Abstract
Purpose
To identify predictive ultrasound signs for unfavorable outcome in fetal gastroschisis (GS).
Methods
This is a retrospective cohort study among pregnant women with the prenatal diagnosis of GS between 1998 and 2011 at the University of Wuerzburg, Germany. Analysis included prenatal ultrasound scans, neonatal intensive care unit (NICU) records, and pediatric records. The collected variables included maternal and fetal demographics, as well as an analysis of predictors for unfavorable fetal outcome. Unfavorable outcome was defined by more than 2 postnatal surgical interventions, intestinal resections, and long time to oral feeding (≥4 weeks).
Results
35 cases of fetal GS were diagnosed, whereby 23 cases met the inclusion criteria and were evaluated by prenatal ultrasound and postnatal outcome. Based on the postnatal situation, 15 patients were classified in a good prognosis group and 8 patients in a poor prognosis group. Fetuses with poor prognosis were presented later during pregnancy (21.1 ± 6 vs. 26.9 ± 5.3 weeks; p < 0.01) and delivered at earlier gestational age (35.6 ± 0.8 vs. 33.4 ± 1.4 weeks; p < 0.01) with lower birth weight (2074 ± 306.3 vs. 2559 ± 255.4 g; p < 0.01). There were no differences in prenatal findings like growth restriction, amniotic fluid index, or Doppler results between good and poor prognosis group. However, early detected and long-lasting bowel dilatation was associated with poor prognosis.
Conclusion
Late presentation and early gestational age at delivery are associated with poor prognosis in neonates with GS. Furthermore, early onset as well as long duration of bowel dilatation is associated with poor fetal outcome, while other ultrasound characteristics are not able to predict poor prognosis of GS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
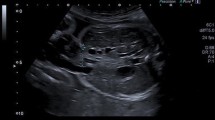
Gastroschisis (GS) is a defect of the right anterior abdominal wall through which intestinal loops, liver, or spleen can prolapse (Fig. 1). In contrast to exomphalos, there is no membrane covering the abdominal contents. GS affects around one in 3000–8000 pregnancies with an increasing prevalence worldwide [1].
Several hypotheses concerning the pathogenesis of GS exist, which include a failure of the mesoderm, rupture of the amnion of the umbilical ring, the abnormal involution of the right umbilical vein, and a disruption of the right vitelline or yolk sac artery [2]. Gastroschisis more frequently affects young primigravids with low socio-economic status, educational attainment, and poor maternal diet [3]. Furthermore, a number of sympathomimetic drugs (e.g., cocain) and nicotine abusus have been described to increase the occurrence of GS [4–6].
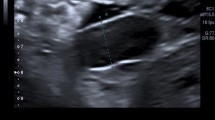
The diagnosis is nowadays mostly made by prenatal ultrasound with a detection rate of 90 % in Europe [7]. When the physiological prolapse of the bowel should have already returned into the abdominal cavity at around 11 weeks of gestation, GS can be diagnosed by bowel floating freely in the amniotic fluid (Fig. 2). The prolapse is typically seen on the right abdominal wall next to the umbilical cord insertion with an absence of peritoneal covering. Prenatal management for GS includes counseling by a multidisciplinary team of obstetricians, neonatologists, and pediatric surgeons. Differentiated ultrasound is a major part of the prenatal management to prevent stillbirth and bowel damage. Postnatally, neonatologists attend to airway stabilization and circulation as required while protecting the bowel with sterile film to avoid infection, kinking of intestinal loops, and high loss of fluid by eviscerated bowel. Depending on the degree of disproportion, returning the bowel to the abdominal cavity is performed either in a single operation (primary abdominal wall closure) or in several surgical interventions such as plastic closure (Silo bag, Schuster`s plastic) or patch repair (goretex patch) [8]. The aim of this study was to identify ultrasound signs that can predict a favorable or unfavorable diagnosis for fetal gastroschisis and therefore might help obstetricians and pediatric surgeons in counseling parents with fetal diagnosis of GS regarding perinatal and postnatal management.
Methods
In this retrospective study, data were collected of patients with prenatal diagnosis of GS at our institution between 1998 and 2011. Cases with terminations of pregnancy were excluded as well as fetuses with additional anomalies, twin pregnancies, and with missing postnatal follow-up. Analysis comprised prenatal ultrasound scans, neonatal intensive care unit (NICU) records, and pediatric care unit records. Fetal biometry, maximal dilatation of intraabdominal and extraabdominal bowel, and the presence or absence of oligohydramnios or polyhydramnios were assessed by prenatal ultrasound. Color and pulsed wave Doppler were used to measure the pulsatility index of the umbilical artery. Furthermore, maternal records were reviewed for gestational age at delivery, mode of delivery, birth weight, and APGAR scores at 5 and 10 min. Number and extent of surgical interventions, length of time to oral feeding, and days in the NICU were retrieved from pediatric records.
Based on the postnatal situation, we hypothesized that two groups with a different prognosis exist: Group 1 includes infants with favorable outcome which is characterized by low number of surgical procedures (≤2), no bowel resection, and length of time to oral feeding up to 4 weeks. Group 2 includes infants with unfavorable prognosis with more than 2 surgical interventions, intestinal resections, and long time to oral feeding (≥4 weeks).
Statistical analysis was performed on SPSS Statistics 22 Software using Mann–Whitney-U test, Student’s t test, and Fisher`s exact test. Statistical significance is assumed for p < 0.05.
Results
Between 1998 and 2011, 35 cases of fetal gastroschisis were prenatally diagnosed by ultrasound. Twelve (34.3 %) cases had to be excluded either because of termination of pregnancy (8.6 %), incomplete data (8.6 %), associated extraabdominal congenital anomalies (14.3 %), twin pregnancy (2.9 %), or intrauterine death (2.9 %). Thus, 23 cases of gastroschisis met the inclusion criteria and were evaluated statistically. Associated extraabdominal anomalies were cleft lip palate, dolichocephalic head shape with dilated cerebrospinal fluid spaces, and dilatation of renal pelvis.
The mean maternal age was 21.6 (±3.7) years and 18 out of 23 (78 %) mothers were nulliparous and 21.7 % were smokers. Gestational age at presentation of mothers to our department was 23.1 weeks (±6.3) and 34.8 weeks (±1.5) at delivery. Detailed ultrasound examination during pregnancy was able to detect a mean bowel dilatation of 19 (±5.7) mm. Bowel dilatation of ≥15 mm was measured in 20 cases (86.9 %) and initially detected after a mean of 31.3 (±3.9) weeks. In 5 (21.7 %) cases, an abnormal amount of amniotic fluid was detected. Two patients presented with polyhydramnios, one with oligohydramnios and in one case initial oligohydramnios turned into polyhydramnios during pregnancy. Intrauterine growth restriction was registered in 4 of 23 cases (17.4 %) and Doppler examination was pathologic in 4 (17.4 %) cases. All infants were delivered by cesarean section, 19 (82.6 %) of which were performed electively. In 2 cases, cesarean section was performed earlier due to premature rupture of the membrane and in 2 cases because of the onset of preterm labor. Nine (39.1 %) children were male, and 14 (60.9 %) were female. The mean birth weight of all included neonates was 2390.6 g (±356.6), and the mean APGAR value 5 min after delivery was 9.0 (±0.8) (Table 1). Postnatally, a mean of 2.65 (±2.9) operations was performed. In 7 (30.4 %) cases, bowel resection had to be performed. 20 (87 %) children received a primary closure of the abdomen, and of these, 8 (40 %) received several more operations. The mean stay on ICU was 43.8 days (±48.5) and 76.0 days (±75.0) in hospital. Two (8.7 %) children died within 12 months after delivery due to necrotising enterocolitis or ischemia of small bowel.
We defined two groups with favorable outcome or unfavorable outcome regarding the number of surgical interventions, bowel resections, and time to oral feeding. Neonates with favorable outcome received a mean of 1.1 (±0.3) operations which were all performed within 12 h but did not receive any bowel resections. The time to oral feeding was 22.4 (±8.7) days. However, patients with poor prognosis received a mean of 5.6 (±3.3) operations and 7 of them (87.5 %) were bowel resected. The time to oral feeding in this group was 124.5 (±73.6) days (Table 2). We investigated potential signs in ultrasound that might be related to favorable or unfavorable prognosis of infants with GS. We found out that neither a pathologic Doppler (25 vs. 13.3 %, p = 0.5), nor an abnormal amniotic fluid amount (25 vs. 13.3 %, p = 0.44), nor the maximal dimension of bowel dilatation [21.4 mm (±5.8) vs. 17.7 mm (±5.4), p = 0.15] is a prediction marker for bad or good prognosis of GS. However, neonates with poor prognosis developed bowel dilatation (≥15 mm) significantly earlier than neonates with good prognosis (28.1 ± 4.4 vs. 33.0 ± 2.3 weeks; p = 0.02), and dilatation (>15 mm) persisted significantly longer [40.5 (±27.2) days vs. 17.4 (±14.9) days; p = 0.02] (Table 3). Furthermore, they were presented significantly later during gestation (21.1 ± 6 vs. 26.9 ± 5.3 weeks; p < 0.01) and were delivered significantly earlier than children with good prognosis (35.6 ± 0.8 vs. 33.4 ± 1.4 weeks; p < 0.01). Even after exclusion of cases that were delivered by secondary cesarean section, infants with poor prognosis (n = 5) were delivered significantly earlier by elective cesarean section than infants with good prognosis (n = 14) (251.9 ± 5.5 vs. 239.4 ± 8.3; p < 0.01). Patients with poor prognosis showed significantly lower birth weight with a mean of 2074 g (±306.3) vs. 2559 g (±255.4); p < 0.01. Moreover, infants with poor prognosis stayed longer in hospital and on ICU than infants with good prognosis (p < 0.01) (Table 4).
Discussion
Gastroschisis is a congenital defect of the abdominal wall with prolapsing intestinal loops that requires postnatal intensive care and prolonged parenteral nutrition. However, mortality rate is described to be around 5–10 % and neonates with gastroschisis normally develop well [9]. Even though prenatal diagnosis and surveillance via ultrasound play a crucial role in GS, it still remains unclear if ultrasound findings can predict adverse neonatal outcome. Therefore, we retrospectively investigated all cases of gastroschisis in our department between 1998 and 2011 regarding maternal and fetal demographics, fetal outcome, and prenatal ultrasound findings.
Patients with the diagnosis of fetal GS were mainly young primigravids which matches earlier published and recognized risk factors for the development of GS. Interestingly, nicotine or drug abuse was registered in 21.7 % of all cases in our cohort, and there was no prediction factor for adverse outcome of neonates with gastroschisis. It is conceivable that there is a higher number of unreported cases of nicotine abuse because of a concealment due to social rejection. Furthermore, in the presented study, around 14 % of all reported cases of gastroschisis were excluded due to associated anomalies, even though it is widely known that there is a lower incidence of associated anomalies with gastroschisis in comparison to other abdominal wall defects. These results are in line with other publications that show associated anomalies of kidney, limb, and CNS with a similar incidence [10, 11]. The importance and meaningfulness of detailed ultrasound to predict fetal outcome in case of gastroschisis is controversly discussed in literature. Our data suggest that regardless of ultrasound findings, i.e., dimension of maximal bowel dilatation, amniotic fluid amount, Doppler or growth restriction, no predictions of favorable or unfavorable outcome can be made. This is in accordance to findings of Overcash et al. who were also able to show that ultrasound findings such as IUGR, bowel dilatation, and oligohydramnios were not predictive for adverse neonatal outcome [12]. On the other hand, Aina-Mumuney et al. presented a study of 34 cases with GS identifying associations between stomach dilatation and postnatal mortality and morbidity [13]. Furthermore, also Santiago-Munoz et al. describe associations of ultrasound findings such as stomach dilatation and large abdominal defects with adverse neonatal outcome [14]. But in our study, poor fetal outcome was associated with early onset of bowel dilatation and infants presented dilatation of the bowel for a significantly longer period of time. This is in accordance to Nick et al., who described an association of intraabdominal bowel dilatation during the second trimester of pregnancy and adverse outcome [15].
In our study, only 17 % of all fetuses with GS were prenatally diagnosed with growth restriction which was not associated with unfavorable diagnosis. These findings are in accordance to findings of Puligandla et al. who describe growth restriction in only 15 % of all cases and comparable mortality and morbidity to normally grown neonates [16]. Fetuses in our study were more often female (60.9 %) than male which is in disparity to current literature stating that GS is associated with male sex [5]. In our study, we were able to show that neonates with worse outcome were significantly later presented to our department with GS during gestation which could allow the conclusion of a later detection of GS during gestation when compared to infants with good outcome. Moreover, neonates with poor outcome were significantly earlier delivered than neonates with favorable prognosis. In the poor prognosis group 3, neonates were delivered by secondary cesarean section due to premature labor or rupture of the membrane, whereas only one of all cases in the good prognosis group was delivered by secondary cesarean section. But when cases of secondary cesarean section are excluded in both groups, the gestational age at birth is still significantly higher in the good prognosis group. This fact might be based on earlier indication of elective cesarean section in the poor prognosis group. These results are comparable with Cain et al. [17]. As a consequence, neonates with unfavorable outcome presented lower birth weights than neonates with favorable outcome. Even though initial assessment by APGAR score did not differ in the two prognosis groups, children with unfavorable outcome stay significantly longer in NICU and in hospital in general. These findings are again similar to those of Overcash et al. [12].
Regarding the delivery mode, all cases of GS in our department were delivered by cesarean section. Even though there is no evidence for better outcome due to CS, we performed elective CS in 82.6 % of all cases.
The presented study is limited by the retrospective design and therefore lacks the advantages of a large prospective randomized controlled multicenter study design. The intention was to analyze a local cohort over a long period of time (13 years) in order to characterize management strategies, outcomes, and advances in prenatal care in our institution. Furthermore, it was of major interest to compare prenatal ultrasound findings in neonates with good or poor prognosis to investigate potential prediction factors of prenatal ultrasound diagnostic.
In summary, we present a retrospective single-center analysis of ultrasound prediction markers for fetal outcome of gastroschisis. Even if our data show that established criteria of ultrasound like IUGR, Doppler, or amniotic fluid amount are not associated with poor fetal outcome, it is likely that an early detected and long-lasting bowel dilatation of more than 15 mm might be associated with poor fetal outcome. On the other hand, early detection and diagnosis of gastroschisis, as well as a late delivery, might lead to better fetal outcome. As a result, early prenatal diagnosis, counseling, and referral to a specified center should be a major focus, while detailed ultrasound during pregnancy can help obstetricians only to limited extent when counseling parents regarding the likelihood of postnatal adaption impairments, bowel complications, or length of hospitalization.
References
Loane M et al (2007) Increasing prevalence of gastroschisis in Europe 1980–2002: a phenomenon restricted to younger mothers? Paediatr Perinat Epidemiol 21(4):363–369
David AL, Tan A, Curry J (2008) Gastroschisis: sonographic diagnosis, associations, management and outcome. Prenat Diagn 28(7):633–644
Torfs CP et al (1998) Association between mothers’ nutrient intake and their offspring’s risk of gastroschisis. Teratology 58(6):241–250
Goldbaum G, Daling J, Milham S (1990) Risk factors for gastroschisis. Teratology 42(4):397–403
Torfs CP et al (1994) A population-based study of gastroschisis: demographic, pregnancy, and lifestyle risk factors. Teratology 50(1):44–53
Schulz AC et al (2012) A classic twin study of isolated gastroschisis. Fetal Pediatr Pathol 31(5):324–330
Garne E et al (2007) Gastrointestinal malformations: impact of prenatal diagnosis on gestational age at birth. Paediatr Perinat Epidemiol 21(4):370–375
Orion KC et al (2011) Outcomes of plastic closure in gastroschisis. Surgery 150(2):177–185
Bradnock TJ et al (2011) Gastroschisis: one year outcomes from national cohort study. BMJ 343:d6749
Mastroiacovo P et al (2007) Gastroschisis and associated defects: an international study. Am J Med Genet A 143A(7):660–671
Ruano R et al (2011) The association of gastroschisis with other congenital anomalies: how important is it? Prenat Diagn 31(4):347–350
Overcash RT et al (2014) Factors associated with gastroschisis outcomes. Obstet Gynecol 124(3):551–557
Aina-Mumuney AJ et al (2004) A dilated fetal stomach predicts a complicated postnatal course in cases of prenatally diagnosed gastroschisis. Am J Obstet Gynecol 190(5):1326–1330
Santiago-Munoz PC et al (2007) Outcomes of pregnancies with fetal gastroschisis. Obstet Gynecol 110(3):663–668
Nick AM et al (2006) Second-trimester intra-abdominal bowel dilation in fetuses with gastroschisis predicts neonatal bowel atresia. Ultrasound Obstet Gynecol 28(6):821–825
Puligandla PS et al (2004) The significance of intrauterine growth restriction is different from prematurity for the outcome of infants with gastroschisis. J Pediatr Surg 39(8):1200–1204
Cain MA et al (2014) Perinatal outcomes and hospital costs in gastroschisis based on gestational age at delivery. Obstet Gynecol 124(3):543–550
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no potential conflict of interest.
Rights and permissions
About this article
Cite this article
Stüber, T.N., Frieauff, E., Weiß, C. et al. Prenatal sonographic ultrasound predictors for the outcome in fetal gastroschisis: a retrospective analysis. Arch Gynecol Obstet 293, 1001–1006 (2016). https://doi.org/10.1007/s00404-015-3936-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-015-3936-2