Abstract
Objective
The aim of this study is to prospectively evaluate and compare the accuracy of high-frequency TVS and of two type of MRI (dynamic contrast-enhanced MRI or diffusion-weighted MRI), in association with HE4 in preoperative endometrial cancer (EC) staging.
Study design
Starting from January 2012 to February 2015, all patients with EC at prior endometrial biopsy, referred to the Division of Gynaecologic Oncology of the University Campus Bio-Medico of Rome, were prospectively included in the study. All of them underwent complete surgical staging hysterectomy and bilateral oophorectomy, pelvic and lumboaortic lymphadenectomy, according to 2011 NCCN guidelines. The day before surgery, patients underwent to transvaginal ultrasonography (TVS), HE4 serum dosage, and using a computer-based random procedure, to dynamic contrast-enhanced MRI (Group A) or to diffusion-weighted MRI (Group B), to assess myometrial invasion and cervical involvement.
Results
Starting from January 2012 to February 2015, a total of 79 patients were considered for the analysis and randomly divided into Group A (n = 38) and Group B (n = 41). Regarding myometrial invasion, MRI and TVS resulted comparable in terms of preoperative detection. Concerning the cervical infiltration, the association between TVS and HE4 is characterized by a better preoperative diagnostic validity (TVS + HE4 96.3 vs. 91 % for MRI and 85 % for the TVS).
Conclusion
Our results, even the low number of enrolled patients, are promising and may lead to a greater efficiency and lower health care costs in identifying those women who require radical surgery and pelvic lymphadenectomy and should be addressed, in specialized centers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endometrial carcinoma (EC) is the most common gynaecologic malignancy and the seventh neoplasia worldwide, with the highest incidence in North America and Europe [1, 2].
Considering that clinical staging results incorrect in over 20 % of cases, EC is staged according to a surgical system, including hysterectomy, bilateral adnexectomy, peritoneal washing cytology and lymphadenectomy [3].
Careful preoperative evaluation is essential, as women with EC surely would benefit from being addressed in gynecologic oncology centers of excellence for treatment; this would optimize the costs of health care and the likelihood of making a complete surgical staging and an optimal surgical treatment.
In particular, an accurate preoperative staging of disease would assist in planning the optimal course of treatment. For example, the ability to distinguish between patients with a myometrial invasion superior or inferior of 50 % (FIGO Stage IA vs. Stage IB), would allow the identification of those women who may or may not need lymphadenectomy, and thus might require a higher level of surgical expertise. Furthermore, tumor extension to the cervical stroma (Stage II) warrants radical hysterectomy and knowledge of this condition could help in planning the required surgery. This issue is becoming even more relevant as less invasive surgical techniques, such as laparoscopy, are emerging as alternative procedures [4–11].
Several techniques have been studied as preoperative tools for EC staging. Magnetic resonance imaging (MRI), computed tomography (CT) and transvaginal sonography (TVS) have all been proven to be accurate in the assessment of depth of myometrial infiltration [12].
In particular, it has been demonstrated that contrast-enhanced MRI performs better than helical CT in the preoperative EC staging [13]. However, MRI is expensive, time consuming and has limited availability, so that it may be not appropriate for all patients.
A handful of relatively small-scale prospective studies have compared the diagnostic accuracy of TVS and MRI in the preoperative local staging of endometrial cancer with conflicting results. The sensitivity of these techniques in identifying the myometrial infiltration is reported as 91 % for MRI with contrast, 83 % for MRI without contrast, 85 % for TVS and 79 % for CT. However, there has been no prospective comparison of the accuracy of TVS compared to MRI in the assessment of cervical invasion [14–16]. However, the few retrospective studies report sensitivity in identifying cervical involvement of 79 % for MRI with contrast and 93 % for TVS). For this reason, the use of further clinical methods that may increase the diagnostic power of these imaging techniques is desirable [17, 18].
The role of tumor markers in EC is still debated. CA15-3 and CA125 have been found to be elevated in only 36 % and 24.6 % of EC patients, respectively. Human epididymis protein 4 (HE4) is a novel tumor marker emerging in EC management [19–24].
Currently, only few studies in literature investigated the role of HE4 in the staging of endometrial cancer, suggesting that HE4 may be a useful preoperative tool [25–27].
Thus the challenge to find an effective preoperative tool for endometrial cancer diagnosis and staging is still open. In this prospective study, we evaluated and compared the accuracy of high-frequency TVS and of two type of MRI (dynamic contrast-enhanced MRI or diffusion-weighted MRI), in association with HE4 in preoperative EC staging.
Materials and methods
Starting from January 2012 to February 2015, all patients with endometrial cancer diagnosed at prior endometrial biopsy, referred to the Division of Gynaecologic Oncology of Campus Bio-Medico University of Rome, were prospectively included in the study. The institutional internal review board approved the study.
Inclusion criteria for enrollment were as follows: (1) age between 18 and 80 years; (2) Eastern Cooperative Oncology Group performance status 0–2 according to World Health Organization criteria; (3) informed consent obtained from the patients.
Exclusion criteria included: (1) abnormal cardiac, hematological, renal, respiratory, and/or hepatic functions; (2) presence of a secondary malignancy; (3) concomitant benign and/or malignant adnexal pathologies, (4) claustrophobia, (5) any mental illness.
All patients underwent complete surgical staging which consisted of hysterectomy, bilateral oophorectomy, pelvic and lumboaortic lymphadenectomy, according to 2011 NCCN guidelines.
According to our protocol, a detailed anamnesis was taken for each patient, recording: age, parity, menarche, Body Mass Index (BMI), comorbidities and previous medical history.
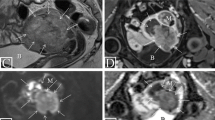
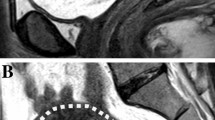
The day before surgery, transvaginal ultrasonography (TVS) was performed to all patients with a commercially available machine, GE Voluson E8, using a 7.5 MHz vaginal probe. At TVS, The uterus was scanned in the sagittal plane from cornu to cornu and in the (oblique) transverse plane from the cervix to the fundus. Having established an overview of the whole uterus, the image was magnified to contain only the uterine corpus. We defined the tumor mass in the sagittal plane, and we measured the tumor/uterine AP ratio at the point where we found the deepest myometrial invasion. We chose to use the tumor/uterine AP ratio to describe tumor size, instead of endometrial thickness or the tumor/uterine ratio of the 3D volume, because we found that this was the best objective parameter related to tumor size to predict myometrial invasion according to receiver-operating characteristics (ROC) curves. In fact, the area under the curve (AUC) tumor/uterine ratio, endometrial thickness and tumor/uterine ratio of the 3D volume resulted 0.79, 0.74 and 0.77, respectively. Invasion of the cervix (Stage II) was evaluated using B-mode sonography when the neoplastic tissue extended caudally merging with the endocervical mucosa. A slight pressure applied to the transvaginal probe often allowed the sonographer to differentiate between mere protrusion of the endometrial tissue to the cervical canal and true infiltration of the endocervical mucosa.
Using a computer-based random procedure, all patients preoperatively underwent to dynamic contrast-enhanced MRI (Group A) or to diffusion-weighted MRI (Group B), to assess myometrial invasion and cervical involvement.
At our institution, the MRI studies are performed on a 1.5-T magnet (Signa Excite; GE Healthcare, Waukesha, Wis) with an eight-channel cardiac array coil. All imaging study is performed with the patient supine. Axial, axial oblique and sagittal fast recovery fast spin echo T2-weighted images and axial T1-weighted images of the pelvis are obtained. All axial oblique images are obtained in a plane perpendicular to the endometrial cavity [5, 12, 14].
Sagittal and axial oblique diffusion-weighted MRI of the pelvis is performed with b values of 0, 500 (sagittal), and 800 (axial oblique) s/mm2.
Dynamic contrast-enhanced MR images are obtained with a three-dimensional gradient recalled echo T1-weighted LAVA (liver acquisition volume acceleration) sequence (GE Healthcare) after the administration of 0.1 mmol/kg of gadolinium at a rate of 2 mL/s [5, 12, 14].
Images are acquired prior to contrast injection and then during multiple phases of enhancement in both sagittal and axial oblique planes (sagittal: 25 s, 1 min, and 2 min after injection; axial oblique: 4 min after injection). The day before surgery, blood samples were obtained for HE4 dosage. All sera were acquired following a standard collection protocol. Briefly, samples were collected in a red top vacutainer, clotted 60–90 min and centrifuged for 10 min at 1300×g.
Serum fractions were aliquoted and stored at −80 °C until analysis. HE4 levels were determined using the HE4 EIA assay (Fujirebio Diagnostics). The HE4 EIA is a solid phase, noncompetitive immunoassay based upon the direct “sandwich” technique using two monoclonal antibodies, 2 H5 and 3D8, directed against two epitopes in the CWFDC domain of HE4. During the enzyme reaction, a blue color developed if the antigen was present. The intensity of the color was directly proportional to the amount of HE4 present in the samples.
Patients were included in the study only if both physicians, performing TVS and MRI, could give an unequivocal opinion on myometrial and cervical infiltration by the neoplasm. The sensitivity, specificity, positive (PPV) and negative (NPV) predictive values, and accuracy for MRI, TVS and HE4 in assessing the depth of myometrial invasion and eventual extension to the cervix were calculated, with histological diagnosis as the ‘gold standard’. All continuous data were expressed as mean and SD. Calculation of sensitivity, specificity, PPV, NPV was performed for the three features. Significance was set at the P ≤ 0.05 level in all analyses. Statistical analysis was performed using software Medcalc © statistical software ver. 12.4.0.0 in stepwise mood.
Results
Starting from January 2012 to February 2015, 89 patients have been enrolled and randomized. Six patients were excluded for suffering of claustrophobia during MRI procedure and four were excluded for being not completely staged. Therefore, a total of 79 patients, divided into Group A (n = 38) and Group B (n = 41), were considered for the analysis (Fig. 1).
Clinical, ultrasound and operative patient’s characteristics are illustrated in Table 1.
Homogeneity of the two sets was assessed and no significant differences were observed in the features of the two randomized case series regarding age, HE4 levels and FIGO stages. In Group A, the diagnostic performances of TVS and dynamic contrast-enhanced MRI in predicting myometrial involvement are summarized in Table 2.
The depth of myometrial infiltration was correctly assessed by TVS in 35/38 cases (92.1 %), overestimated in one case (3 %) and underestimated in two (7 %). Dynamic contrast-enhanced MRI correctly assessed myometrial infiltration in 31/38 cases (81.6 %), overestimated it in 3 (8 %) and underestimated it in 4 (10 %). Results of the different combinations of these techniques (even along with HE4) are reported in Table 2.
Concerning cervical involvement, TVS and MRI results are reported in Table 3. It was correctly assessed by TVS in 35/38 cases (92.1 %), overestimated in one case (3 %) and underestimated in two (7 %). Dynamic contrast-enhanced MRI correctly assessed cervical involvement in 29/38 cases (76.3 %), overestimated it in 6 (16 %) and underestimated it in 3 (8 %). Results of the different combinations of these techniques (even along with HE4) are reported in Table 3.
In Group B, the diagnostic performances of TVS and diffusion-weighted MRI in predicting deep myometrial involvement are summarized in Table 4.
The depth of myometrial infiltration was correctly assessed by TVS in 39/41 (95.1 %) cases, never overestimated and underestimated in two (5 %). Diffusion-weighted MRI correctly assessed myometrial infiltration in 35/41 cases (85.4 %), overestimated it in 1 (2 %) and underestimated it in 5 (12 %). Results of the different combinations of these techniques (even along with HE4) are reported in Table 4.
Concerning cervical involvement, TVS and MRI results are reported in Table 5. Cervical involvement was correctly assessed by TVS in 40/41 cases (97.6 %), never overestimated and underestimated in one case (2 %). Diffusion-weighted MRI correctly assessed cervical involvement in 35/41 cases (85.4 %), overestimated it in 4 (9 %) and underestimated it in 2 (5 %) Results of the different combinations of these techniques (even along with HE4) are reported in Table 5.
Mean HE4 levels for all 79 patients was 119.4 pmol/L. In our population, based on ROC curve, we found that the HE4 value of >63 pmol/L is the best cut-off to indentify patients with myometrial invasion >50 % with a sensitivity of 79.2 %, a specificity of 74.2 % (PPV = 82.6 % and NPV = 69.7 %). Concerning cervical involvement, we found that the HE4 value of >108 pmol/L is the best cut-off to identify patients stage II with a sensitivity of 76.2 %, a specificity of 77.6 % (PPV = 55.2 % and NPV = 90 %).
Discussion
EC treatment and prognosis are influenced by prognostic factors, such as the depth of myometrial infiltration, the extension to the cervix and the presence of lymph node metastases. This consolidated the view that an appropriate knowledge of the extension of the local–regional tumor disease can affect the survival (70–80 % at 5 years) [28, 29].
The preoperative knowledge of prognostic factors can also widely influence the treatment at choice, sometimes requiring a more radical surgical approach to improve the prognosis (disease- free survival of 72 % at 5 years) [30, 31]. On the other hand, considering that lymphadenectomy (pelvic and paraaortic) has no therapeutic role in EC but merely diagnostic/prognostic (as it permits an accurate staging of the patient), and considering that it can lead to a series of complications (such as lymphedema) that may seriously impact on patient’s quality of life, to avoid overtreatment, international guidelines suggests that it can be spared, or at least tailored, in cases at low-risk for nodal metastasis (<50 % myometrial invasion, tumor <2 cm, G1/G2 histology). For this reason, finding a diagnostic tool that is able to preoperatively identify these patients would be of great benefit in avoiding overtreatment. Unfortunately, there are no standardized techniques for preoperative evaluation that have adequate sensitivity and specificity in predicting a state of advanced disease, and besides, there are no guidelines or algorithms to be used in the preoperative evaluation of EC.
TVS, MRI with and without contrast and CT have been studied in a recent meta-analysis, which showed that although the percentages of sensitivity and specificity indicate a good performance of all the methods, the most favorable trends were recorded for MRI and TVS. However, these data remain unsatisfactory as regarding sensitivity in assessing myometrial infiltration (91 vs. 85 %, respectively, for MRI and TVS) and in evaluating cervical infiltration (MRI 79 vs. 93 % TVS). For this reason, we still could not rely on such techniques in the preoperative evaluation of the patient and in the surgical planning [17] and the use of other clinical methods that may increase the diagnostic power is needed.
Basing on our results, HE4 has proved to be useful in EC staging, as it improves the accuracies obtained for both MRI and TVS. In fact, the use of tumor marker seems to compensate the limit of TVS and MRI that are operator-dependent. Although there are no statistically significant differences regarding the capacity of the two techniques associated to HE4 in detecting myometrial infiltration, there is an increase of 5 % in sensitivity and specificity if compared with the ultrasound and about a 12 % for sensitivity and 14 % of specificity with regard to the MRI.
Different results are highlighted regarding the cervical infiltration, in which the association between TVS and HE4 results to be statistically superior.
The combination of these three techniques (TVS, MRI and HE4) appears to significantly improve the sensitivity and specificity in detecting myometrial infiltration, but considering the high costs of MRI, its limited availability, and sometimes its medical contraindications, the TVS along with HE4 may be considered the best valid alternative in EC staging before surgery.
As concerning cervical infiltration, our data show a better result in terms of sensitivity and specificity if compared to those reported in the literature; in fact, the association between TVS and HE4 is characterized by a really high preoperative diagnostic validity (TVS + HE4 96.3 vs 91 % for MRI and 85 % for the TVS) [17]. One possible bias of our study may be represented by the low number of enrolled patients. However, our results are promising and may lead to lower health care costs and to a greater efficiency in identifying those women who would require radical surgery and lymphadenectomy that should be addressed to highly specialized centers. Moreover, this association allows a correct staging in patients who cannot undergo to MRI due to contraindications (claustrophobia, bearers of a pacemaker, obese, suffering from mental illness) or due to the reduced accessibility to centers with adequate devices.
In our study, the association of TVS and HE4 had shown a good sensibility and specificity in the preoperative EC evaluation. Using this preoperative diagnostic approach, we can be able to distinguish between early from advanced stage disease, avoiding surgical under or overtreatment and sending the patient to highly specialized center if needed. Furthermore having the same efficacy of RMI, this diagnostic tool can lead to reduced health care costs; moreover, it can be used in not-specialized center where MRI is not always available and in cases in which the use of MRI is contraindicated. Of course further larger studies will be needed to confirm these encouraging results.
References
Parkin DM, Bray F, Pisani P, Ferlay J (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ (2005) Cancer statistics 2005. CA Cancer J Clin 55:10–30
Creasman WT (1989) FIGO stage 1988 revision. GynecolOncol 35:125–127
Creasman WT, Morrow CP, Bundy BN, Homesly HD, Graham JE, Heller PB (1987) Surgical pathologic spread patterns of endometrial cancer. Cancer 60:2035–2041
Rubin SC, Hoskins WJ, Saigo PE, Nori D, Mychalczak B, Chapman D, Lewis JL Jr (1992) Management of endometrial adenocarcinoma with cervical involvement. Gynecol Oncol 45:294–298
Morrow CP, Curtin JP, Townsend DG (1998) Tumors of the endometrium. In: Morrow CP, Curtin JP (eds) Synopsis of gynecologic oncology (5th edn). Churchill Livingstone, New York, NY, pp 151–185
Varpula MJ, Klemi PJ (1993) Staging of uterine endometrial carcinoma with ultra-low field (0.02 T) MRI: a comparative study with CT. J Comput Assist Tomogr 17:641–647
Kinkel K, Kaji Y, Yu KK, Segal MR, Powell CB, Hricak H (1999) Radiologic staging in patients with endometrial cancer: a metaanalysis. Radiology 212:711–718
Ayhan A, Taskiran C, Celik C, Yuce K (2004) The long term survival of women with surgical stage II endometrioid type endometrial cancer. Gynecol Oncol 93:9–13
Nagar H, Dobbs S, McClelland HR, Price J, McCluggage WG, Grey A (2006) The diagnostic accuracy of magnetic resonance imaging in detecting cervical involvement in endometrial cancer. Gynecol Oncol 103:431–434
Magrina JF (2005) Outcomes of laparoscopic treatment for endometrial cancer. Curr Opin Obstet Gynecol 17:343–346
Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ (2005) Cancer statistics 2005. CA Cancer J Clin 55:10–30
Kinkel K, Kaji Y, Yu KK, Segal MR, Powell CB, Hricak H (1999) Radiologic staging in patients with endometrial cancer: a metaanalysis. Radiology 212:711–718
DelMaschio A, Vanzulli A, Sironi S, Spagnolo D, Belloni C, Garancini P, Taccagni GL (1993) Estimating the depth of myometrial involvement by endometrial carcinoma: efficacy of transvaginal sonography vs. MR imaging. AJR Am J Roentgenol 160:533–538
Cagnazzo G, D’Addario V, Martinelli G, Lastilla G (1992) Depth of myometrial invasion in endometrial cancer: preoperative assessment by transvaginal ultrasonography and magnetic resonance imaging. Ultrasound Obstet Gynecol 2:40–43
Yamashita Y, Mizutani H, Torashima M, Takahashi M, Miyazaki K, Okamura H, Ushijima H, Ohtake H, Tokunaga T (1993) Assessment of myometrial invasion by endometrial carcinoma: transvaginal sonography vs contrast-enhanced MR imaging. AJR Am J Roentgenol 161:595–599
Kinkel K, Kaji Y, Yu KK, Segal MR, Lu Y, Powell CB, Hricak H (1999) Radiologic staging in patients with endometrial cancer: a meta-analysis. Radiology 212:711–717
Epstein E, Van Holsbeke C, Mascilini F, Måsbäck A, Kannisto P, Ameye L, Fischerova D, Zannoni G, Vellone V, Timmerman D, Testa AC (2011) Gray-scale and color Doppler ultrasound characteristics of endometrial cancer in relation to stage, grade and tumor size. Ultrasound Obstet Gynecol 38(5):586–593
Moore RG, Brown AK, Miller MC, Badgwell D, Lu Z, Allard WJ et al (2008) Utility of a novel serum tumor biomarker HE4 in patients with endometrioid adenocarcinoma of the uterus. Gynecol Oncol 110(2):196–201
Bignotti E, Ragnoli M, Zanotti L, Calza S, Falchetti M, Lonardi S et al (2011) Diagnostic and prognostic impact of serum HE4 detection in endometrial carcinoma patients. Br J Cancer 104:1418–1425
Drapkin R, von Horsten HH, Lin Y, et al (2005) Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res 65(6):2162–2169
Kobel M, Kalloger SE, Boyd N et al (2008) Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med 5:e23
Plotti F, Capriglione S, Terranova C, Montera R, Aloisi A, Damiani P, Muzii L, Scaletta G, Benedetti-Panici P, Angioli R (2012) Does HE4 have a role as biomarker in the recurrence of ovarian cancer? Tumour Biol 33(6):2117–2123
Duk IM (1994) CA125: a useful marker in endometrial carcinoma. Am J Obstet Gynaecol Oncol 54(3):321–326
Angioli R, Plotti F, Capriglione S, Montera R, Damiani P, Ricciardi R, Aloisi A, Luvero D, Cafà EV, Dugo N, Angelucci M, Benedetti-Panici P (2013) The role of novel biomarker HE4 in endometrial cancer: a case control prospective study. Tumour Biol 34(1):571–576
Moore RG, Miller CM, Brown AK, Robison K, Steinhoff M, Lambert-Messerlian G (2011) Utility of tumor marker HE4 to predict depth of myometrial invasion in endometrioid adenocarcinoma of the uterus. Int J Gynecol Cancer 21(7):1185–1190
Capriglione S, Plotti F, Miranda A, Ricciardi R, Scaletta G, Aloisi A, Guzzo F, Montera R, Angioli R (2015) Utility of tumor marker HE4 as prognostic factor in endometrial cancer: a single-center controlled study. Tumour Biol 36(6):4151–4156
Epstein E, Van Holsbeke C, Mascilini F, Måsbäck A, Kannisto P, Ameye L, Fischerova D, Zannoni G, Vellone V, Timmerman D, Testa AC (2011) Gray-scale and color Doppler ultrasound characteristics of endometrial cancer in relation to stage, grade and tumor size. Ultrasound Obstet Gynecol 38(5):586–593
Larson DM, Connor GP, Broste SK, Krawisz BR, Johnson KK (1996) Prognostic significant of gross myometrial invasion with endometrial cancer. Obstet Gynecol 88:394–398
Rutledge FN, Freedman RS, Gershenson DM (1987) Gynecologic cancer: diagnosis and treatment strategies. University of Texas Press, Austin, p 3
Yokoyama Y, Maruyama H, Sato S, Saito Y (1997) Indispensability of pelvic and paraaortic lymphadenectomy in endometrial cancers. Gynecol Oncol 64:411–417
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest or financial ties to disclose. This article does not contain any studies with human participants or animals performed by any of the authors. Therefore, informed consent was not necessary.
Rights and permissions
About this article
Cite this article
Angioli, R., Plotti, F., Capriglione, S. et al. Preoperative local staging of endometrial cancer: the challenge of imaging techniques and serum biomarkers. Arch Gynecol Obstet 294, 1291–1298 (2016). https://doi.org/10.1007/s00404-016-4181-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-016-4181-z