Abstract
Purpose
This is the first study to determine the cytomegalovirus (CMV) seronegativity rate for women of childbearing age in Saxony-Anhalt and to determine the prevalence of clinically relevant congenital CMV (cCMV) infection in Central Germany, because there are no valid data available.
Methods
The retrospective study was undertaken between January 2005 and December 2015. For the first time in Germany, the following seven data sources were used to analyze the prevalence of clinically relevant cCMV infection and the rate of CMV seronegative women of childbearing age: CMV Screening in maternity unit, University Women’s Hospital, Social Paediatrics Centre (SPC), Malformation Monitoring Centre (MMC), Newborn Hearing Screening (NHS), Neonatal Intensive Care Unit (NICU), and In-house Doctor Department. Key parameters were anti-CMV IgG and IgM, CMV PCR of urine, and clinically relevant symptoms caused by CMV.
Results
Between 46 and 52% of women of childbearing age were CMV seronegative. The prevalence of clinically relevant cCMV infection was between 0.008 and 0.04%.
Conclusions
The CMV seronegativity rate of women of childbearing age was confirmed to be in the middle range of estimated data from other sources in Germany. Data from the NICU, SPC, NHS, and MMC show the prevalence of clinically relevant cCMV infection. The risk of all cCMV infections is underestimated. Thus, the true prevalence of clinically relevant and subclinical cCMV infections is >0.04%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Congenital cytomegalovirus (cCMV) infection is the most frequent congenital infection worldwide [1]. Moreover, cCMV infection is the most common nongenetic cause of sensorineural hearing loss (SNHL) in childhood [2]. It is also associated with permanent neurological complications such as vision loss, motor disabilities, or delayed neurological development [3]. The significance of cCMV infection becomes clear when comparing its prevalence with the prevalence of other congenital anomalies. More infants are affected by cCMV infection compared to Down syndrome or fetal alcohol syndrome [4]. However, women of childbearing age have a poor awareness of the risks of cCMV infection [5]. It is lower than the awareness of less common childhood illnesses or infectious diseases [3].
CMV seronegativity (anti-CMV IgM and IgG negative) of pregnant women can be seen as a risk factor for cCMV infection, because they have no CMV IgG antibodies protecting against primary CMV infection. Thus, they are at risk for primary CMV infection during pregnancy, which carries the highest risk for the fetus. As of recent, there are no valid data on the CMV seronegativity rate of women of childbearing age in Germany. It is estimated to 40–60% as in other developed countries [6,7,8]. It is important to verify the estimated data for CMV seronegative women.
The virus transmission rate in primary CMV infection is 40–50% [9, 10]. After the intrauterine virus transmission, a distinction needs to be drawn between a clinically relevant cCMV infection with symptoms and a subclinical cCMV infection without apparent clinical effects.
10–15% of the newborns will be symptomatic at birth [11]. Those newborns with a clinically relevant cCMV infection are suffering from microcephalus, intrauterine growth retardation (IUGR), hepatosplenomegaly, jaundice, petechia, or chorioretinitis [8, 12]. Impaired placental capacity to provide oxygen and nutrients to the fetus due to an indirect effect of intrauterine infection causes this wide range of neurological manifestations [13].
In fact, the majority of the symptomatic infants develop late complications such as progressive SNHL, vision impairment or motor disabilities, and mental retardation [14, 15]. Approximately 15–25% of congenital hearing loss and hearing disorders by the age of 4 years are caused by cCMV infection [16, 17]. Direct viral damage to the fetal cochlear cells plays an important role in the pathogenesis of SNHL [13].
If CMV IgG seroconversion is detected, the diagnostic of primary infection is straightforward. However, as CMV IgM may be persisting for months, due to a secondary infection or a cross reaction, the presence of IgM alone does not reflect a primary infection. Therefore, the diagnosis is based on the association of IgM with low avidity IgG index and positive CMV DNA detection in maternal blood [18, 19].
However, women with protective CMV IgG antibodies are still at risk for reinfection or reactivation of a latent CMV infection. If the mothers have a secondary CMV infection during pregnancy, most newborns will be clinically unapparent [20]. 8–15% of those infants will develop sequelae—most frequently manifested as SNHL—as well [8, 12, 21].
As the overall burden of the disease associated with cCMV was estimated to cost approximately $1.9 billion/year to the US health care system, this should be considered a public health problem and prevention is needed [22]. However, there is no effective vaccine for universal immunization available [23, 24].
Maternal-fetal virus transmission may follow either primary or recurrent maternal infection. Therefore, the current focus in primary prevention is to avoid infection during pregnancy. This could be realized by detailed hygienic counseling on the common ways of infection [18, 25, 26].
To prevent cCMV complications, maternal and neonatal treatment are the currently used options.
In case of maternal infection, the focus in secondary prevention is to avoid transmission.
Although the 2014 published double-blind randomized, placebo-controlled phase 2 trial could not prove hyperimmune globulin treatment effectiveness to prevent congenital infection, this could not close the chapter [24, 27, 28]. As this treatment option remains highly controversial, hyperimmune globulins are used off-label in individual cases in Germany because of the very encouraging results of the non-randomized trial published before by Nigro et al. [29, 30].
In cases of fetal infection proven by amniocentesis, treatment may involve the application of anti-viral drugs. Results of a non-randomized, open-label phase 2 study for in utero treatment with valacyclovir show a positive effect to improve the outcome [31].
While there are limited data regarding dose and safety for the neonatal treatment, ganciclovir, valganciclovir, foscarnet, and cidofovir have been the best studied anti-viral drugs in infants. A systematic review concerning postnatal anti-viral therapy for cCMV identified valganciclovir as the most reliable drug in immunocompetent infants (using prolonged administration and having a low rate of side effects) [32].
What should the infected pregnant woman be offered? Literature review showed that the only option currently validated for cCMV treatment remains postnatal treatment (valganciclovir and ganciclovir) for severe symptomatic newborns. Due to the irreversible nature of neonatal brain and sensorineural damage, the treatment effect will be very limited with modest improvement of hearing impairment and neurodevelopmental delay. Nevertheless, this clearly indicates that strategies of antenatal interventions need to be explored further to avoid vertical transmission and irreversible lesions [2, 24].
There are no controlled studies showing a benefit of preventive therapy of asymptomatic CMV infected infants. Only one paper is available from Lackner et al. who treated 12 neonates with ganciclovir for 21 days and 11 were observed without therapy. Only in two untreated children, 10 year follow-up showed SNHL, while all treated children had no hearing impairment [33].
A routine systematic CMV screening for infected neonates or for primary infection in pregnant women is not implemented in Germany or any country to date. CMV testing during pregnancy is part of the individual health services (IGeL) in Germany and, therefore, has to be requested by the patient and is at their own expense [24, 26, 34]. In Israel, most obstetricians test women for CMV during pregnancy due to fear of litigation, but no screening program has been implemented. In Israel, the prevalence of cCMV infection is about 0.7% [35, 36].
While the current literature focuses on proactive approach to screen after birth, there are no valid cCMV prevalence data for central Germany [37,38,39]. Therefore, it is important to present this retrospective study using routine clinical data from multiple data sources to obtain data on proportion of CMV seronegative woman in childbearing age, CMV IgG seroconversion in pregnant woman, and proportion of affected children due to cCMV.
Methods
For the first time in Germany, seven different data sources were used to:
-
1.
evaluate the CMV seronegativity rate of women of childbearing age using serology data of healthcare workers in the University Hospital and data of CMV screening in the University Women’s Hospital Magdeburg;
-
2.
evaluate the prevalence of symptomatic cCMV infection in Saxony-Anhalt.
We manually and electronically evaluated routine clinical hospital data from patients who were treated (including outpatient department) from different time periods from 2005 to 2015 at the University Hospital. Data were obtained from maternity and paediatric records, laboratory data files, and hospital documentary program (Medico, Medizinische Dokumentation, Cerner Deutschland). Furthermore, we included data from Social Paediatrics Centres (SPCs), Newborn Hearing Screening (NHS) program, and the Malformation Monitoring Centre (MMC) of Saxony-Anhalt. Data were transferred to a specifically prepared form with Excel tables and processed in the statistical software SPSS (Version Statistics 24, IBM). The description of baseline characteristics was done using n (%). The prevalence was calculated overall, for each source, with a 95% confidence interval (CI). The study was approved by the institutional ethics committee.
The following data sources were used (Table 1):
CMV seronegativity
To determine the CMV seronegativity rate, the following two data sources were used:
-
(a)
Information on CMV serology of female health care workers obtained from the In-house Doctor Department of the University Hospital Magdeburg. Between 01/01/2009 and 31/12/2014, there was an average of 2998 female health care workers at the University Hospital Magdeburg. About 2% of these employees got pregnant each year (average 57 women per year). Thus, a total of 388 women were tested for CMV. All included women were between 18 and 49 years old. A CMV serology was defined as “CMV seronegative” if no anti-CMV IgG and IgM was proven.
There are no follow-up data on those pregnancies. At the In-house Department, this serological screening was done to distinguish between high risk and low risk for cCMV and subsequently about the working permit during pregnancy.
-
(b)
CMV serology testing was obtained from the maternity unit of the University Women’s Hospital Magdeburg. The Women’s Hospital introduced a routine CMV screening for all hospitalized pregnant women up to the 34th week of gestational age regardless of the reason for hospitalization in 2014. Selection criteria were a pregnancy up to the 34th week of gestational age and a hospitalization in this period at the University Women’s Hospital. 456 pregnant women were hospitalized and screened for CMV IgM and IgG between 01/01/2014 and 31/12/2015. The pregnant women were between 15 and 44 years old. For methods and case definition, see Tables 2 and 3. This routine screening for women up to the 34th week of gestational age was done based on the AWMF guideline [18]. This will have impact on the processing of human milk (breastfeeding) in case of preterm or low birth weight infants.
Table 2 Reference range for CMV serology and PCR Table 3 Definition of CMV cases
Prevalence of clinically relevant cCMV infection
The following six different data sources were used to determine the prevalence of clinically relevant cCMV infection:
-
(c)
810 CMV serologies and/or PCR of urine results of newborns admitted to NICU of the University Hospital Magdeburg born between 1/01/2007 and 31/12/2014 (in total 1869 admitted newborns) were analyzed. The catchment area for this NICU was Northern Saxony-Anhalt with 68,973 live births in this period. A CMV testing was arranged in case of cCMV-like symptoms such as preterm delivery, low birth weight, anemia, jaundice, microcephalus, seizures, or intracerebral abnormalities in ultrasound examination. A clinically relevant cCMV infection in this data set is defined with postnatal symptoms (as described before) due to cCMV infection in need of therapy. Inclusion criteria were a positive CMV testing within 12 weeks after birth and the treatment in the NICU in this period. For serological methods and case definition, see Tables 2 and 3.
-
(d)
33,365 infants (>99% of all live births see, Table 1) born between 1/01/2011 and 31/12/2012 in Saxony-Anhalt were tested in the NHS program. Consecutively, the data of children having hearing impairment at the age of three years of this birth cohort were included. Hearing impairment was defined as bilateral or unilateral hearing loss of 35 decibel or more. These data were provided from the NHS Program Saxony-Anhalt. The differentiation between SNHL and other hearing disorders was done at the Paediatric Audiology Centres. The information was obtained from the medical history taken by the staff at the Paediatric Audiology Centres and from the NHS program using information given by the maternity record book or child health book. Inclusion criteria were a prenatal or postnatal (first 12 weeks after birth) history of cCMV infection. However, not all infants with SNHL were tested for cCMV after noticeable findings in the NHS. There are no follow-up data included.
-
(e)
Data from the SPC in Halle and Magdeburg (there are the only SPCs in Saxony-Anhalt) of 84,886 infants born between 1/01/2010 and 31/12/2014 in Saxony-Anhalt were analyzed. The two SPCs offer care to all infants with developmental disabilities or developmental delay in Saxony-Anhalt. Inclusion criteria were as follows: born in Saxony-Anhalt in the study period, neurodevelopmental disabilities due to cCMV infection (proven prenatal or within 12 weeks after birth). For case overview, see Table 4.
Table 4 Details of registered cCMV cases in the MMC -
(f)
Data from the MMC (population-based birth defect registry program) Saxony-Anhalt were obtained for the birth cohort born between 1/01/2005 and 31/12/2014 (total number of 172,544 live born). Inclusion criteria were as follows: mother is residence in Saxony-Anhalt and pregnancy in the study period, all pregnancies affected by birth defects and a diagnosed cCMV infection (prenatal or postnatal). Diagnosis was made by the physician. For case overview, see Table 4. Cases registered via SPC overlap the cases registered via MMC. MMC data do include all pregnancy outcomes (including termination of pregnancy after prenatal diagnosis and spontaneous abortion).
-
(g)
The data of the routine CMV screening in the University Women’s Hospital (see above group b) were analyzed again. 456 CMV serum results of pregnant women (<34th week of pregnancy) from the maternity unit between 1/01/2014 and 31/12/2015 were analyzed for seroconversion. For methods and case definition, see Tables 2 and 3. No pregnancy outcome data were available.
-
(h)
502 CMV serology results of hospitalized pregnant women (at any week of gestation) between 1/01/2006 and 31/12/2014 tested for CMV IgM and IgG were included. In this period, 8650 pregnant women were treated at the University Women’s Hospital Magdeburg. These women were tested for CMV in case of clinical symptoms, IUGR, or prenatal abnormal ultrasound findings. For methods and case definition, see Tables 2 and 3.
Results
CMV seronegativity
-
(a)
Of 388 pregnant women tested for anti-CMV IgM and IgG at the In-house Doctor Department, 202 (52.1%; 95% CI 47.09–57.03) women were CMV seronegative.
-
(b)
Of 456 pregnant women (<34th week of pregnancy) screened at the University Women’s Hospital, 211 (46.3%; 95% CI 41.7–50.85) were CMV seronegative.
In those pregnant women of childbearing age tested, the CMV seronegativity rate was 46–52%.
Prevalence of cCMV infection
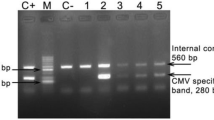
For details, see Fig. 1.
-
(c)
810 infants in the NICU were tested for cCMV. 13 (1.6%) infants had a positive urine and/or serum in the first 14 day postnatal. Three more children had positive urine and/or serum in the first 12 week postnatal. To summarize, 16 (2.0%) out of 810 tested children had a seropositive cCMV infection. Referring to all 1869 newborns admitted to NICU in this period, there was a prevalence of clinically relevant cCMV infection of 0.86% (95% CI 0.44–1.27) (8.6 per 1000 births) in this cohort. Referring to all births in Northern Saxony-Anhalt in this period, the prevalence of clinically relevant cCMV infection in need of therapy was 0.023% (95% CI 0.01–0.03) (0.23 per 1000 births).
-
(d)
33,365 newborns of the birth cohort 2011 and 2012 in Saxony-Anhalt were tested in the NHS. In this period, there were in total 33,725 births in Saxony-Anhalt. 122 cases of relevant hearing loss were reported in the follow-up at the age of three years. 37 (30.3%) of those children had an SNHL, only 15 (12.3%) of them had a history of cCMV infection. Thus, the prevalence of SNHL with a positive history of cCMV infection was 0.04% (95% CI 0.02–0.07) (0.4 per 1000 births) of all births.
-
(e)
In the Halle and Magdeburg SPC, eight infants with clinically relevant cCMV infection symptoms had been registered in the study period. These infants suffered from severe neurodevelopmental impairment and had an impaired motor and/or cognitive development. In total 84,886 children were live born between 2010 and 2014 in Saxony-Anhalt. Therefore, the prevalence of clinically relevant cCMV infection was calculated as 0.009% (95% CI 0.003–0.02) (0.09 per 1000 births).
-
(f)
Between 2005 and 2014, 14 infants with clinically relevant cCMV infection were registered in the MMC Saxony-Anhalt. As a result, the prevalence of severe clinically relevant cCMV infection in cases with birth defects was 0.008% (95% CI 0.004–0.01) (0.08 per 1000 births). All eight registered cases of cCMV from the SPCs were also registered in the MMC. In addition, six cCMV cases were registered in the MMC during the study period. These cases were notably more severe cases, resulting to stillbirth or miscarriage. For more details, see Table 4.
-
(g)
In the CMV screening group of the maternity unit at the University Women’s Hospital, 456 pregnant women up to the 34th week of gestational age were tested between 2014 and 2015. 13 serological results were IgG and IgM positive. Seven out of those 456 serologies were IgG positive and indefinite for IgM. In six out of those seven cases of indefinite serology, no follow-up serologies were available. One control examination followed; IgM was again indefinite. An amniocentesis was carried out in one case and showed a positive CMV PCR. In total, there were 20 (4.4%; 95% CI 2.51–6.27) cases at risk for a primary CMV infection (showing seroconversion). Primary CMV infection during pregnancy results in the highest risk for the fetus. Referring to a virus transmission rate of 50% (known from the literature [11, 40]), 10 (2.2%) newborns (22 per 1000 live births) with cCMV infection were expected.
-
(h)
Out of the 502 CMV serologies between 2006 and 2014, 39 (7.8%) cases of CMV infection were recorded (seroconversion and recurrent). In one case, pregnancy resulted in spontaneous abortion in the 20th week of gestational age. The differentiation between a primary and a recurrent CMV infection in this case was not possible. One case was a primary CMV infection in the 25th week of gestational age. The IgG avidity was low and we had a positive CMV PCR in the amniotic fluid. The differentiation between a primary and a recurrent CMV infection was not possible in the majority of 37 cases. There was a positive anti-CMV IgG in every case. The IgM was positive in 16 cases. 21 serologies showed an indefinite IgM. The recommended control examination was done in 12 cases. IgG avidity was analyzed in two cases. The IgG avidity was high in both cases. That meant that a primary infection was >4 months ago. However, those serologies were tested shortly before delivery (≥24th week of gestation). Thus, a risk for primary CMV infection in the first or second trimester was high. Such as in the CMV screening, we assumed that, if all those cases were a primary CMV infection (highest risk for the fetus), there were 39 (7.8%; 95% CI 5.43–10.11) cases of CMV infection out of 502 tested pregnant women. In case of primary infection, referred to virus transmission rate of 50% (obtained from the literature [11, 40]), we expect a maximum of 20 children with cCMV infection in this period. We calculated a maximum prevalence of cCMV infection of 0.23% (2.3 per 1000 live births) out of all 8650 hospitalized pregnant women at the University Women’s Hospital in this period.
Discussion
Since CMV is the most frequent congenital infection worldwide, it is important to obtain epidemiological data of the cCMV infection as precisely as possible. This is the first retrospective study in Germany using seven different data sources to determine the prevalence of severe symptomatic cCMV infection and the rate of CMV seronegative women of childbearing age. Most of the cCMV infections are asymptomatic at birth. However, those infants can develop sequelae. It is necessary to know the amount of cCMV infections. There are no recent valid data for Germany. We analyzed different data sets of medical sectors where cCMV infected children can become clinically apparent. The postnatal diagnosis of cCMV infection depends on the treating physician. This is the limitation of the data sources. More precise data can only be collected in a prospective study.
The rates of 46–52% CMV seronegative women were comparable with estimated data for Germany (40–60%) mentioned elsewhere [6,7,8]. Comparable to our data, another study from Germany from 2015 could show that more than 50% of women of childbearing age have no anti-CMV IgG [41]. Thus, approximately half of all women of childbearing age had no anti-CMV IgG protecting against the primary CMV infection during pregnancy. The other half of the women of childbearing age had protective CMV IgG antibodies. They are still at risk for a secondary CMV infection during pregnancy. CCMV infection follows either maternal primary or secondary infections. It was previously assumed that primary infection has the highest risk for the fetus for severe sequelae [42]. However, studies of high-seroprevalence regions showed that secondary CMV infection during pregnancy is the main source of cCMV infection in those populations [43, 44]. Thus, reinfection with a new strain of CMV is a risk for almost half of all women with (for primary CMV infection) “protective” CMV IgG in the low-seroprevalence region of Germany.
The data of the NICU (group c) showed an overestimated proportion of cCMV infection because of the “high-risk cohort” of infants admitted to an NICU. We assume an accumulation of sick infants and preterm infants on NICU. Therefore, we defined “high-risk cohort” to emphasize the difference compared to an “average-risk cohort” from crude population. Thus, the prevalence of all cCMV infections (asymptomatic at birth, mild symptomatic, abortion, term infants, etc.) should have been calculated with the whole birth cohort of 68,973 infants in Northern Saxony-Anhalt, because it is the only level 1 perinatal centre in Northern Saxony-Anhalt. We stated that all preterm infants with severe symptoms of cCMV infection were treated in the NICU. The calculated prevalence of clinically relevant cCMV infection is 0.023%; reflecting a lower limit (only the severe symptomatic infants due to cCMV). Referred to all births in Germany (average of 685,433 per year between 1st January 2005 and 31th December 2015 [45]), we should, therefore, expect at least 160 newborns suffering from clinically relevant cCMV infection in need of neonatal intensive care each year.
The data of the SPC and the MMC Saxony-Anhalt showed the prevalence of clinically relevant cCMV infection with severe structural neuronal abnormalities. This was also a prevalence reflecting a lower limit, because a proven CMV infection resulting in a structural damage of the brain or severe neurodevelopmental disabilities was inclusion criteria for being registered in the MMC and the SPC. The implementation of a CMV testing depends on the treating physician, because there is no routine CMV screening in Germany. Some of the clinically relevant cCMV infections show only unspecific symptoms or no symptoms at all at infancy. In some cases, symptoms will be developed in the first years of life and a cCMV infection cannot be proven several months after birth (Guthrie card has to be destroyed after 3 month due to German law). Thus, we stated that those cCMV cases were not detected and the crude prevalence of clinically relevant cCMV infection may be higher than 0.009–0.008%. This is comparable to a study from Australia on cCMV infection among infants with cerebral palsy. Newborn screening cards of infants with cerebral palsy were retrospectively analyzed for CMV DNA. Many of cCMV cases were detected in which no treating physician arranged a postnatal test of CMV DNA [46].
Furthermore, the data of the NHS showed the prevalence of clinically relevant cCMV infection. A meta-analysis had shown that 0.05% of all newborns suffer from SNHL caused by CMV [47]. This is similar to the calculated prevalence of 0.04% in our samples. However, only the infants with SNHL having a medical history of cCMV infection could be included in this 0.04%. The crude prevalence of clinically relevant cCMV infection leading to SNHL could be higher when screening all infants with SNHL detected in NHS program. Nevertheless, we are expecting more than 270 children to suffer from SNHL caused by cCMV in Germany each year based on our data.
This becomes more obvious when comparing our data with a study from Kimberlin et al. from 2015 [2]. The group stated that 24% of hearing loss at the age of 4 years was caused by cCMV. In our study, only 12.3% were caused by cCMV. As there is no routine cCMV screening in Germany for all newborns, there is no screening for cCMV in the NHS program either. We assume that there are SNHL cases with an unknown medical history of cCMV infection. This fact may explain the discrepancy between the data. There could be more precise data of SNHL caused by cCMV infection when testing neonatal Guthrie cards after failing in NHS. A study in Belgium tested this procedure and they could show that 7.3% of SNHL was caused by cCMV infection [48]. This is less than we could prove and much less than we were expecting for all cCMV cases. It is possible that there are more cases of cCMV infection causing SHNL in Germany than in Belgium. However, it is also possible that we had some false positive cases. As shown in the data of Boudewyns et al. and in the light of our results and other available evidence, proportion on SNHL due to cCMV remains controversial [48].
We assume that the CMV screening group of the maternity unit showed an overestimated prevalence of cCMV infection. The CMV cases were the total amount of primary and secondary CMV infection. The virus transmission rate in a recurrent CMV infection is significantly lower than in primary infection [10, 49]. Thus, the prevalence of cCMV infection is <2.2%. Furthermore, in our CMV screening data (during pregnancy), it was obvious that some CMV retesting was not undertaken for various reasons (prompt discharge from the hospital, external delivery, etc.). This resulted in a missed opportunity to follow the advised steps of the AWMF guideline in the early pregnancy (for example, hygienic counseling or prenatal diagnosis in amniotic fluid) [18].
Authors emphasize that the risk of cCMV infection might have been underestimated during clinical daily practice and the 2014 guidelines were not followed there. This retrospective study cannot prove the importance to detect seroconversion, but this might change in case of an effective treatment to reduce the burden of disease due to cCMV infection also in asymptomatic infants to stop sequelae (SNHL) [36].
Approximately 94% of all pregnant women at the University Women’s Hospital were not tested for CMV infection. It is impossible to detect all CMV infections when the testing is based on clinical or ultrasound findings, because not all infected women have symptoms and not all infected children have prenatal ultrasound findings [10, 50]. Thus, CMV infection during pregnancy is underdiagnosed in the daily clinical practice and the calculated prevalence of 0.23% (group h) is only the lower limit.
The data of all samples showed that the crude prevalence of clinically relevant and subclinical cCMV infection was higher than 0.04% and lower than 2.2%. Goderis et al. showed in a meta-analysis of 37 studies that the prevalence of all cCMV infections is 0.58% [47]. It is supposed that the prevalence in Germany is 0.2–0.5% [12, 51]. Due to our data and after literature review, we suppose that cCMV infection is an underestimated risk [15, 52, 53].
Strengths and limitations
It is important to present this data collection on perinatal CMV, even though there are no pregnancy follow-up data provided for the data sets. Due to using data from different sources, we are able to calculate prevalence reflecting the upper limit or prevalence for the more severe affected infants from NICU, SPC, and MMC or prevalence reflecting the lower limit cases from the NHS program (SNHL).
To calculate the prevalence on symptomatic infants, a prospective study will be needed. Furthermore, to take into account the sequelae due to cCMV, a kind of screening (maternal, neonatal) will be needed to be able to calculate the crude prevalence.
Conclusions
There are three key points of this retrospective study:
-
The first one is that the prevalence of clinically relevant cCMV infection is underestimated. We assume that cCMV is underdiagnosed in daily clinical practice. Precise results for prevalence of clinically relevant and subclinical cCMV infection are only determinable with a prospective study.
-
The second key point is that a CMV screening is necessary to detect asymptomatic infants who are also at risk of long-time sequelae due to cCMV infections. However, there is only a proven benefit of postnatal therapy for symptomatic infants to date. The benefit for asymptomatic children could not be proven to date [54].
-
The third key point is that according to the guidelines for diagnosis, women should be made aware of cCMV infection risk (seronegativity). They will benefit from hygienic counseling for primary prevention of cCMV [25].
References
Numazaki KCS (1997) Current aspects of diagnosis and treatment of cytomegalovirus infections in infants. Clin Diagn Virol 8:169–181
Kimberlin DW, Jester PM, Sánchez PJ et al (2015) Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med 372(10):933–943. doi:10.1056/NEJMoa1404599
Jeon J, Victor M, Adler SP et al (2006) Knowledge and awareness of congenital cytomegalovirus among women. Infect Dis Obstet Gynecol 2006:80383. doi:10.1155/IDOG/2006/80383
Cannon MJ, Davis KF (2005) Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health 5:70. doi:10.1186/1471-2458-5-70
Price SM, Bonilla E, Zador P et al (2014) Educating women about congenital cytomegalovirus: assessment of health education materials through a web-based survey. BMC Womens Health 14:144. doi:10.1186/s12905-014-0144-3
Meyer-Wittkopf M (2015) Die Cytomegalie-Infektion in der Schwangerschaft. Hebamme 28(02):90–96. doi:10.1055/s-0035-1547437
Knabl J (2011) CMV-Infektion und -Screening in der Schwangerschaft. Z Geburtshilfe Neonatol 35:719–721
Nyholm JLS (2010) Prevention of maternal cytomegalovirus infection: current status and future prospects. Int J Women’s Health 2:23–35
Meyer-Wittkopf M, Buxmann H, Gonser M et al (2009) Neues zu prä- und perinatalen Cytomegalovirus-Infektion. Frauenarzt 6:525–527
Revello MG (2004) Pathogenesis and prenatal diagnosis of human cytomegalovirus infection. J Clin Virol 29(2):71–83. doi:10.1016/j.jcv.2003.09.012
Ornoy A, Diav-Citrin O (2006) Fetal effects of primary and secondary cytomegalovirus infection in pregnancy. Reprod Toxicol 21(4):399–409. doi:10.1016/j.reprotox.2005.02.002
Hamprecht K, Jahn G (2007) Human cytomegalovirus and congenital virus infection (Humanes Cytomegalovirus und kongenitale Infektion). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 50(11):1379–1392. doi:10.1007/s00103-007-0194-x
Adler SP, Nigro G, Pereira L (2007) Recent advances in the prevention and treatment of congenital cytomegalovirus infections. Semin Perinatol 31(1):10–18. doi:10.1053/j.semperi.2007.01.002
Turner KM, Lee HC, Boppana SB et al (2014) Incidence and impact of CMV infection in very low birth weight infants. Pediatrics 133(3):e609–e615. doi:10.1542/peds.2013-2217
Sorichetti B, Goshen O, Pauwels J et al (2016) Symptomatic Congenital Cytomegalovirus Infection Is Underdiagnosed in British Columbia. J Pediatr 169:316–317. doi:10.1016/j.jpeds.2015.10.069
Fowler KB (2013) Congenital cytomegalovirus infection: audiologic outcome. Clin Infect Dis 57(Suppl 4):S182–S184. doi:10.1093/cid/cit609
Grosse SD, Ross DS, Dollard SC (2008) Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: a quantitative assessment. J Clin Virol 41(2):57–62. doi:10.1016/j.jcv.2007.09.004
Gesellschaft für Virologie (GfV), Deutsche Vereinigung zur Bekämpfung von Viruskrankheiten (DVV) (2014) S2 k-Leitlinie Labordiagnostik schwangerschaftsrelevanter Virusinfektionen, AWMF Registernummer 0093/001. http://www.awmf.org/uploads/tx_szleitlinien/093-001l_S2k_Labordiagnostik_schwangerschaftsrelevanter_Virusinfektionen_2014-05.pdf
Revello MG, Fabbri E, Furione M et al (2011) Role of prenatal diagnosis and counseling in the management of 735 pregnancies complicated by primary human cytomegalovirus infection: a 20-year experience. J Clin Virol 50(4):303–307. doi:10.1016/j.jcv.2010.12.012
Fowler Karen B, Sergio Stagno, Pass Robert F, Britt William J, Boll Thomas J, Alford Charles A (1992) The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med 10:663–667
Naing ZW, Scott GM, Shand A et al (2016) Congenital cytomegalovirus infection in pregnancy: a review of prevalence, clinical features, diagnosis and prevention. Aust N Z J Obstet Gynaecol 56(1):9–18. doi:10.1111/ajo.12408
Yow MD, Demmler GJ (1992) Congenital cytomegalovirus disease–20 years is long enough. N Engl J Med 326(10):702–703. doi:10.1056/NEJM199203053261010
Kharfan-Dabaja MA, Boeckh M, Wilck MB et al (2012) A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis 12(4):290–299. doi:10.1016/S1473-3099(11)70344-9
Leruez-Ville MVY (2016) Optimum treatment of congenital cytomegalovirus infection. Expert Rev Anti Infect Ther 14:479–488
Vauloup-Fellous C, Picone O, Cordier A et al (2009) Does hygiene counseling have an impact on the rate of CMV primary infection during pregnancy? Results of a 3-year prospective study in a French hospital. J Clin Virol 46(Suppl 4):S49–S53. doi:10.1016/j.jcv.2009.09.003
Kling C, Kabelitz D (2015) Congenital HCMV and assisted reproduction: why not use the chance for primary screening? Arch Gynecol Obstet 291(6):1205–1211. doi:10.1007/s00404-014-3583-z
Revello MG, Lazzarotto T, Guerra B et al (2014) A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med 370(14):1316–1326. doi:10.1056/NEJMoa1310214
Hamprecht K, Kagan KO, Goelz R (2014) Hyperimmune globulin to prevent congenital CMV infection. N Engl J Med 370(26):2543. doi:10.1056/NEJMc1405377#SA1
Nigro G, Adler SP, La Torre R et al (2005) Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med 353(13):1350–1362. doi:10.1056/NEJMoa043337
Schampera MS, Schweinzer K, Abele H et al (2017) Comparison of cytomegalovirus (CMV)-specific neutralization capacity of hyperimmunoglobulin (HIG) versus standard intravenous immunoglobulin (IVIG) preparations: impact of CMV IgG normalization. J Clin Virol 90:40–45. doi:10.1016/j.jcv.2017.03.005
Leruez-Ville M, Ghout I, Bussieres L et al (2016) In utero treatment of congenital cytomegalovirus infection with valacyclovir in a multicenter, open-label, phase II study. Am J Obstet Gynecol 215(4):462.e1. doi:10.1016/j.ajog.2016.04.003
Mareri A, Lasorella S, Iapadre G, Maresca M, Tambucci R, Nigro G (2016) Anti-viral therapy for congenital cytomgealovirus infection: pharmacokinetics, efficacy and side effect. J Matern Fetal Neonatal Med 29:1657–1664
Lackner A, Acham A, Alborno T et al (2009) Effect on hearing of ganciclovir therapy for asymptomatic congenital cytomegalovirus infection: four to 10 year follow up. J Laryngol Otol 123(4):391–396. doi:10.1017/S0022215108003162
Karacan M, Batukan M, Cebi Z et al (2014) Screening cytomegalovirus, rubella and toxoplasma infections in pregnant women with unknown pre-pregnancy serological status. Arch Gynecol Obstet 290(6):1115–1120. doi:10.1007/s00404-014-3340-3
Rahav G (2007) Congenital Cytomegalovirus Infection-a Question of Screening. Isr Med Assoc J 9:392–394
Walker SP, Palma-Dias R, Wood EM et al (2013) Cytomegalovirus in pregnancy: to screen or not to screen. BMC Pregnancy Childbirth 13:96. doi:10.1186/1471-2393-13-96
Cannon MJ, Griffiths PD, Aston Van et al (2014) Universal newborn screening for congenital CMV infection: what is the evidence of potential benefit? Rev Med Virol 24(5):291–307. doi:10.1002/rmv.1790
Ross SA, Ahmed A, Palmer AL et al (2014) Detection of congenital cytomegalovirus infection by real-time polymerase chain reaction analysis of saliva or urine specimens. J Infect Dis 210(9):1415–1418. doi:10.1093/infdis/jiu263
Ronchi A, Shimamura M, Malhotra PS et al (2017) Encouraging postnatal cytomegalovirus (CMV) screening: the time is NOW for universal screening! Expert Rev Anti Infect Ther 15(5):417–419. doi:10.1080/14787210.2017.1303377
Davis NL, King CC, Kourtis AP (2017) Cytomegalovirus infection in pregnancy. Birth Defects Res 109(5):336–346. doi:10.1002/bdra.23601
Kling C, Kabelitz D (2015) HCMV seroprevalence in couples under infertility treatment. Arch Gynecol Obstet 292(2):439–443. doi:10.1007/s00404-015-3640-2
de Vries JJ, van Zwet EW, Dekker FW et al (2013) The apparent paradox of maternal seropositivity as a risk factor for congenital cytomegalovirus infection: a population-based prediction model. Rev Med Virol 23(4):241–249. doi:10.1002/rmv.1744
Manicklal S, Emery VC, Lazzarotto T et al (2013) The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev 26(1):86–102. doi:10.1128/CMR.00062-12
Yamamoto AY, Mussi-Pinhata MM, Boppana SB et al (2010) Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. Am J Obstet Gynecol 202(3):297.e1–8. doi:10.1016/j.ajog.2009.11.018
German Federal Statistical Office Livebirths in Germany. https://www-genesis.destatis.de/genesis/online/data;jsessionid=322C9013455F1273FF5FB0D682D9AF2F.tomcat_GO_2_2?operation=abruftabelleBearbeiten&levelindex=2&levelid=1472552892299&auswahloperation=abruftabelleAuspraegungAuswaehlen&auswahlverzeichnis=ordnungsstruktur&auswahlziel=werteabruf&selectionname=12612-0001&auswahltext=&werteabruf=Werteabruf. Accessed 30 Aug 2016
Smithers-Sheedy H, Raynes-Greenow C, Badawi N et al (2016) Congenital cytomegalovirus among children with cerebral palsy. J Pediatr. doi:10.1016/j.jpeds.2016.10.024
Goderis J, de Leenheer E, Smets K et al (2014) Hearing loss and congenital CMV infection: a systematic review. Pediatrics 134(5):972–982. doi:10.1542/peds.2014-1173
Boudewyns A, Declau F, Smets K et al (2009) Cytomegalovirus DNA detection in Guthrie cards: role in the diagnostic work-up of childhood hearing loss. Otol Neurotol 30(7):943–949. doi:10.1097/MAO.0b013e3181b76b22
Kenneson A, Cannon MJ (2007) Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 17(4):253–276. doi:10.1002/rmv.535
Guerra B, Simonazzi G, Puccetti C et al (2008) Ultrasound prediction of symptomatic congenital cytomegalovirus infection. Am J Obstet Gynecol 198(4):380.e1–7. doi:10.1016/j.ajog.2007.09.052
Vochem M (2003) CMV infections of the neonate: prevalence, diagnosis, therapy (CMV-Infektionen bei Neugeborenen: pravalenz, Diagnostik und Therapie). Z Geburtshilfe Neonatol 207(3):114–118. doi:10.1055/s-2003-40978
McMullen BJ, Palasanthiran P, Jones CA, Hall BV, Robertson PW, Howard J, Rawlinson WD (2011) Congenital cytomegalovirus- time to diagnosis, management and clinical sequelae in Australia: opportunities for earlier identification. Med J Aust 12:625–629
Townsend CL, Peckham CS, Tookey PA (2011) Surveillance of congenital cytomegalovirus in the UK and Ireland. Arch Dis Child Fetal Neonatal Ed 96(6):F398–F403. doi:10.1136/adc.2010.199901
De Vries J, Vossen A, Kroes A, Van der Zeijst B (2011) Implementing neonatal screening for congenital cytomegalovirus: addressing the deafness of policy makers. Rev Med Virol 21:54–61
Author information
Authors and Affiliations
Contributions
HR: conceived the study, data collection and management, data analysis, and manuscript writing. AR: conceived the study, data collection and management, project development, manuscript writing, and editing. BB: data collection and revision of manuscript. SC: data collection and revision of manuscript. BD: data collection and revision of manuscript. JF: data collection and revision of manuscript. SF: data collection and revision of manuscript. CF: data collection and revision of manuscript. AL: data analysis and revision of manuscript. IP: data collection and revision of manuscript. CS: data collection and revision of manuscript. AR: conceived the study, data collection, and manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest and they had full control of all primary data, and they agree to allow the Journal to review the data if requested.
Ethical approval
For this type of study, formal consent is not required. Ethical approval for this study was obtained from the ethics committee of the Medical Faculty (EK17/16).
Rights and permissions
About this article
Cite this article
Rütten, H., Rissmann, A., Brett, B. et al. Congenital cytomegalovirus infection in Central Germany: an underestimated risk. Arch Gynecol Obstet 296, 231–240 (2017). https://doi.org/10.1007/s00404-017-4435-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-017-4435-4