Abstract
Introduction
Minimal invasive approaches have proven beneficial for patients undergoing myomectomy and hysterectomy, but necessary tissue morcellation carries the risk of cell dissemination in rare cases of inadvertent malignancy. Performing the morcellation process within a contained bag system may prevent spilling and therefore enhance safety of the laparoscopic procedures.
Material and methods
The present study describes the development and experimental evaluation of a new bag system in vitro and in vivo in a pig model of laparoscopic supracervical hysterectomies.
Results
The main results on n = 8 procedures with in-bag morcellation compared to n = 8 controls without bag indicate reproducible feasibility and protective effect of the new bag, which is the first published to our knowledge that does not require puncturing in a standard multiport laparoscopy setting. Overall surgery time was significantly prolonged in the bag group by 12.86 min (P = 0.0052; 95 % confidence interval 4.64–21.07), but peritoneal washings were negative for muscle cells in all cases with bag use, compared to positive cytology in 5/8 cases without bag (P = 0.0256).
Conclusion
Clinical trials will now be necessary to investigate the reproducibility of these encouraging data in human application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Minimally invasive approaches have proven beneficial for patients undergoing myomectomy as well as hysterectomy in comparison to open techniques, as they reduce significantly the surgery-associated morbidity [1, 2]. To retrieve surgical specimens from the abdomen, however, the minimally invasive concept requires morcellating them intra-abdominally. This applies obviously to enucleated fibroids and supracervically amputated uteri, but may also become necessary during total laparoscopic hysterectomy, if the specimen is too large to pass the vaginal opening [3]. Manual morcellation has been clinical routine long before power-driven morcellators were introduced in 1993 to ease the procedure [4].
Using laparoscopic morcellation for two decades, not only benefits but also potential risks associated with this technique have been reported. Among other, i.e., mainly mechanical, risks [5, 6], the potential of harmful cell spreading from the morcellated tissue throughout the abdominal cavity was reported. When seeding, this may lead to benign conditions such as so-called parasitic leiomyoma or peritoneal adenomyosis [7–11], but may also result in peritoneal dissemination of inadvertently morcellated malignancy, e.g., uterine sarcoma mistaken for benign leiomyoma [11–13]. Despite controversial discussion [14], evidence suggests that the prognosis of malignant disease may thus be worsened iatrogenically [15–18].
Prevalence data of such unsuspected malignancy at the time of hysterectomy and power morcellation to date derive from retrospective analyses and vary largely. In a cohort of 36,470 patients from a US insurance database, it was determined at 0.27 % [19], while it was found at only 0.13 % of 10,731 morcellated uteri in a German single center series [20]. Estimates from meta-analyses and literature reviews by international gynecological societies range from 0.1 to 0.25 % [21–26].
The risk of inadvertently morcellating and spreading malignant tissue led to strong warnings against the use of power morcellators to treat uterine fibroids by the US Federal Drug Administration (FDA) in April and November 2014 [27, 28], thereby questioning essential aspects of the minimally invasive concept for myomectomy and hysterectomy. On the other hand, it was estimated using a decision analysis model [23, 29] that converting all hysterectomies currently undergoing power morcellation to open procedures would result in a substantial increase in morbidity and mortality from open surgery.
Acknowledging that there will remain a risk of unsuspected malignancy despite all efforts in preoperative differential diagnostics, the question arises how to reduce the risk of tissue morcellation instead of abandoning the benefits of minimally invasive treatment [21–26, 29–31]. Morcellating within an intra-abdominally placed, closed containment system has been suggested for this purpose as a potential method to prevent cell spilling [22, 23, 25, 32, 33].
The aim of the present experimental study was therefore, to develop an innovative bag system for closed power morcellation and pre-clinically evaluate its ability to reduce the risk of fluid or cell spilling during laparoscopic supracervical hysterectomy.
Materials and methods
Bag system
The material was to be compatible for use in human, inflatable, transparent, water and cell tight, not inflammable and not melting under endoscopic light exposure. These clinical requirements were fulfilled by a 50 µm polyurethane film, not allowing molecule migration above 2–5 Å (2–5 × 10−10 m), with stress failure at 60 MPa.
The dimensions were defined according to human anatomy and an average pneumoperitoneum volume with the aim of allowing visualized contained power morcellation using an otherwise unmodified technique in a multiport laparoscopic approach.
Feed sizes of 340 × 250 mm were calculated to allow a pseudo-pneumoperitoneum capacity of 2.5 l, while the bag could still be folded suitably to enter a 12 mm trocar. An opening of 160 mm enables the surgeon to even bring in fibroid or uterine specimens clinically considered as large. This site was well designated for suprapubic morcellator access after pulling the opening outside the abdominal wall. A separate 16 mm-wide tubular opening was created for optic trocar access. This second opening was designed to be pulled out at the umbilical site, giving access to an umbilical optic trocar with usual CO2 insufflation (Fig. 1). To protect the optic against contamination, keeping it uncontaminated for eventually continuing the surgery after morcellation, a rigid sleeve was additionally designed at 250 mm length and 11 mm diameter with a window at its tip (Fig. 2).
After completing power morcellation, the optic should be withdrawn from the bag with its sleeve, and the possibly contaminated sleeve removed.
To remove the bag, it is pulled through toward the suprapubic side. The bag was designed to prevent contamination also during this phase of the procedure, when the former umbilical opening is to be pulled through the peritoneal cavity. To achieve this goal, the respective tubular opening was designed at a length of 190 mm, not only to suit different anatomies but also to allow everting its outer part. By the eversion, contamination could be prevented during the procedure and optic sleeve removal. The protected, everted part of the tubular opening is unrolled after removing the optic and sleeve, and occluded by tying a double knot (Fig. 3). Thus, the potentially contaminated section of the bag should be securely tight, while the part of the bag, which gets into contact with the peritoneal cavity during the pull-through, should have remained uncontaminated.
Experimental model
The properties of the new bag system (manufacturer: A.M.I. GmbH, Austria) were first tested in vitro, morcellating beef tongues in a laparoscopy training device using standard laparoscopy equipment including a Rotocut morcellator (KARL STORZ GmbH & Co. KG, Germany). Typical multiport laparoscopy was simulated with an umbilical 11 mm trocar and 10 mm optic, two laterally positioned 6 mm trocars and one suprapubically positioned 12 mm trocar, which served to introduce the bag. Specimens to be morcellated measured 10 × 6 × 5 cm. Three surgical procedures were simulated consecutively in this setting.
As a second step, experimental evaluation was continued in vivo after obtaining ethical committee approval performing laparoscopic supracervical hysterectomy on n = 16 PIC-variety farmhouse pigs.
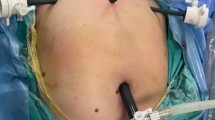
The animals were randomly distributed into two groups: in group A (n = 8), power morcellation and tissue extraction were performed using the new containment bag system (“bag group”), and group B (n = 8) was operated conventionally without bag (“control group”) (Fig. 4).
All operative procedures were performed by the same team of surgeons (SR, AH).
Three different types of power morcellators were used: Morcellex (Gynecare), Lina Xcise (Lina Medical) and Rotocut (Karl Storz). Each type was used in each group in a pre-defined number (Morcellex x3, Lina Xcise x3, Rotocut x2), but in a randomly assigned order.
Animal surgical procedure
The animal was positioned in lithotripsy position. The pneumoperitoneum was established via Veress needle at the umbilical site, an 11 mm trocar inserted transumbilically and the optic (0°) introduced. Under visual control (HD), another 11 mm suprapubic trocar and two 6 mm lateral trocars were positioned and the peritoneal cavity inspected and rinsed with physiologic NaCl. The fluid was extracted by suction for peritoneal cytology. Supracervical hysterectomy started with dividing the adnexa from the uterine horns, followed by dissection of the parametria and cutting the uterine vessels after coagulation and completed by dissecting the uterine corpus from the cervix. The entire procedure was performed using bipolar scissors for preparation and cutting, eventually replaced by bipolar clamps for coagulation in case of bleeding. The tissue was manipulated with a traumatic 5 mm grasping forceps (equipment and instrumentation: Karl Storz, Germany).
In group A, the containment bag was now introduced suprapubically after replacing the 11 mm trocar at this site by a blunt 12 mm introductory trocar. The uterine specimen was put into the bag via its large opening and the same pulled back through the suprapubic port site, while removing the introductory trocar again. Using a traumatic forceps via a lateral trocar, the second, small opening of the bag was then led through the umbilical trocar, while the trocar itself was removed for later use. At this time, both openings of the bag were situated extraperitoneally. Via the small bag opening at the umbilical site, the optic was introduced with its cover sheath, using the blunt introductory trocar now at this site. Pseudo-pneumoperitoneum in the bag was established by inflating the bag via this trocar, maintaining 12 mmHg during the entire procedure. The morcellator was inserted via the large opening suprapubically into the bag, and in-bag morcellation of the uterine specimens performed in the usual manner (Fig. 5). After completion, the morcellator was removed from the bag and the bag instilled with blue dye solution for tightness control. Now, the optic and its cover sheath were withdrawn together with the introductory trocar, the small bag opening closed securely by knotting, and the bag extracted from the peritoneal cavity by pulling it through from its suprapubic part. The original umbilical trocar and the optic (after removing the protective sheath) were re-inserted and the procedure continued by re-establishing the pneumoperitoneum.
In group B, tissue morcellation and extraction were performed in a routine manner via suprapubic access.
In both groups, at this time of the procedure, the peritoneal cavity was rinsed again with physiologic NaCl and the fluid again suctioned to obtain a second, post-morcellation peritoneal cytology. The procedure was ended by removing the pneumoperitoneum and closing the abdominal wall incisions by sutures.
Evaluation parameter and statistical analysis
Bag handling, pseudo-pneumoperitoneum, feasibility of power morcellation and visualization quality were qualitatively registered as described subjectively by the surgeons during both, in vitro and in vivo experiments.
Bag integrity including security of knot occlusion was checked by visual inspection for lesions and blue dye spilling. Additionally, the bags were postoperatively inflated with pressurized air at 20 mbar (15 mmHG) under laboratory conditions.
Regarding the in vivo evaluation, animal weight, initial CO2 volume of the pneumoperitoneum, weight of morcellated tissue and amount of fluid collected for cytology were registered for baseline characteristics comparison of both groups for possible heterogeneity using unpaired t test.
Time of surgery, time of bag use and overall volume of used CO2 were measured to analyze the impact of bag use for the duration of surgery. Comparisons between the two groups were analyzed using unpaired t test.
Peritoneal cytology was analyzed for the presence of smooth muscle cells in the bag group (group A) versus the control group (group B) prior and post-morcellation as an indicator for possible cell spilling. Examination was done by two cytologists independently (CN, FO) blinded to the procedures and the experimental groups. The collected fluid specimens were centrifuged, suspended in CytoLytR, centrifuged, decanted and resuspended in PeservCytR, then processed using ThinPrepR 5000 automated slide processor (Hologic) and stained with Pap stain using the Tissue Stainer COT 20 (Medite). Positive controls were prepared similarly. Cellularity was first graded semi-quantitatively as none (0), low (1+), moderate (2+) or high (3+). Additional centrifugation steps with resuspension in CytoLytR solution were performed for bloody specimens. Cell blocks were then prepared by centrifuging and decanting the residual solution to yield a cell pellet, which was suspended using thrombin and 1 ml citrate and then embedded in paraffin wax, sectioned and stained with hematoxylin and eosin using standard technique. Slides were examined for the presence of mesothelial cells, lymphocytes, granulocytes and spindle cells using the above-described score. Samples scored positive for spindle cells based on morphological criteria were subjected to immunocytochemistry for smooth muscle cell identification. For this purpose, cell block sections were deparaffinized, washed in ethanol and in phosphate-buffered saline, and then incubated with the primary antibodies ActinSMA (DAKO-Clone 1A4 RTU), desmin (DAKO-Clone D33 RTU) and caldesmon (Ventana-Clone E89 RTU). Standard detection technique was performed using ultraView Universal Alkaline Phophatase Red Detection Kit (Ventana). Slides were counterstained with Bluing Reagent. Differences in smooth muscle cell presence was compared for Group A and B in a contingency table and analyzed using Fisher’s exact test for significance.
Results
Feasibility
Handling of the bag system proved intuitively feasible and was successful in all cases, in vitro and in vivo, during all steps of the procedures. The same applies for specimen positioning into the bag. Also in all cases, sufficient pseudo-pneumoperitoneum, subjectively similar to the usual pneumoperitoneum in human procedures, could be established within the bag, using routine pressure-controlled CO2 insufflation in the same way as in control cases. Visualization was clear and not impaired while using the protection sleeve. Power morcellation proved feasible without the need to modify the technique as compared to the conventionally performed cases. No differences were found between the three morcellator devices as far as in-bag procedure feasibility was concerned. No differences in feasibility were noted between the two surgeons.
Bag integrity
Visual inspection after use did not reveal any lesions of the bags. Fluid tightness was proved in all cases by visual control for eventual blue dye spilling. Bags did not lose blue dye-stained fluid, nor was spilled blue dye found in the peritoneal cavity. All used bags proved also tight during pressurized air inflation.
Baseline characteristics of comparison groups
The analysis of baseline characteristics did not reveal any heterogeneity among both groups (Table 1). The median weight of the animals in group A was 32 kg (minimum 30, maximum 34) and in group B 32.5 kg (minimum 31, maximum 35). The difference was not significant (P = 0.1450; 95 % confidence interval of the mean difference −0.389 to 2.389).
Mean volumes of CO2 at initial pneumoperitoneum were 3.988 ± 0.511 l in group A and 3.913 ± 0.422 l in group B. The mean difference of 0.075 (95 % confidence interval −0.428 to 0.578) l was not statistically significant (P = 0.7537).
Mean weights of morcellated tissue were 17.263 ± 8.766 g in group A and 26.575 ± 15.285 g in group B. The mean difference of 9.313 g (95 % confidence interval −22.674 to 4.049) was not statistically significant (P = 0.1572).
Time of surgery and CO2 volume use (Table 2)
The mean overall duration of surgery was 31.86 ± 8.90 min in group A versus 19.00 ± 4.51 min in group B. The mean difference of 12.86 min (95 % confidence interval 4.64–21.07) was highly significant (P = 0.0052).
The time of bag use in group A ranged from 10 to 36 min with a median of 13.5 min. The case in which the bag was in place during 36 min was the first in group A and thus the initial bag application. If the time of bag use was calculated without this case, it ranged from 10 to 20 min among the other seven cases with a median of 12 min. The mean time of surgery in group A exclusive bag use time was 18.29 + 6.29 min, not significantly different from the overall time in group B (P = 0.8112). The mean difference was 0.71 (95 % confidence interval −7.09 to 5.66).
The mean overall volumes of CO2 used to maintain pneumoperitoneum during the entire surgery were 79.500 ± 28.597 l in group A and 44.350 ± 21.677 l in group B. The mean difference of 35.150 (95 % confidence interval 7.076–63.224) proved statistically significant (P = 0.0180).
Peritoneal cytology
Prior to morcellation, mean fluid amounts collected for peritoneal cytology were 935.63 ± 48.72 ml in group A and 976.63 ± 59.14 ml in group B. The mean difference of 41 ml was not significant (P = 0.1524; 95 % confidence interval −99.10 to 17.10). After morcellation, the mean amounts were 1007.25 ± 40.61 and 992.75 ± 45.47 ml with a not significant difference of 14.50 ml (P = 0.5121; 95 % confidence interval −31.73 to 60.73) (Table 1).
Cellularity was found positive in all peritoneal fluid specimens prior to morcellation, regardless of the experimental groups (group A: score 3+ n = 4, score 2+ n = 4; group B: score 3+ n = 4, score 2+ n = 4). Post-morcellation, the cellularity of the specimens was obtained in 15/16 cases (group A: score 3+ n = 4, score 2+ n = 3; score 1+ n = 1; group B: score 3+ n = 6, score 1+ n = 1). In one case of group B, the fluid was found to be acellular except for the presence of erythrocytes. Morphologically, the cells found prior to morcellation could all be differentiated into mesothelial cells, lymphocytes or granulocytes, but no spindle cells. Post-morcellation, all specimens in group A and 7/8 in group B as well contained mesothelial cells, lymphocytes or granulocytes, and additionally spindle cells (Fig. 6) were detected in 5 cases of group B versus none of group A. These were identified as smooth muscle cells by immunocytochemical staining in four cases with positive SMA (Fig. 7). In one case SMA was negative, but desmin and caldesmon were positive. The difference between the two groups proved statistically significant (P = 0.0256). The results are summarized in Table 3.
Immunocytochemical identification of smooth muscle cells by SMA antibody staining: same case as in Fig. 6 post-morcellation without bag (ActinSMA, 20×)
Discussion
Tissue morcellation during hysterectomy or myomectomy is consistently considered contraindicated in settings where uterine malignancy is either known or suspected. But despite meticulous preoperative diagnostic workup, there remains a risk of inadvertently morcellating malignancy in presumed benign tissue [21, 22, 25, 26, 31, 34]. Cell spread throughout the peritoneal cavity could potentially be prevented in such circumstances by using a retrieval bag for tissue morcellation and extraction [24–26, 32, 33, 35].
The first attempts date back to 1993 in urology [36]. Then, and during subsequent years, laparoscopic techniques were clinically and experimentally evaluated for nephrectomy in early and low-grade renal cell carcinoma. Morcellation in a bag was intended to prevent iatrogenic dissemination and especially port site metastases. Oncological results were discussed controversially [37–39]. As far as technical aspects are concerned, bags were inserted via a port site and, after positioning the specimen and exteriorization of the bag’s mouth, morcellation was performed mechanically or power driven, under direct inspection or with the help of a laparoscopy optic inserted beneath the morcellator into the bag [39–42]. In general surgery, a comparable technique has been reported for splenectomy with manual morcellation in an exteriorized bag after laparoscopic preparation [43].
Several approaches have been described in gynecology to date using different ways of bag insertion, morcellation and tissue extraction. These include abdominal port site [44–49] or transvaginal [50] insertion of the bag, open abdominal (mini-laparotomic) [49] or vaginal in-bag morcellation [51–55], each manually under direct vision, and laparoscopic power morcellation within a pseudo-pneumoperitoneum in the bag inside the abdomen [44–50]. The latter technique provides the possibility of containing morcellation, when vaginal access is not suitable.
Bags used for the respective approaches have different sizes and materials, but all have a common geometry consisting of a sac with one mouth. After bag insertion into the peritoneal space, the specimen is positioned in the bag via this mouth and the same exteriorized onto the abdominal wall, thus following the principles of usual retrieval bags [44, 47, 48]. The challenge at this point is to provide safe morcellation which requires access for the morcellator device, enough space and visual control. If direct vision is not an option, because vaginal access is not practicable and laparotomy needs to be avoided, laparoscopic guidance is needed.
In analogy to the vaginal approach, single incision umbilical trocars provide suitable simultaneous access for morcellator and optic, as described by Cohen [44]. Multiport approach requires at least one puncture of the intraperitoneally insufflated bag in all so far published systems [44–50]. Puncturing the bag, however, carries the risk of leakage and spilling, and thus opposes the intent of contained morcellation [21, 23, 25, 26, 56].
To our knowledge, the bag system reported in the present study is the first providing multiport access without puncturing.
The initial procedure compares to that yet reported with bag insertion into the peritoneal space and specimen positioning into the bag. In contrast to others, our pre-folded system fits through a 12 mm suprapubic trocar and intraperitoneal orientation is facilitated by a ring, keeping the bag mouth open to ease the entry of the specimen. Up to this point, the procedure does not differ from usual multiport laparoscopy approach. The bag’s mouth, where the specimen has been placed in, is now exteriorized suprapubically and the respective trocar removed. This site will allow morcellator access at its typical position without the necessity of modifying the classical, trained approach.
In contrast to earlier reported systems, the bag here described has a second preformed mouth resembling a tube, designed for optic trocar access. This tube is pulled out transumbilically and replaces the optic trocar. Re-inserting this trocar and the optic now bluntly inside the tube gives visual access to the bag interior in a setting completely comparable to usual multiport laparoscopy. Also pseudo-pneumoperitoneum is established in a trained manner via the umbilical optic trocar.
Systems requiring bag puncture achieve to prevent spilling from the puncture hole by using a balloon tip trocar [44–50]. Our system provides at this point an intact closed, contained environment for subsequent morcellation.
One aspect of the potential of cell dissemination has not been addressed in earlier concepts to our knowledge. The laparoscopy optic may be contaminated by aerosolized tissue components and dissemination might occur during continuation of the surgical procedure. To overcome this risk, a window-tipped sleeve has been designed to cover the optic, which is eliminated after completing the morcellation-associated part of the procedure. Concerns about impaired visualization did not prove relevant during our experimental evaluation.
Contained power morcellation was feasible without problems in all cases of our experimental series. No differences were noted between the two operating surgeons, or with regard to the morcellator types.
Important prerequisites consist of adequate size and geometry of the bag, allowing tissue handling and correct visualization [21, 23]. Earlier reports used bags at volumes between 1000 and 3100 ml [44, 47–50]. The bag presented here provides a capacity of 2500 ml, which proved suitable to establish a pseudo-pneumoperitoneum totally comparable to clinical routine. Morcellation, therefore, did not differ from routine use either. The experimental model of performing the surgery in pigs proved well selected with regard to the question of space, with average pneumoperitoneum volumes measuring around 3.9 l. Limitations of the experimental model may consist of the smaller tissue sizes and shape of the morcellate, and clinical trials will have to prove equal success in human.
Using the bags takes time. Earlier clinical trials reported 26–30 min additional duration of surgery [46, 49]. In our experimental setting, the time consumed for handling the bags ranged from 10 to 36 min with an average of 12.9 min. Timelines are also reflected in augmented CO2 consumption for maintaining the pneumoperitoneum. The significant difference in this regard may also be increased by CO2 loss during bag insertion, removal, and re-establishing the pneumoperitoneum afterward. The rest of the procedure including the morcellation process, however, was not influenced with reference to time measurements, which did not differ statistically.
As claimed before [21–23], the transparency of the bag facilitated simultaneous visualization inside the bag and of the surrounding organs, thus allowing the surgeon to control morcellation in a previously trained routine manner, protecting adjacent tissue from the morcellator blade by keeping adequate distance. As the presented bag system does not require puncture from outside, there is no necessity of obscured instrument action, which had been criticized compared to other techniques [23, 56].
Further dissemination risk exists during bag removal [25]. In approaches with bag puncture, the described strategies to prevent spilling from the hole include grasping at this site as well as suctioning to create negative pressure by keeping aerosols within the bag [44, 47, 48, 50]. The present approach does not have to fear leakage from a puncture, but from the constructive second opening of the bag. This challenge was solved by designing a special method for occlusion. Before the bag is removed by pulling it toward the suprapubic site, the tubular optic trocar opening at the umbilical site is occluded by knotting it twice. Because the inner surface may have been contaminated while withdrawing the optic and its sleeve, the tubular part is originally everted and its uncontaminated part not unrolled until knotting.
In fact, tightness was successfully ensured in all cases. Visual inspection did not reveal any lesion, formally confirmed by the absence of blue dye spilling or gas leakage.
As previously described in vitro by Cohen [57], cytologic analyses of fluid washings searching for muscle cells may indicate possible cell dissemination. Thus, the most important result of the present study consists of the complete absence of disseminated myometrial cells in peritoneal washings after morcellation within bags, while spindle cells were found in five of eight (4 of them smooth muscle actin positive) animals of the control group without bags. Negative findings in all cases of both groups prior to morcellation indicate that this was the most probable source.
Whereas cell dissemination was neither detectable in all cases without bags, the observed difference between the experimental groups with complete absence of spread cells when morcellation took place within the bag suggests a significant protective effect of the evaluated system.
A clinical trial will now be necessary to investigate the reproducibility of these encouraging experimental data in human application.
References
Bhave Chittawar P, Franik S, Pouwer AW, Farquhar C (2014) Minimally invasive surgical techniques versus open myomectomy for uterine fibroids. Cochrane Database Syst Rev 21;10:CD004638. doi: 10.1002/14651858.CD004638.pub3
Nieboer TE, Johnson N, Lethaby A, Tavender E, Curr E, Garry R, van Voorst S, Mol BW, Kluivers KB (2009) Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev 8;(3):CD003677. doi: 10.1002/14651858.CD003677.pub4
Condous G, Bignardi T, Alhamdan D, Van Calster B, Van Huffel S, Timmerman D, Lam A (2009) What determines the need to morcellate the uterus during total laparoscopic hysterectomy? J Minim Invasive Gynecol 16(1):52–55. doi:10.1016/j.jmig.2008.09.618 Epub 2008 Nov 8
Steiner RA, Wight E, Tadir Y, Haller U (1993) Electrical cutting device for laparoscopic removal of tissue from the abdominal cavity. Obstet Gynecol 81(3):471–474
Driessen SR, Arkenbout EA, Thurkow AL, Jansen FW (2014) Electromechanical morcellators in minimally invasive gynecologic surgery: an update. J Minim Invasive Gynecol 21(3):377–383. doi:10.1016/j.jmig.2013.12.121 Epub 2014 Jan 21
Milad MP, Milad EA (2014) Laparoscopic morcellator-related complications. J Minim Invasive Gynecol 21(3):486–491. doi:10.1016/j.jmig.2013.12.003 Epub 2013 Dec 10
Kho KA, Nezhat C (2009) Parasitic myomas. Obstet Gynecol 114(3):611–615. doi:10.1097/AOG.0b013e3181b2b09a
Hilger WS, Magrina JF (2006) Removal of pelvic leiomyomata and endometriosis five years after supracervical hysterectomy. Obstet Gynecol 108(3 Pt 2):772–774
Donnez O, Squifflet J, Leconte I, Jadoul P, Donnez J (2007) Posthysterectomy pelvic adenomyotic masses observed in 8 cases out of a series of 1405 laparoscopic subtotal hysterectomies. J Minim Invasive Gynecol 14(2):156–160
Pereira N, Buchanan TR, Wishall KM, Kim SH, Grias I, Richard SD, Della Badia CR (2015) Electric morcellation-related reoperations after laparoscopic myomectomy and nonmyomectomy procedures. J Minim Invasive Gynecol 22(2):163–176. doi:10.1016/j.jmig.2014.09.006 Epub 2014 Sep 11
Tan-Kim J, Hartzell KA, Reinsch CS, O’Day CH, Kennedy JS, Menefee SA, Harrison TA (2014) Uterine sarcomas and parasitic myomas after laparoscopic hysterectomy with power morcellation. Am J Obstet Gynecol. doi:10.1016/j.ajog.2014.12.002
Lieng M, Berner E, Busund B (2015) Risk of morcellation of uterine leiomyosarcomas in laparoscopic supracervical hysterectomy and laparoscopic myomectomy, a retrospective trial including 4791 women. J Minim Invasive Gynecol 22(3):410–414. doi:10.1016/j.jmig.2014.10.022 Epub 2014 Nov 1
Seidman MA, Oduyebo T, Muto MG, Crum CP, Nucci MR, Quade BJ (2012) Peritoneal dissemination complicating morcellation of uterine mesenchymal neoplasms. PLoS ONE 7(11):e50058. doi:10.1371/journal.pone.0050058 Epub 2012 Nov 26
Pritts EA, Parker WH, Brown J, Olive DL (2015) Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 22(1):26–33. doi:10.1016/j.jmig.2014.08.781 Epub 2014 Sep 3
Perri T, Korach J, Sadetzki S, Oberman B, Fridman E, Ben-Baruch G (2009) Uterine leiomyosarcoma: does the primary surgical procedure matter? Int J Gynecol Cancer 19(2):257–260. doi:10.1111/IGC.0b013e31819a1f8f
Park JY, Park SK, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH (2011) The impact of tumor morcellation during surgery on the prognosis of patients with apparently early uterine leiomyosarcoma. Gynecol Oncol 122(2):255–259. doi:10.1016/j.ygyno.2011.04.021 Epub 2011 May 12
George S, Barysauskas C, Serrano C, Oduyebo T, Rauh-Hain JA, Del Carmen MG, Demetri GD, Muto MG (2014) Retrospective cohort study evaluating the impact of intraperitoneal morcellation on outcomes of localized uterine leiomyosarcoma. Cancer 120(20):3154–3158. doi:10.1002/cncr.28844 Epub 2014 Jun 12
Bogani G, Cliby WA, Aletti GD (2015) Impact of morcellation on survival outcomes of patients with unexpected uterine leiomyosarcoma: a systematic review and meta-analysis. Gynecol Oncol 137(1):167–172. doi:10.1016/j.ygyno.2014.11.011 Epub 2014 Nov 20
Wright JD, Tergas AI, Burke WM, Cui RR, Ananth CV, Chen L, Hershman DL (2014) Uterine pathology in women undergoing minimally invasive hysterectomy using morcellation. JAMA 312(12):1253–1255. doi:10.1001/jama.2014.9005
Bojahr B, De Wilde RL, Tchartchian G (2015) Malignancy rate of 10,731 uteri morcellated during laparoscopic supracervical hysterectomy (LASH). Arch Gynecol Obstet. doi:10.1007/s00404-015-3696-z
http://www.sgo.org/wp-content/uploads/2014/04/SGO-Testimony-to-FDA-on-Power-Morcellation-FINAL.pdf. Accessed 12 Apr 2015
http://www.acog.org/Resources-And-Publications/Task-Force-and-Work-Group-Reports/Power-Morcellation-and-Occult-Malignancy-in-Gynecologic-Surgery. Accessed 12 Apr 2015
http://www.aagl.org/aaglnews/aagl-statement-to-the-fda-on-power-morcellation. Accessed 12 Apr 2015
http://www.aagl.org/aaglnews/member-update-5-aagl-response-to-fda-guidance-on-use-of-power-morcellation-during-tissue-extraction-for-uterine-fibroids. Accessed 12 Apr 2015
Brölmann H, Tanos V, Grimbizis G, Ind T, Philips K, van den Bosch T, Sawalhe S, van den Haak L, Jansen FW, Pijnenborg J, Taran FA, Brucker S, Wattiez A, Campo R, O’Donovan P, de Wilde RL, European Society of Gynaecological Endoscopy (ESGE) steering committee on fibroid morcellation (2015) Options on fibroid morcellation: a literature review. Gynecol Surg 12(1):3–15 Epub 2015 Feb 7
Beckmann MW, Juhasz-Böss I, Denschlag D, Gaß P, Dimpfl T, Harter P, Mallmann P, Renner SP, Rimbach S, Runnebaum I, Untch M, Brucker SY, Wallwiener D (2015) Surgical methods for the treatment of uterine fibroids—risk of uterine sarcoma and problems of morcellation: position paper of the DGGG. Geburtshilfe Frauenheilkd 75(2):148–164
http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.html. Accessed 12 Apr 2015
http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm424443.html. Accessed 12 Apr 2015
Brown J (2014) AAGL advancing minimally invasive gynecology worldwide: statement to the FDA on power morcellation. J Minim Invasive Gynecol 21(6):970–971. doi:10.1016/j.jmig.2014.08.780 Epub 2014 Sep 4
http://www.acog.org/About-ACOG/News-Room/Statements/2014/ACOG-Statement-on-Power-Morcellation. Accessed 12 Apr 2015
Singh SS, Scott S, Bougie O, Leyland N; SOGC Clinical Practice-Gynaecology Committee, Leyland N, Wolfman W, Allaire C, Awadalla A, Bullen A, Burnett M, Goldstein S, Lemyre M, Marcoux V, Potestio F, Rittenberg D, Singh SS, Yeung G; GOC Executive Committee, Hoskins P, Miller D, Gotlieb W, Bernardini M, Hopkins L (2015) Technical update on tissue morcellation during gynaecologic surgery: its uses, complications, and risks of unsuspected malignancy. J Obstet Gynaecol Can;37(1):68–81. English, French. Erratum. In: J Obstet Gynaecol Can;37(2):107
Kho KA, Anderson TL, Nezhat CH (2014) Intracorporeal electromechanical tissue morcellation: a critical review and recommendations for clinical practice. Obstet Gynecol 124(4):787–793. doi:10.1097/AOG.0000000000000448
Stine JE, Clarke-Pearson DL, Gehrig PA (2014) Uterine morcellation at the time of hysterectomy: techniques, risks, and recommendations. Obstet Gynecol Surv 69(7):415–425. doi:10.1097/OGX.0000000000000088
AAGL Advancing Minimally Invasive Gynecology Worldwide (2011) AAGL position statement: route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol 18(1):1–3. doi:10.1016/j.jmig.2010.10.001 Epub 2010 Nov 6
http://www.aagl.org/wp-content/uploads/2014/05/Tissue_Extraction_TFR.pdf
Urban DA, Kerbl K, McDougall EM, Stone AM, Fadden PT, Clayman RV (1993) Organ entrapment and renal morcellation: permeability studies. J Urol 150(6):1792–1794
Barrett PH, Fentie DD, Taranger LA (1998) Laparoscopic radical nephrectomy with morcellation for renal cell carcinoma: the Saskatoon experience. Urology 52(1):23–28
Tsivian A, Sidi AA (2003) Port site metastases in urological laparoscopic surgery. J Urol 169(4):1213–1218
Wu SD, Lesani OA, Zhao LC, Johnston WK, Wolf JS Jr, Clayman RV, Nadler RB (2009) A multi-institutional study on the safety and efficacy of specimen morcellation after laparoscopic radical nephrectomy for clinical stage T1 or T2 renal cell carcinoma. J Endourol 23(9):1513–1518. doi:10.1089/end.2009.0387
Parekh AR, Moran ME, Newkirk RE, Desai PJ, Calvano CJ (2000) Tissue removal utilizing steiner morcellator within a LapSac: effects of a fluid-filled environment. J Endourol 14(2):185–189
Landman J, Collyer WC, Olweny E, Andreoni C, McDougall E, Clayman RV (2000) Laparoscopic renal ablation: an in vitro comparison of currently available electrical tissue morcellators. Urology 56(4):677–681
Cai Y, Jacobson A, Marcovich R, Lowe D, El-Hakim A, Shah DK, Smith AD, Lee BR (2003) Electrical prostate morcellator: an alternative to manual morcellation for laparoscopic nephrectomy specimens? An in vitro study. Urology 61(6):1113–1117 discussion 1117
Greene AK, Hodin RA (2001) Laparoscopic splenectomy for massive splenomegaly using a Lahey bag. Am J Surg 181(6):543–546
Cohen SL, Einarsson JI, Wang KC, Brown D, Boruta D, Scheib SA, Fader AN, Shibley T (2014) Contained power morcellation within an insufflated isolation bag. Obstet Gynecol 124(3):491–497. doi:10.1097/AOG.0000000000000421
Einarsson JI, Cohen SL, Fuchs N, Wang KC (2014) In-bag morcellation. J Minim Invasive Gynecol 21(5):951–953. doi:10.1016/j.jmig.2014.04.010 Epub 2014 Apr 25
Vargas MV, Cohen SL, Fuchs-Weizman N, Wang KC, Manoucheri E, Vitonis AF, Einarsson JI (2015) Open power morcellation versus contained power morcellation within an insufflated isolation bag: comparison of perioperative outcomes. J Minim Invasive Gynecol 22(3):433–438. doi:10.1016/j.jmig.2014.11.010 Epub 2014 Nov 29
Cholkeri-Singh A, Miller CE (2015) Power morcellation in a specimen bag. J Minim Invasive Gynecol 22(2):160. doi:10.1016/j.jmig.2014.10.012 Epub 2014 Oct 18
Kanade TT, McKenna JB, Choi S, Tsai BP, Rosen DM, Cario GM, Chou D (2014) Sydney contained in bag morcellation for laparoscopic myomectomy. J Minim Invasive Gynecol 21(6):981. doi:10.1016/j.jmig.2014.07.005 Epub 2014 Jul 15
Srouji SS, Kaser DJ, Gargiulo AR (2015) Techniques for contained morcellation in gynecologic surgery. Fertil Steril 103(4):e34. doi:10.1016/j.fertnstert.2015.01.022 Epub 2015 Feb 21
McKenna JB, Kanade T, Choi S, Tsai BP, Rosen DM, Cario GM, Chou D (2014) The Sydney contained in bag morcellation technique. J Minim Invasive Gynecol 21(6):984–985. doi:10.1016/j.jmig.2014.07.007 Epub 2014 Jul 15
Günthert AR, Christmann C, Kostov P, Mueller MD (2015) Safe vaginal uterine morcellation following total laparoscopic hysterectomy. Am J Obstet Gynecol; 212(4):546.e1–4. doi: 10.1016/j.ajog.2014.11.020. Epub 2014 Nov 25
Montella F, Riboni F, Cosma S, Dealberti D, Prigione S, Pisani C, Rovetta E (2014) A safe method of vaginal longitudinal morcellation of bulky uterus with endometrial cancer in a bag at laparoscopy. Surg Endosc 28(6):1949–1953. doi:10.1007/s00464-014-3422-0 Epub 2014 Feb 25
Favero G, Miglino G, Köhler C, Pfiffer T, Silva e Silva A, Ribeiro A, Le X, Anton C, Baracat EC, Carvalho JP (2015) Vaginal morcellation inside protective pouch: a safe strategy for uterine extration in cases of bulky endometrial cancers. Operative and oncological safety of the method. J Minim Invasive Gynecol. doi:10.1016/j.jmig.2015.04.015 Epub ahead of print
Bogani G, Uccella S, Cromi A, Serati M, Casarin J, Sturla D, Ghezzi F (2014) Electric motorized morcellator versus transvaginal extraction for myoma retrieval after laparoscopic myomectomy: a propensity-matched analysis. J Minim Invasive Gynecol 21(5):928–934. doi:10.1016/j.jmig.2014.04.012 Epub 2014 Apr 26
Bogani G, Serati M, Uccella S, Ghezzi F (2014) In-bag morcellation for presumed myoma retrieval at laparoscopy. Cancer 120(24):4004–4005. doi:10.1002/cncr.28959 Epub 2014 Aug 7
Rardin CR (2014) Mitigating risks of specimen extraction: is in-bag power morcellation an answer? Obstet Gynecol 124(3):489–490. doi:10.1097/AOG.0000000000000434
Cohen SL, Greenberg JA, Wang KC, Srouji SS, Gargiulo AR, Pozner CN, Hoover N, Einarsson JI (2014) Risk of leakage and tissue dissemination with various contained tissue extraction (CTE) techniques: an in vitro pilot study. J Minim Invasive Gynecol 21(5):935–939. doi:10.1016/j.jmig.2014.06.004 Epub 2014 Jun 11
Acknowledgments
The authors thank Reinhold Zimmermann, Esra Foditsch, Ioan Hutu, Irina Patras, Bogdan Hoinoiu and Romana Korlat for their invaluable help during conception, realization and analysis of the described experiments.
Conflict of interest
The present study was financially supported by A.M.I., Feldkirch, Austria. We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rimbach, S., Holzknecht, A., Nemes, C. et al. A new in-bag system to reduce the risk of tissue morcellation: development and experimental evaluation during laparoscopic hysterectomy. Arch Gynecol Obstet 292, 1311–1320 (2015). https://doi.org/10.1007/s00404-015-3788-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-015-3788-9