage I, G1. Subsequently, 7 days later, a laparoscopic staging with omentectomy, appendectomy, peritoneal biopsies, pelvic and paraaortic lymphadenectomy was performed in September 2015. The duration of surgery was 5 h and 16 min. Postoperatively, the patient developed severe pain in both lower legs. Serum CK and myoglobin levels were massively elevated, i.e., 1109 U/L and 18151 µg/L, respectively. Measurement of intracompartment pressure showed a value of 70 mmHg. The diagnosis of an acute compartment syndrome was established, and immediate fasciotomy in both lower legs was performed (Fig. 1). Based on this index event, we performed serum CK and myoglobin measurements routinely after all laparoscopies starting in November 2015.
Systematic review of the literature
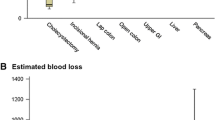
In a systematic literature search of the databases PubMed and Cochrane Central Register of Controlled Trials (search date 19-01-2017) using the search terms “gynecologic surgical procedures” (MeSH Terms) OR [“gynecologic” (All Fields) AND “surgical” (All Fields) AND “procedures” (All Fields)] OR “gynecologic surgical procedures” (All Fields) OR [“gynecologic” (All Fields) AND “surgery” (All Fields)] OR “gynecologic surgery” (All Fields)] AND [“compartment syndromes” (MeSH Terms) OR [“compartment” (All Fields) AND “syndromes” (All Fields)] OR “compartment syndromes” (All Fields) OR [“compartment” (All Fields) AND “syndrome” (All Fields)] OR “compartment syndrome” (All Fields), we identified 45 studies. After screening all abstracts of these studies, 21 citations were identified reporting on CS after gynecologic surgeries [1, 2, 5, 10, 11, 16,17,18, 25,26,27,28,29,30,31,32,33,34,35,36,37]. Studies not reporting on CS or studies reporting on CS after non-gynecologic surgeries were excluded. Cross-reference searching identified a further 16 studies [3, 4, 6,7,8,9, 12,13,14,15, 20, 38,39,40,41,42]. Thus, in summary, 37 studies were selected for this review. Among the 37 studies, we found 29 case reports, five retrospective cohort studies, one survey, and one narrative review. One publication contained a case report and a retrospective cohort study. Figure 2 shows a diagram of the literature search. Table 1 shows the study characteristics and outcomes of patients with CS after gynecologic laparoscopy described in all 37 studies. The total number of women described in these 37 studies was 86. 46 of these women developed CS after open gynecologic surgery or without surgery and 40 of these women developed CS after gynecologic laparoscopy. The median duration of surgery was 5.4 (0.5–12) h. Most, but not all patients [49/86 (57%)], were placed in a dorsal lithotomy position during surgery. Fasciotomy with or without necrosectomy was the most common treatment, performed in 30/86 (35%) cases. The median CK and myoglobin serum levels among women with CS were 19,223 (177–27,412) U/L and 1248 (285–1360) µg/L, respectively. We identified two cases with a fatal outcome. However, in both cases, the fatal outcome was related to causes other than CS, namely liver cirrhosis and hemorrhage. Therefore, the mortality of CS in the literature was zero. However, the long-term morbidity among all reported cases was considerable with 14/86 (16%) women reporting permanent neurologic sequelae after CS.
Evaluation of serum CK and myoglobin
Characteristics of patients and performed surgeries are shown in Table 2. Median (range) duration of surgery was 56 (7–306) min. Duration of surgery was longer than 180 min in 34 patients (11.3%). Overall, median CK and myoglobin levels were 68 (14–1576) U/L and 45 (14–1040) µg/L, respectively. The 95th and 99th percentile of serum CK levels were 158 (142.8–202) U/L and 391.5 (259.3–2427.3) U/L, respectively. The 95th and 99th percentile of serum myoglobin levels were 152.3 (121.8–206.3) µg/L and 298.9 (213.4–1577.1) µg/L, respectively. In our series, the highest postoperative serum CK and myoglobin levels were 1576 U/L and 1040 µg/L, respectively, and were observed in a patient with metastasized malignant disease, followed by serum CK and myoglobin levels of 403 U/L and 300 µg/L, respectively, as the second highest serum levels in patients without developing CS. Clinical and serological parameters grouped according to duration of surgery< vs. ≥180 min are shown in Table 3. Serum myoglobin, but not CK, levels were significantly elevated in patients undergoing surgeries ≥180 min. Considering variables as continuous parameters, duration of surgery and patients’ age were correlated with serum myoglobin, but not CK, levels in a univariate and multivariate analysis (Table 4).
Discussion
In a systematic review of the literature, we identified 86 women with CS after gynecologic surgery with 40 of these occurring after gynecologic laparoscopy. The median duration of surgery was 5.4 (0.5–12) h, and the median CK and myoglobin serum levels were 19,223 (177–27,412) U/L and 1248 (285–1360) µg/L, respectively. The long-term morbidity was considerable with 14/86 (16%) women reporting permanent neurologic sequelae. In addition, we have established normal postoperative serum levels of CK and myoglobin in a cohort study of women undergoing gynecologic laparoscopy.
A number of previously published papers are focusing on the reduction of complications in gynecologic surgery [43, 44]. Occurrence of CS is a very rare but devastating event in gynecologic surgery. Prevention strategies for a CS include minimization of operating time in lithotomy position, intermittent mobilization of legs during surgery, use of vacuum mattresses in order to prevent malposition of the patient during surgery, lowering the legs below the level of the right cardiac atrium, and intermittent compression devices [45]. Although the benefits of these interventions have not been proven in prospective, randomized trials, expert opinion suggests incorporating these procedures into daily clinical practice [45]. Furthermore, it has been stressed that gynecologists should be aware of this rare complication.
The diagnosis of a CS is notoriously difficult and can only be achieved by the invasive measurement of ICP. Predictive markers for the development of CS are warranted, because they would be less invasive and easily evaluable in clinical practice. Raising the suspicion of postoperative CS by means of a readily available, cheap, and noninvasive serum test could lead to a more rapid diagnosis and a better prognosis of affected patients, because the success of therapy, i.e., fasciotomy, is negatively associated with the elapsed time after surgery [1, 2].
Serum parameters are commonly used as predictive and prognostic parameters. Serum CK and myoglobin levels have been shown to be elevated in patients with CS. In our index patient, both serum parameters were markedly elevated. Although normal values for serum CK and myoglobin levels are readily available, we have no data concerning postoperative normal values. It can reasonably be hypothesized that established normal values, i.e., preoperative values, cannot be used for postoperative prediction/exclusion of compartment syndrome.
Postoperative serum myoglobin, but not CK, levels were found to be correlated with duration of surgery and patients’ age. In a multivariate analysis duration of surgery and patients’ age were associated with serum myoglobin levels. In our series of 300 patients undergoing gynecologic laparoscopy, only one patient had serum CK and myoglobin levels greater than 1000 U/L or 1000 µg/L, respectively. Of note, this was a patient with metastasized malignant disease undergoing diagnostic laparoscopy. ICP values in this patient were normal. This observation suggests that the normal values established in our study may not apply to this category of patients.
Measurement of serum parameters indicative of CS might be especially valuable in patients who are not able to report pain, swelling, or other symptoms, because they are sedated or suffer from dementia. When measurement of serum CK and myoglobin levels is only performed in patients at “high risk” of CS, it can be hypothesized that only few patients will present with markedly elevated serum levels possibly making measurement of ICP necessary, whereas the risk of CS with “normal values” of CK and myoglobin seems low. Thus, we suggest to adopt a risk-based approach with the use of postoperative CK and myoglobin measurements only in patients after prolonged surgery or those with a combination of other risk factors such as intraoperative hypotension, hypovolemia, cardiovascular disease, and diabetes mellitus.
We have shown in a literature review that cases of CS are usually correlated with a marked increase in CK and myoglobin levels and have established upper limits of postoperative CK and myoglobin serum levels after gynecologic laparoscopy. Although it would not be prudent to recommend a general screening for CS after laparoscopy due to the rarity of the event, such measurements might be reasonable in patients at risk of CS. Markedly elevated CK and myoglobin serum levels should raise the suspicion of this devastating condition.
References
Bauer EC, Koch N, Janni W, Bender HG, Fleisch MC (2014) Compartment syndrome after gynecologic operations: evidence from case reports and reviews. Eur J Obstet Gynecol Reprod Biol 173:7–12
Bauer EC, Koch N, Erichsen CJ, Juettner T, Rein D, Janni W et al (2014) Survey of compartment syndrome of the lower extremity after gynecological operations. Langenbecks Arch Surg 399:343–348
Veisi F, Zangeneh M, Malekkhosravi S, Rezavand N (2013) Abdominal compartment syndrome due to OHSS. J Obstet Gynaecol India 63:350–353
Chikazawa K, Netsu S, Akashi K, Suzuki Y, Konno R, Motomatsu S (2016) Delayed diagnosis of single compartment muscle contusion after radical hysterectomy in the lithotomy position: a case report. Int J Surg Case Rep 26:199–201
Stornelli N, Wydra FB, Mitchell JJ, Stahel PF, Fabbri S (2016) The dangers of lithotomy positioning in the operating room: case report of bilateral lower extremity compartment syndrome after a 90-min surgical procedure. Patient Saf Surg 10:18
Boesgaard-Kjer DH, Boesgaard-Kjer D, Kjer JJ (2013) Well-leg compartment syndrome after gynecological laparoscopic surgery. Acta Obstet Gynecol Scand 92:598–600
Radosa JC, Radosa MP, Sütterlin M (2011) Acute lower limb compartment syndrome after cesarean section: a case report. J Med Case Rep 5:161
Roman H, Rozsnayi F, Puscasiu L, Resch B, Belhiba H, Lefebure B et al (2010) Complications associated with two laparoscopic procedures used in the management of rectal endometriosis. JSLS 14:169–177
Tomassetti C, Meuleman C, Vanacker B, D’Hooghe T (2009) Lower limb compartment syndrome as a complication of laparoscopic laser surgery for severe endometriosis. Fertil Steril 92:2038.e9-12
Nakamura K, Aoki H, Hirakawa T, Murata T, Kanuma T, Minegishi T (2008) Compartment syndrome with thrombosis of common iliac artery after gynecologic surgery. Obstet Gynecol 112:486–488
Soltsman S, Russo P, Greenshpun A, Ben-Ami M (2008) Abdominal compartment syndrome after laparoscopic salpingectomy for ectopic pregnancy. J Minim Invasive Gynecol 15:508–510
Olah KS (2008) Adductor compartment syndrome: an unusual complication of the trans-obturator tape procedure. J Obstet Gynaecol 28:363–364
Yanazume S, Yanazume Y, Iwamoto I, Tsuji T, Yoshinaga M, Douchi T (2006) Severe leg compartment syndrome associated with dorsal lithotomy position during radical hysterectomy. J Obstet Gynaecol Res 32:610–612
Cohen SA, Hurt WG (2001) Compartment syndrome associated with lithotomy position and intermittent compression stockings. Obstet Gynecol 97:832–833
von Gruenigen VE, Coleman RL, King MR, Miller DS (1999) Abdominal compartment syndrome in gynecologic surgery. Obstet Gynecol 94:830–832
Honda T, Tokushige M, Uda S, Egawa H, Suginami H (1995) A case of laparoscopic complication: injury of the left common iliac vessels and subsequent acute compartment syndrome of the left leg. J Obstet Gynaecol 21:273–275
Schwartz LB, Stahl RS, DeCherney AH (1993) Unilateral compartment syndrome after prolonged gynecologic surgery in the dorsal lithotomy position. A case report. J Reprod Med 38:469–471
Adler LM, Loughlin JS, Morin CJ, Haning RV Jr (1990) Bilateral compartment syndrome after a long gynecologic operation in the lithotomy position. Am J Obstet Gynecol 162:1271–1272
Beraldo S, Dodds SR (2006) Lower limb acute compartment syndrome after colorectal surgery in prolonged lithotomy position. Dis Colon Rectum 49:1772–1780
Wassenaar EB, van den Brand JG, van der Werken C (2006) Compartment syndrome of the lower leg after surgery in the modified lithotomy position: report of seven cases. Dis Colon Rectum 49:1449–1453
Chase J, Harford F, Pinzur MS, Zussman M (2000) Intraoperative lower extremity compartment pressures in lithotomy-positioned patients. Dis Colon Rectum 43:678–680
Mabee JR, Bostwick TL (1993) Pathophysiology and mechanisms of compartment syndrome. Orthop Rev 22:175–181
Wallimann T, Hemmer W (1994) Creatine kinase in non-muscle tissues and cells. Mol Cell Biochem 133:193–220
Ordway GA, Garry DJ (2004) Myoglobin: an essential hemoprotein in striated muscle. J Exp Biol 207:3441–3446
Na Q, Liu CX, Cui H, Chen J, Liu SS, Li QL (2012) Successful treatment of two patients with postpartum disseminated intravascular coagulation complicated by abdominal compartment syndrome. Gynecol Obstet Invest 73(4):337–340
Sanda RB, Aziz R, Bhutto A, Seliem SI (2011) Abdominal compartment syndrome complicating massive hemorrhage from an unusual presentation of ruptured ectopic pregnancy. Ann Afr Med 10(3):252–255
Zavras N, Christianakis E, Ereikat K, Mpourikas D, Velaoras K, Alexandrou J (2012) Intracranial hypertension secondary to abdominal compartment syndrome in a girl with giant ovarian cystic mass. Indian J Pediatr 79(4):541–542
Lawrenz B, Kraemer B, Wallwiener D, Witte M, Fehm T, Becker S (2011) Lower extremity compartment syndrome after laparoscopic radical hysterectomy: brief report of an unusual complication of laparoscopic positioning requirements. J Minim Invasive Gynecol 18(4):531–533
Deras P, Amraoui J, Boutin C, Laporte S, Ripart J (2010) Rhabdomyolysis and compartment syndrome of two forearms after robotic assisted prolonged surgery. Ann Fr Anesth Reanim 29(4):301–303
Lee KC, Kim HY, Lee MJ, Koo JW, Lim JA, Kim SH (2010) Abdominal compartment syndrome occurring due to uterine perforation during a hysteroscopy procedure. J Anesth 24(2):280–283
Tsutsui S, Hoshi T, Miyabe M, Tanaka M (2009) Compartment syndrome in the left lower leg following semiradical hysterectomy under general anesthesia combined with epidural anesthesia. Masui 58(4):496–498
Otsuka RA, Amaro JL, Tanaka MT, Epacagnan E, Mendes JB Jr, Kawano PR, Fugita OE (2008) Laparoscopic repair of vesicovaginal fistula. J Endourol 22(3):525–527
Khen-Dunlop N, Lortat-Jacob S, Thibaud E, Clément-Ziza M, Lyonnet S, Nihoul-Fekete C (2007) Rokitansky syndrome: clinical experience and results of sigmoid vaginoplasty in 23 young girls. J Urol 177(3):1107–1111
Byers BD, Silva PH, Kost ER (2007) Delivery complicated by postpartum hemorrhage and lower extremity compartment syndrome. Obstet Gynecol 109(2 Pt2):507–509
Kendrick JE 4th, Leath CA 3rd, Melton SM, Straughn JM Jr (2006) Use of a fascial prosthesis for management of abdominal compartment syndrome secondary to obstetric hemorrhage. Obstet Gynecol 107(2 Pt 2):493–496
Raatz D, Börner P (2001) Laparoscopy assisted radical vaginal hysterectomy (LAVRH) for cervical carcinoma–perioperative parameters and complications. Zentralbl Gynakol 123(3):136–142
Jacobs D, Azagra JS, Delauwer M, Bain H, Vanderheyden JE (1992) Unusual complication after pelvic surgery: unilateral lower limb crush syndrome and bilateral common peroneal nerve paralysis. Acta Anaesthesiol Belg 43(2):139–143
Jyothi NK, Cox C (2000) Compartment syndrome following postpartum haemorrhage. BJOG 107:430–432
Kemp B, Schroeder W, Raumanns J, Magin M, Rath W (1996) Compartment syndrome after Wertheim-Meigs operation. J Gynecol Surg 12:279–286
Szalay G, Meyer C, Alt V, Heiss C (2010) Acute compartment syndrome of the lower leg as an interdisciplinary complication after long duration intervention in the lithotomy position. Zentralbl Chir 135:163–167
Tonnies P, Stogbauer R, Watermann D, Hermann C (1999) Compartment syndrome of the lower leg after prolonged gynecological surgeries in lithotomy position. Zentralbl Gynakol 121:149–152
Ulrich D, Bader AA, Zotter M, Koch H, Pristauz G, Tamussino K (2010) Well-leg compartment syndrome after surgery for gynecologic cancer. J Gynecol Surg 26:261–262
Kundu S, Weiss C, Hertel H, Hillemanns P, Klapdor R, Soergel P (2017) Association between intraabdominal pressure during gynaecologic laparoscopy and postoperative pain. Arch Gynecol Obstet 295:1191–1199
Elessawy M, Schollmeyer T, Mettler L, Jonat W, Schem C, von Hehn U, Alkatout I (2015) The incidence of complications by hysterectomy for benign disease in correlation to an assumed preoperative score. Arch Gynecol Obstet 292:127–133
DGGG Leitlinienprogramm (2015) Empfehlung zur Verhinderung von lagerungsbedingten Schäden in der operativen Gynäkologie. AWMF-Registernummer: 015/077. http://www.awmf.org/uploads/tx_szleitlinien/015-077l_S1Verhinderung_Lagerungssch%C3%A4den_Operationen_Gyn%C3%A4kologie_2015-04.pdf. Accessed 17 May 2017
Author information
Authors and Affiliations
Contributions
KH-F involved in protocol/project development, data collection and management, data analysis, and manuscript writing/editing. JL contributed to protocol/project development, data collection and management, and manuscript writing/editing. GB-P performed data collection and management and manuscript writing/editing. FR participated in protocol/project development and manuscript writing/editing. VU involved in data collection and management, data analysis, and manuscript writing/editing. LH and CT performed protocol/project development, data analysis, and manuscript writing/editing.
Corresponding author
Ethics declarations
Funding
No funding has been received for the present study.
Conflict of interest
All authors have no actual or potential conflict of interest including any financial, personal, or other relationships with other people or organizations within two years of beginning the submitted work that could inappropriately influence, or be perceived to influence, their work to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
IRB approval
IRB approval was obtained for the present study.
Rights and permissions
About this article
Cite this article
Hefler-Frischmuth, K., Lafleur, J., Brunnmayr-Petkin, G. et al. Compartment syndrome after gynecologic laparoscopy: systematic review of the literature and establishment of normal values for postoperative serum creatine kinase and myoglobin levels. Arch Gynecol Obstet 296, 285–293 (2017). https://doi.org/10.1007/s00404-017-4440-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-017-4440-7