Abstract
Hyperemesis gravidarum (HG) is described as unexplained excessive nausea and vomiting during pregnancy. Some gut hormones that regulate appetite may have important role in etiopathogenesis of HG and weight changes during pregnancy. In this study, levels of gut satiety hormones were evaluated in pregnant women with HG.
Methods
This prospective case–control study was conducted in 30 women with HG and 30 healthy pregnant women without symptoms of HG. Fasting venous blood samples were taken from all subjects for measurement of plasma gut hormone levels; obestatin (pg/mL), peptide YY (PYY), pancreatic polypeptide (PP) and cholecystokinin (CCK).
Results
Plasma PYY and PP levels were significantly higher in HG group. The most important parameter in diagnosis of HG was plasma PP level. Simple use of PP level led to the diagnosis 91.1 % of HG cases correctly. The single most important parameter in the prediction of HG was also PP level.
Conclusion
Anorexigenic gut hormones might have important role in etiopathogenesis of hyperemesis gravidarum and weight changes during pregnancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperemesis gravidarum (HG) is described as unexplained excessive nausea and vomiting during pregnancy, leading to fluid, electrolyte imbalance, nutritional deficiency and weight loss. It occurs about 0.5–2 % of pregnant women, and is the most common cause of admission to the hospital in early pregnancy [1–3].
Many etiopathogenic factors have been considered for HG, including endocrine–hormonal factors such as higher levels of hCG, progesterone and thyroid hormones during early pregnancy, hepatic dysfunction, changes in lipid metabolism, upper gastrointestinal system dysmotility, and psychological factors. However, no specific causative factor has been established [1–4].
The regulation of body weight is under homeostatic control. But in especially early pregnancies this homeostasis may change towards negative energy balance. Appetite is affected by many factors including the hormones. The gastrointestinal–pancreatic system (GIS) is a significant endocrine organ in the body and it is the source of important appetite-regulating hormones. There is a communication between the GIS and appetite centers of central nervous system (CNS) that occurs via the vagus nerve to the brainstem, and via spinal afferents to the spinal cord [5]. This gut–brain axis is important for appetite regulation and has both neural and humoral components [6, 7].
Acyl-ghrelin, obestatin, cholecystokinin (CCK), pancreatic polypeptide (PP) and peptide YY (PYY) are some of the appetite-regulating hormones secreted from GIS. They act as meal initiators and terminators. Of these, ghrelin is the only known orexigenic gut hormone, whereas the others are satiety factors.
Nowadays, some peripheral hormones secreted from GIS which regulate appetite become popular. Most of the studies on these hormones focused on their efficiency on treatment of obesity. However, these hormones may also have important role in etiopathogenesis of HG and weight changes during pregnancy. Appetite hormones secreted from GIS could be responsible from HG. In this study, levels of gut satiety hormones were evaluated in pregnant women with HG.
Materials and methods
This prospective case–control study was conducted involving 60 pregnant women in their first trimester of pregnancy. The women were selected from the outpatient clinic of Maternity Clinic and the in-patient wards of the Hospital of Obstetric and Gynecology at Turgut Ozal University Hospital between July 2012 and October 2013. The patients were divided into two groups according to the presence of HG. Thirty women with HG and 30 healthy pregnant women without symptoms of HG were taken into the study. HG was defined as persistent nausea and vomiting associated with ketosis and weight loss >5 % of pre-pregnancy weight. Inclusion criteria were: singleton pregnancy with a live embryo, healthy women without any medical disorders, age range between 18 and 35 years, weight within 20 % of normal weight for height at the beginning of pregnancy, and gestation time between 6 and 14 weeks. The exclusion criteria were: smokers or drug users in the index pregnancy, cases with any systemic disease and/or psychological disorder that can cause vomiting, and multiple gestations. In addition, those women with uncertain dates or early pregnancy loss were also excluded from the study. All participants were followed at least until the second trimester anomaly scan (at 20–24 weeks). Cases with any abnormality during pregnancy were excluded from the study. Approval for this study was obtained from the Local Institutional Review Board of the Faculty of Medicine, Fatih University. Informed consent was obtained from all participants.
All relevant data including demographic information (age, gravida, parity, body mass index (BMI), obstetric history, gestational week) were collected for further analysis. The gestational age was calculated by the modified Naegele’s rule. Last menstrual period-derived gestational age was compared with ultrasound-derived gestational age using CRL [8] and if there was a marked discrepancy of 2 weeks or more than the woman was excluded from the study.
Fasting venous blood samples were taken from all subjects for measurement of plasma gut hormone levels; obestatin (pg/mL), PYY (ng/mL), PP (ng/mL) and CCK (ng/mL) levels by enzyme immunoassay (EIA). Serum TSH (μIU/mL) level was measured by electrochemiluminescence immunoassay (ECLIA); serum FT3 (pg/mL), serum FT4 (ng/dL); serum human chorionic gonadotrophins (serum hCG) were measured quantitatively by the Sandwich principle. Blood urea (mg/dL), serum creatinine (mg/dL), serum sodium (mmol/L), serum potassium (mmol/L), serum glucose, AST, ALT and complete blood count were also measured to detect the severity of emesis in addition to ketones in a morning urine sample measured by urine stripes.
Statistical analysis
Data were analyzed with the SPSS software version 15.0 for Windows (SPSS Inc., Chicago, IL, USA). Groups were controlled in terms of conformity to normal distribution by graphical check and Shapiro–Wilk test. Median (IQR) was used for groups that were not distributed normally. The Mann–Whitney test was used to compare groups. Mean ± SD and Student t test were used for groups that were distributed normally. Receiver-operating characteristics (ROC) curve analysis was performed to determine cutoff points in prediction of HG. Logistic regression analysis was done for determination of parameters that can predict HG. p value of ≤0.05 was taken as significant.
Results
The study was conducted involving 60 pregnant women in their first trimester of pregnancy. Thirty women with HG and 30 healthy pregnant women without any HG symptoms were entered the study. During follow-up period, 6 women in the HG group and 14 women in the control group were lost. The remaining 24 women with HG and 16 healthy control were taken into the study.
Groups were compared with each other in terms of age, gravida, parity, gestational age and BMI. There was no statistically significant difference between groups (p > 0.05) (Table 1). Mean weight of the women were 66.9 ± 12.2 and 63.0 ± 11.9 kg at the beginning of the pregnancy and during the emesis period in the HG group, respectively.
The groups were compared with each other for plasma GIS hormone levels. Plasma obestatin and CCK levels were higher in HG group than control group. But this difference did not reach statistical significance (p = 0.576 and p = 0.429, respectively). On the other hand, plasma PYY and PP levels were significantly higher in HG group (p = 0.030 and p < 0.001, respectively) (Table 2).
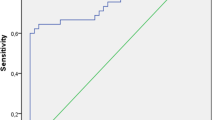
ROC analysis was performed to find most effective parameter in diagnosis of HG. The most effective parameter was plasma PP level (AUC = 0.911, p < 0.001 and CI % 95 = 0.825–0.998) (Table 3). Simple use of PP level led to diagnosis of 91.1 % of HG cases correctly. Sensitivity and specificity values at different cutoff levels of PP value are given in Table 4.
Logistic regression analysis was done to find most effective parameter in prediction of HG. The analysis revealed that the single most important parameter in the prediction of HG was PP level (p = 0.009) (Table 5).
Discussion
The GIS is the largest endocrine organ of the body. More than 30 hormone genes are expressed and more than 100 bioactive peptides are produced by the GIS [9]. In addition to its digestive and absorptive functions, GIS also has an important role in the energy balance via gut hormones. There is a perfect interaction between GIS and the CNS to control adjustments in food intake [10, 11]. Postprandial satiety is regulated by a sensory system that communicates between the gut and appetite-regulating centers in the brain, with the hypothalamus being the most important one [12]. While release of satiety hormones from GIS occurs in the postprandial state, secretion of the orexigenic hormone occurs in the fasting state [13, 14]. So, understanding of the gut–brain axis might be important in development of new strategies for treatment and prevention of nutritional-appetite disorders.
It has been shown by many studies that gut hormones administered at physiological concentrations can influence appetite in rodent models and humans [15–17]. Following GI or bariatric surgery, circulating gut hormone levels are changed [18, 19]. The reduction in appetite and body weight, which are associated with GI or bariatric surgery may be partly due to the changes in the release of some gut hormones. Gut peptides are known to cross the blood–brain barrier and induce changes in neural activation. They act both indirectly on the vagus nerve and directly on target areas of the hypothalamus [18–20]. Dysregulation in gut hormone secretion could therefore be associated with HG.
In this study, we examined gut satiety hormones of GIS in HG cases. Obestatin is one of them. It is produced in the stomach and small intestine [21]. Obestatin opposes the actions of ghrelin which increases appetite [22]. It is originally produced from the same ghrelin gene. After differential modification, obestatin and ghrelin which have opposing action bind and activate different receptors [23]. Obestatin levels are lower in obese subjects when compared with lean subjects, and hormone levels show a significant increase in the obese patients 6 months after gastric banding surgery [24]. In the study of Gungor et al., they measure serum obestatin levels in HG patients and compare with normal pregnant women. They did not find any difference between groups in terms of obestatin level [25]. In this study, we found that although obestatin levels were higher in HG women, this difference was not statistically significant.
The other gut appetite-suppressing hormone, which we studied, is PYY. It is secreted by the distal small intestine and large intestine [26]. It inhibits appetite through feedback into the hypothalamus by binding to the hypothalamic receptors [27]. In contrast to the anorectic effects observed by peripheral and hypothalamic intra-arcuate PYY administration, direct administration of PYY into the third ventricle of the brain or paraventricular nucleus increases in food intake [28, 29]. Therefore, PYY appears to have differing effects on food intake depending on the site of administration. High PYY levels have been linked to decreased appetite and food intake [30]. PYY is elevated in patients with malabsorptive disorders and in patients with anorexia nervosa [31, 32]. In humans, PYY infusion reduces appetite and food intake [15, 33]. PYY level is also found to be elevated in HG cases [34]. In our study, we also measured the PYY level and its level is significantly higher in HG cases. So, it can be concluded that PYY might be involved in pathophysiology of HG.
The other studied satiety hormone is PP. It is synthesized by endocrine F cells of the pancreatic islets [26]. In a similar manner to PYY, PP demonstrates differential effects on food intake depending on the site of administration. When given peripherally, it acts as an anorectic hormone, whereas CNS administration stimulates food intake [35]. In normal-weight human subjects, intravenous infusion of PP results in a 25 % reduction in daily food intake [36]. In another study, exogenous infusion of peptide YY decreased food intake by 36 % and decreased subjective hunger [33]. Circulating PP levels seem to be inversely proportional to adiposity; higher levels are reported in subjects with anorexia nervosa [37]. In this study, we found that PP level was significantly higher in HG group. In regression analysis, PP level predicted 91.1 % of HG correctly. All these mean PP might also be involved in the pathogenesis of the HG.
CCK is another important appetite suppressant hormone that was measured in this study. It is secreted postprandially predominately from the I cells in the duodenum and jejunum [38]. It is reported to reduce food intake in humans and rodents [39, 40]. CCK also coordinates the release of digestive enzymes from the pancreas, stimulates gall bladder contraction, increases intestinal motility and inhibits gastric emptying [41]. It mediates satiety by acting on the CCK receptors distributed in the central nervous system similar to other gut hormones. In our study, we found that CCK level was higher in HG cases although the difference was not statistically significant.
In normal physiology, the secretion of appetite-regulating gut hormones changes according to metabolic need. The pregnancy itself might alter GIS hormone secretion. In the 1st trimester, there is a negative energy balance which could be induced by pregnancy-associated changes in GIS function. It might be a protective mechanism for the fetus. This is the time in which mother and fetus need no additional energy but they must be protected from any harmful substance or deleterious agent to keep developing fetus safe. After first trimester, organogenesis is completed and physiological mechanisms are activated to increase maternal appetite for the contribution of adequate energy intake for the mother and fetus. Relation of gut hormones and neonatal weight is demonstrated by the study of Valsamakis et al. They showed that circulating maternal pro-appetite gut hormone, active ghrelin is correlated positively with neonatal visceral energy storage (as expressed by neonatal waist) in the second and third trimester [42]. In the study of Sodowski et al., they examined the patterns of basal and postprandial plasma concentrations of certain gut hormones affecting food intake. They demonstrated that pregnant women with overweight and obesity exhibit significant changes in gut hormones affecting food intake, and these changes might contribute, at least in part, to the development of obesity in pregnancy [43]. These studies together with ours prove the effect of GIS hormones on maternal eating habits and weight changes.
Normally, release of gut satiety hormones is triggered by the presence of food in the gut. But in HG cases although fasting present, levels of PYY and PP were still higher. This might be explained by slowing of transit time of food in GIS during pregnancy due to effect of placental hormones mainly progesterone. As the transit time increases, prolonged presence of food in the gut might stimulate long-term satiety hormone secretion. Most prominent symptoms occur during first trimester of pregnancy. After first trimester, adaptation and desensitization might develop and then symptoms decrease despite prolongation of transit time.
During pregnancy senses of smell and taste also change, which may alter eating habits of pregnancy. Pregnant women may produce more saliva, which may also changes appetite. Excessive salivation might be perceived as continuous oral feeding to initiate secretion of gut satiety hormones.
Another important point is that effect of these peptides may be through an afferent gut–brain signal or efferent CNS–gut route. As we mentioned before, some ‘gut’ peptides are actually gut/brain peptides. So a change observed in appetite and weight might be controlled by the enteroendocrine gut or by the CNS or by both. Pregnancy may affect secretion of these hormones both in peripheral and central way.
Brain sensitivity to the gut hormones may change in relation to different physiological states, especially those that involve changes in energy balance (e.g., fasting, obesity). Energy balance is changed dramatically during pregnancy, even it differs between trimesters. Pregnancy might alter receptor number or structure of gut satiety hormones and change sensitivity of CNS to secreted gut hormones.
HG is seen more frequently in women with undesired pregnancy. Gut hormones act as neurotransmitters within the central nervous system to control food intake. Their levels might be dysregulated by stress. The hypothalamus is a crucial region for integrating signals from central and peripheral pathways and plays a major role in appetite regulation. Psychological stress (unplanned pregnancy, marital problems or pregnancy itself) might affect hormone levels in CNS starting an activation from cortex to hypothalamus, brain stem, vagus nerve, ending in the gut.
There are some limitations of our study. First is the number of cases. Although we planned 30 cases for each group, due to high drop out rate case group was formed from 24, control group from 16 patients. This decreased the power of the study. The other important limitation is that we did not measure gut hormone levels at different gestational ages. They could be measured and compared with each other at first, second and third trimester to see their relation with appetite changes as pregnancy progress. Another deficiency of the study is that other satiety hormones and appetite hormone were not measured. Their measurement might be useful for the enlightenment of gut–HG relation.
As a result, in this study, we investigated the role of gut hormones on etiopathogenesis of HG and weight changes during pregnancy. Some gut hormone levels that increased during first trimester might be responsible from the HG. They might be used as potential therapeutic agents for eating disorders. Therapeutic use of antagonists of some anorexigenic hormones could prevent nausea–vomiting, increase appetite and lead to weight gain in women with hyperemesis gravidarum. By this way, we could be able to provide an effective medication to pregnant women with HG. This would improve the quality of life and would prevent high health costs due to prolonged and recurrent hospitalization. The use of gut hormones as therapeutic agents would have the advantage of targeting only appetite control systems. Since these hormones are present natively in the body, side effects and desensitization due to long-term usage of these agents would be less likely. Further work is required to fully understand the multiple signals regulating appetite and body weight.
References
Eliakim R, Abulafia O, Sherer DM (2000) Hyperemesis gravidarum: a current review. Am J Perinatol 17:207–218
Verberg MF, Gillott DJ, Al Fardan N, Grudzinskas JG (2005) Hyperemesis gravidarum, a literature review. Hum Reprod Update 11:527–539
Gazmararian JA, Petersen R, Jamieson DJ, Schild L, Adams MM, Deshpande AD, Franks AL (2002) Hospitalizations during pregnancy among managed care enrollees. Obstet Gynecol 100:94–100
Aka N, Atalay S, Sayharman S, Kiliç D, Köse G, Küçüközkan T (2006) Leptin and leptin receptor levels in pregnant women with hyperemesis gravidarum. Aust NZJ Obst Gyn 46:247–277
Mayer EA (2011) Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci 12:453–466
Stanley S, Wynne K, McGowan B, Bloom S (2005) Hormonal regulation of food intake. Physiol Rev 85:1131–1158
Sahu A (2003) Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol 24:225–253
Hadlock FP, Shah YP, Kanon DJ, Lindsey JV (1992) Fetal crown-rump length: reevaluation of relation to menstrual age (5–18 weeks) with high resolution real-time US. Radiology 182:501–505
Rehfeld JF (1998) The new biology of gastrointestinal hormones. Physiol Rev 78:1087–1108
Pimentel GDMJ, Mota JF, Oyama LM (2009) Oxintomodulina e obesidade. Rev Nutr 22:727–737
Pimentel GD, Zemdegs JC (2010) Foods and nutrients modulates the release of anorexigenic gastrointestinal hormones. Acta Med Port 23:891–900
Murphy KG, Bloom SR (2004) Gut hormones in the control of appetite. Exp Physiol 89:507–516
Leibowitz SF, Wortley KE (2004) Hypothalamic control of energy balance: different peptides, different functions. Peptides 25:473–504
Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG (2000) Central nervous system control of food intake. Nature 404:661–671
Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR (2003) Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med 349:941–948
Cohen MA, Ellis SM, Le Roux CW, Batterham RL, Park A, Patterson M et al (2003) Oxyntomodulin suppresses appetite and reduces food intake in humans. J Clin Endocrinol Metab 88:4696–4701
Owais BC, Katie W, Stephen RB (2008) Can gut hormones control appetite and prevent obesity? Diabetes Care 31:284–289
Sarson DL, Scopinaro N, Bloom SR (1981) Gut hormone changes after jejunoileal (JIB) or biliopancreatic (BPB) bypass surgery for morbid obesity. Int J Obes 5:471–480
Tadross JA, le Roux CW (2009) The mechanisms of weight loss after bariatric surgery. Int J Obes 33(Suppl 1):28–32
Valassi E, Scacchi M, Cavagnini F (2008) Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis 18:158–168
Gourcerol G, St-Pierre DH, Taché Y (2007) Lack of obestatin effects on food intake: should obestatin be renamed ghrelin-associated peptide (GAP)? Regul Pept 141:1–7
Hassouna R, Zizzari P, Tolle V (2010) The ghrelin/obestatin balance in the physiological and pathological control of growth hormone secretion, body composition and food intake. J Neuroendocrinol 22:793–804
Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C et al (2005) Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science 310:996–999
Haider DG, Schindler K, Prager G, Bohdjalian A, Luger A, Wolzt M, Ludvik B (2007) Serum retinol-binding protein-4 is reduced after weight loss in morbidly obese subjects. J Clin Endocrinol Metab 92:1168–1171
Gungor S, Gurates B, Aydin S, Sahin I, Kavak SB, Kumru S, Celik H et al (2013) Ghrelins, obestatin, nesfatin-1 and leptin levels in pregnant women with and without hyperemesis gravidarum. Clin Biochem 46:828–830
Ekblad E, Sundler F (2002) Distribution of pancreatic polypeptide and peptide YY. Peptides 23:251–261
Keire DA, Bowers CW, Solomon TE, Reeve JR Jr (2002) Structure and receptor binding of PYY analogs. Peptides 23:305–321
Morley JE, Levine AS, Grace M, Kneip J (1985) Peptide YY (PYY), a potent orexigenic agent. Brain Res 341:200–203
Stanley BG, Daniel DR, Chin AS, Leibowitz SF (1985) Paraventricular nucleus injections of peptide YY and neuropeptide Y preferentially enhance carbohydrate ingestion. Peptides 6:1205–1211
Vincent RP, le Roux CW (2008) The satiety hormone peptide YY as a regulator of appetite. J Clin Pathol 61:548–552
Adrian TE, Savage AP, Bacarese-Hamilton AJ, Wolfe K, Besterman HS, Bloom SR (1986) Peptide YY abnormalities in gastrointestinal diseases. Gastroenterology 90:379–384
Misra M, Miller KK, Tsai P, Gallagher K, Lin A, Lee N, Herzog DB, Klibanski A (2006) Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 91:1027–1033
Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM et al (2002) Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 418:650–654
Albayrak M, Karatas A, Demiraran Y, Erman H, Topuz S, Bıyık İ, Uzun H, Erkan M (2013) Ghrelin, acylated ghrelin, leptin and PYY-3 levels in hyperemesis gravidarum. J Matern Fetal Neonatal Med 26:866–870
Clark JT, Kalra PS, Crowley WR, Kalra SP (1984) Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology 115:427–429
Batterham RL, Le Roux CW, Cohen MA, Park AJ, Ellis SM, Patterson M, Frost GS, Ghatei MA, Bloom SR (2003) Pancreatic polypeptide reduces appetite and food intake in humans. J Clin Endocrinol Metab 88:3989–3992
Uhe AM, Szmukler GI, Collier GR, Hansky J, O’Dea K, Young GP (1992) Potential regulators of feding behavior in anorexia nervosa. Am J Clin Nutr 55:28–32
Lieverse RJ, Jansen JB, Masclee AM, Lamers CB (1994) Satiety effects of cholecystokinin in humans. Gastroenterology 106:1451–1454
Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA (1985) Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest 75:1144–1152
Kissileff HR, Pi-Sunyer FX, Thornton J, Smith GP (1981) C-terminal octapeptide of cholecystokinin decreases food intake in man. Am J Clin Nutr 34:154–160
Moran TH, Kornbluh R, Moore K, Schwartz GJ (1994) Cholecystokinin inhibits gastric emptying and contracts the pyloric sphincter in rats by interacting with low affinity CCK receptor sites. Regul Pept 52:165–172
Valsamakis G, Papatheodorou DC, Naoum A, Margeli A, Papassotiriou I, Kapantais E, Creatsas G, Kumar S, Mastorakos G (2014) Neonatal birth waist is positively predicted by second trimester maternal active ghrelin, a pro-appetite hormone, and negatively associated with third trimester maternal leptin, a pro-satiety hormone. Early Hum Dev 90:487–492
Sodowski K, Zwirska-Korczala K, Kuka D, Kukla M, Budziszewska P, Czuba B, Włoch A et al (2007) Basal and postprandial gut peptides affecting food intake in lean and obese pregnant women. J Physiol Pharmacol 58(Suppl 1):37–52
Conflict of interest
The authors declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Köşüş, A., Köşüş, N., Usluoğullari, B. et al. Gut satiety hormones and hyperemesis gravidarum. Arch Gynecol Obstet 292, 1225–1230 (2015). https://doi.org/10.1007/s00404-015-3751-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-015-3751-9