Abstract
Adult acne vulgaris affects up to 43–51% of individuals. While there are numerous treatment options for acne including topical, oral, and energy-based approaches, benzoyl peroxide (BPO) is a popular over the counter (OTC) treatment. Although BPO monotherapy has a long history of efficacy and safety, it suffers from several disadvantages, most notably, skin irritation, particularly for treatment naïve patients. In this prospective, randomized, controlled, split-face study, we evaluated the comparative efficacy, safety, and tolerability of a novel 3-step azelaic acid, salicylic acid, and graduated retinol regimen versus a common OTC BPO-based regimen over 12 weeks. A total of 37 adult subjects with self-reported mild to moderate acne vulgaris were recruited. A total of 21 subjects underwent a 2-week washout period and completed the full study with 3 dropping out due to product irritation from the BPO routine, and 13 being lost to follow-up. Detailed tolerability surveys were conducted at Week 4. Additional surveys on tolerability and product preferences were collected monthly, at Week 4, Week 8, and Week 12. A blinded board-certified dermatologist objectively scored the presence and type of acne lesions (open or closed comedones, papules, pustules, nodules, and cysts) at baseline, Week 4, Week 8, and Week 12. Patients photographed themselves and uploaded the images using personal mobile phones. Detailed Week 4 survey results showed across 25 domains of user-assessed product performance, the novel routine outperformed the BPO routine in 19 (76%) which included domains in preference (e.g. “I would use this in the future) and performance (“my skin improved” and “helped my acne clear up faster”). Users of the novel routine reported less facial redness, itching, and burning, though differences did not reach statistical significance. In terms of efficacy, both products performed similarly, reducing total acne lesions by 36% (novel routine) and 40% (BPO routine) by Week 12. Overall, accounting for user preferences and tolerability the novel routine was more preferred than the BPO routine in 79% of domains (22/28). Differences in objective acne lesion reduction were not statistically significant (p = 0.97). In a randomized split-face study, a 3-step azelaic acid, salicylic acid, and graduated retinol regimen delivered similar acne lesion reduction, fewer user dropouts, greater user tolerability, and higher use preference compared to a 3-step BPO routine based in a cohort of participants with mild-to-moderate acne vulgaris.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acne vulgaris is one of the most common human diseases in adults affecting 43–51% of individuals between the ages of 20 to 29 and up to 35% of individuals between the ages of 30 to 39 [1]. The disease can be disfiguring with a profound psychological impact, contributing to both anxiety and depression [2]. Currently, there are a wide range of topical, oral, and energy-based therapies for acne. Topical, over-the-counter (OTC) therapies are popular, representing a global $5 billion USD market [3]. Within this category of topical treatments, benzoyl-peroxide (BPO) based therapies remain one of the most commonly used worldwide.
While BPO as a monotherapy is an effective treatment for acne [4], it has significant side effects that affect adherence, including skin irritation and patient intolerability, particularly among individuals with sensitive skin. In one study, 35% of BPO users noted side effects with a discontinuation rate of 44% at 6 months [5]. Importantly, the prevalence of sensitive skin in the adult population is greater than 70% in a recent systematic review [6]. In addition to tolerability challenges, a recent study conducted by an independent laboratory (Valisure, New Haven, CT) demonstrated that a wide range of widely available acne products with benzoyl peroxide degraded into benzene, a widely known human carcinogen, to well over safe levels when subjected to elevated storage temperatures [18]. Thus, new OTC regimens offering comparable efficacy with greater tolerability, and no risk of benzene degradation, compared to existing BPO therapies would be beneficial to adult patients with mild-to-moderate acne vulgaris.
We hypothesized a novel 3-step regimen with 3 primary anti-acne ingredients (azelaic acid, salicylic acid, and retinol) would offer similar efficacy and greater tolerability compared to a 3-step BPO based product in adult patients with mild-to-moderate acne vulgaris. Therefore, we conducted a randomized controlled split-face study to investigate the efficacy, safety, and tolerability of both OTC regimens.
Materials and methods
Regimens
Two OTC regimens were compared. The first OTC regimen (Geologie, New York City, New York) included 3 separate products: (1) a cleanser (2% salicylic acid), (2) a day cream (5% azelaic acid, 2% hyaluronic acid, and 1% niacinamide), and (3) a night cream with graduated levels of retinol to maximize patient tolerability. From baseline to week 4, subjects were given a night cream with 0.1% retinol. From week 4 to week 8, subjects were given a night cream with 0.2% retinol. Finally, from week 8 to week 12, subjects were given a night cream with 0.3% retinol. The second OTC Regimen (Proactiv Solution, Southaven, Mississippi) also included 3 separate products: (1) a cleanser (2.5% BPO), (2) a toner (glycolic acid), and (3) repairing treatment (2.5% BPO). Proactiv Solution was selected given its high level of popularity as a BPO routine, and a similar 3-step routine as the Geologie Clear System. All products from each OTC regimen were transferred to unlabeled bottles to maximize blinding. Instructions on applications and use were provided per each manufacturers’ instructions. The dermatologist performing skin lesion grading was blinded to treatment laterality.
Study design, enrollment, inclusion / exclusion criteria, and study endpoints
This was a prospective, randomized controlled, double-blinded, single-center study (clinicaltrials.gov: NCT05446402) conducted at Northwestern University in the Department of Dermatology after IRB approval (STU00217056). Patients were recruited in person and via social media (e.g., Facebook). A split-face design was used to compare the novel routine versus the BPO routine. All eligible subjects (> 18 years of age with a clinical diagnosis of mild or moderate acne vulgaris and without an active skin infection or known allergy to the ingredients being evaluated) were recruited and consented. Acne lesion count (open comedones, closed comedones, papules, pustules, nodules, and cysts) was determined by a blinded board-certified dermatologist (PV) at baseline, Week 4, Week 8, and Week 12 of the study via the Facial Lesion Count [7]. Patients self-collected images with standard mobile phones. Subjects were asked to complete both tolerance and product preference surveys at Week 4, Week 8, and Week 12. For tolerability, subjects were asked to rate their level of redness, itching, and burning on a 5-point scale with 1 being very mild and 5 being very severe. For patient preferences, subjects were asked to complete questions related to the product’s effect on their skin, and their propensity to use the product in the future on a 5-point scale. The primary endpoint was acne lesion count with secondary endpoints including both patient preference and tolerability survey results.
As a non-inferiority study, 20 subjects would enable the detection of an absolute difference of 20% with at least 80% power and a 5% level of significance if the standard deviation of this difference is no larger than 50%. Adjusting for a drop rate of 25%, a target of 27 subjects for recruitment and consent was set. The data were analyzed using Stata. Aggregated data for lesion counts were determined for each time point during the study (baseline, Weeks 4, 8, and 12 of treatment use). Descriptive statistics are presented as means. Paired t-tests, used to compare performance with all p-values, were two-tailed assuming equal variances with a level of significance of p ≤ 0.05.
Results
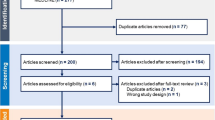
A total of 37 subjects were recruited and consented for the 12-week study. Expansion of the initial target recruitment was required due to drop out of three subjects due to intolerance of the BPO routine and a higher than anticipated lost-to-follow up rate (n = 13) (Fig. 1). Prior to starting both regimens, the final cohort of patients (n = 21) completed a 2-week washout period where no acne treatments, prescription, or OTC medications were used. The final analysis cohort included 10 females, 10 males, and 1 not reported (Table 1). Most subjects were between the ages of 20–29 (n = 10) and 30–39 (n = 6). Self-reported skin type was most reported as a combination of oily and dry (61%; n = 13/21).
Both the novel routine and the BPO routine demonstrated a high degree of efficacy evidenced by reduction in facial lesions. At week 4, both regimens had a similar mean number of acne lesions (novel routine: 7.2 and BPO routine: 7.5) with no statistical difference (p = 0.80). At week 8, the novel routine had an average of 6.6 acne lesions and the BPO routine had an average of 6.5 acne lesions (p = 0.94). At week 12, the novel routine had an average of 4.6 acne lesions and the BPO routine had an average of 4.7 acne lesions (p = 0.93). Over the entire 12 weeks, each product reduced total acne lesions by 36% (novel routine) and 40% (BPO routine).
Detailed patient preference and user tolerability in Week 4 are shown in Fig. 2. Across a total of 25 domains, the novel routine outperformed the BPO routine in 19 domains (76%). The novel routine’s highest performing categories were a patient’s likelihood to use the product in the future, skin feel, and preference. In addition, the novel routine was preferred in domains for efficacy (faster acne clearing, skin less oily) and skin look and feel (softer, smoother, brighter, and more hydrated). The BPO routine was favored in other categories related to acne prevention. When aggregating both user preferences and tolerability the novel routine was more preferred than the BPO routine in 79% of domains (22/28) at Week 4. In Fig. 3, users reported higher scores for the the novel routine in skin feel and future product usage at Week 4 and Week 8. At Week 12, these differences were less apparent (Fig. 3). Additional survey results on tolerability and product preferences were collected at Week 4, Week 8, and Week 12. Detailed Week 4 survey results showed that across 25 domains of user-assessed product performance, in terms of tolerability, users of the novel routine reported less facial redness, itching, burning, and dryness (Fig. 4).
Safety
No adverse events were reported during this study, although three subjects dropped out due to intolerance to the BPO routine during the course of the 12-week study (Figs. 5 and 6).
Week 4, Week 8, and Week 12 objective acne lesion (both inflammatory and non-inflammatory) counts for each regimen. There were no statistically significant differences at any week between the novel routine versus the BPO routine. The final reduction in objective acne lesions from baseline was 36% for the novel routine and 40% for the BPO routine
Discussion
The pathogenesis of acne vulgaris is multi-factorial driven by increased sebum production via hyperplastic sebaceous glands, bacterial colonization by p. acnes, follicular hyperkeratinization, and inflammation [8]. BPO based treatments have long been a cornerstone treatment for acne vulgaris given its ability to reduce antibacterial activity against Cutibacterium acnes and suppress sebum production [9]. However, skin irritation and tolerability remain a key drawback of BPO-based treatments, particularly at higher concentrations[10] —an issue likely exacerbated in the adult acne population with a high underlying prevalence of skin sensitivity. The BPO routine studied here includes 2 products with BPO and glycolic acid toner. For BPO, the predominate mechanism of action is antibacterial [11]. Glyolic acid has largely anti-hyperkeratinization activity[12]. The novel routine was rationally designed to deliver comparable efficacy to BPO based treatments with greater tolerability and less irritation to maximize compliance—this is evident in that no participants dropped out of the study due to intolerance to the novel routine compared to 3 subjects who dropped out due to the BPO routine. Azelaic acid, already FDA-cleared as a topical treatment for acne in a 20% cream formulation, offers multiple effect anti-acne benefits including being bactericidal for C. acnes, anti-inflammatory, and skin lightening for post-inflammatory hyperpigmentation [13]. Salicylic acid, a beta-hydroxy acid, has also long been a mainstay of acne treatments with a positive effect on abnormal keratinization and inflammation. Niacinamide also address abnormal sebum production and anti-inflammatory activities. Retinols address hyper-keratinization, provide antibacterial action, and deliver color correction [12]. Given that retinols, like BPO, have well-established skin irritation and dryness side effects, particularly in treatment naïve or sensitive skin patients, the graduated retinol percentage process (0.1% at month 1, 0.2% at month 2, and 0.3% at month 3) was designed to enhance patient tolerability without loss of efficacy. Combination therapies, such as those offered by the novel routine, have shown to offer better overall performance than monotherapy strategies [14].
The overall results show nearly identical efficacy in reducing objective acne lesion reduction at 12 weeks between both the novel routine and the BPO routine. In contrast, and particularly at the Week 4 time point, the novel routine exhibited higher ratings by user report across multiple domains from direct product preferences to skin appearance and skin feel. These differences were less apparent at Week 8 and Week 12, although the novel routine was still rated higher in skin look and feel, and product perception at the end of the study. In regards to tolerability, the novel routine demonstrated less severe redness, itching, and burning with the greatest differences seen at Week 4. These tolerability differences, analogous to the user preference reports, were less evident at Week 12. Overall, tolerability and user acceptance is critical in the management of acne, a chronic disease typically requiring on-going maintenance treatment. User tolerance is particularly relevant among adults with comorbid sensitive skin. Between 30% to 65% of all patients with acne do not adhere to a treatment regimen and as a result 50% do not receive the full benefits of treatment [15]. For OTC regimens, skin irritation and drying is the most common side effect [16]. To drive greater adherence and overall treatment success, products should be both effective and also tolerable to use [14, 17]. These results suggest that positive early experiences by new users is especially important—the novel routine performed better in 79% of user reported domains in efficacy, product performance, and tolerability at the critical 4 week mark. Though outside the scope of this study, early positive experiences may encourage continued and longer-term adherence to treatment, reducing rates of drop out among new users and providing long term benefits in terms of acne reduction. Traditionally, skin irritation and tolerability remain a key drawback of BPO-based treatments, particularly at higher concentrations [10]—an issue likely exacerbated in the adult acne population with a high underlying prevalence of skin sensitivity. Most recently, BPO acne products may now also present a potential carcinogenicity risk through chemical degradation to benzene [18]. BPO-based acne products can be exposed to higher temperatures in the setting of travel or non-climate controlled storage settings (e.g. in a car). More data is still needed to fully assess the risk, but providers who care for vulnerable populations such as cancer survivors and pregnant persons with acne may recommend non-BPO acne treatments until further data can be obtained.
There are some relevant limitations to note for this study. While a randomized double-blind split-face design lowers the risk of confounders, the final analysis set included only 21 subjects.
The high dropout rate where patients were lost to follow up may reduce the confidence in the overall results—we anticipate this to largely be due to high survey burden. Future work should expand on these initial findings in a larger cohort.
Conclusion
A rationally designed 3-step regimen with azelaic acid, niacinamide, salicylic acid, and graduated nightly retinol resulted in comparable acne lesion reduction with higher overall patient tolerability and patient preferences compared to a 3-step regimen with BPO and glycolic acid in a randomized, double-blind, split-face study of 21 adult patients with acne vulgaris over 12 weeks of therapy.
Ethical approval
SX has stock options and is a consultant of Regimen—the manufacturer of one of the products studied here. JRW has a spouse with stock options and a consultant of Regimen—the manufacturer of one of the products studied here.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Collier CN, Harper JC, Cafardi JA et al (2008) The prevalence of acne in adults 20 years and older. J Am Acad Dermatol 58(1):56–59
Samuels DV, Rosenthal R, Lin R, Chaudhari S, Natsuaki MN (2020) Acne vulgaris and risk of depression and anxiety: A meta-analytic review. J Am Acad Dermatol 83(2):532–541
Insights FB. U.S. Acne Treatment Market Size, Share & COVID-19 Impact Analysis. https://www.fortunebusinessinsights.com/u-s-acne-treatment-market-106565. Published 2023. Accessed October 10, 2023, 2023.
Tanghetti EA, Popp KF (2009) A current review of topical benzoyl peroxide: new perspectives on formulation and utilization. Dermatol Clin 27(1):17–24
Sevimli DB (2019) Topical treatment of acne vulgaris: efficiency, side effects, and adherence rate. J Int Med Res 47(7):2987–2992
Chen W, Dai R, Li L (2020) The prevalence of self-declared sensitive skin: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol 34(8):1779–1788
Lucky AW, Barber BL, Girman CJ, Williams J, Ratterman J, Waldstreicher J (1996) A multirater validation study to assess the reliability of acne lesion counting. J Am Acad Dermatol 35(4):559–565
Williams HC, Dellavalle RP, Garner S (2012) Acne vulgaris. Lancet 379(9813):361–372
Fulton JE Jr, Farzad-Bakshandeh A, Bradley S (1974) Studies on the mechanism of action to topical benzoyl peroxide and vitamin A acid in acne vulgaris. J Cutan Pathol 1(5):191–200
Sagransky M, Yentzer BA, Feldman SR (2009) Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother 10(15):2555–2562
Matin T, Goodman MB (2023) Benzoyl Peroxide. In: StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Marcus Goodman declares no relevant financial relationships with ineligible companies.2023
Araviiskaia E, Dreno B (2016) The role of topical dermocosmetics in acne vulgaris. J Eur Acad Dermatol Venereol 30(6):926–935
Schulte BC, Wu W, Rosen T (2015) Azelaic Acid: Evidence-based Update on Mechanism of Action and Clinical Application. J Drugs Dermatol 14(9):964–968
Gollnick H, Cunliffe W, Berson D et al (2003) Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol 49(1 Suppl):S1-37
Thiboutot D, Dreno B, Layton A (2008) Acne counseling to improve adherence. Cutis 81(1):81–86
Decker A, Graber EM (2012) Over-the-counter Acne Treatments: A Review. J Clin Aesthet Dermatol 5(5):32–40
Flanders PA, McNamara JR (1985) Enhancing acne medication compliance: a comparison of strategies. Behav Res Ther 23(2):225–227
Valisure (2024) Valisure discovers benzoyl peroxide acne treatment products are unstable and form benzene
Acknowledgements
None.
Funding
Regimen.
Author information
Authors and Affiliations
Contributions
AG collected data, analyzed data, and generated figures. SR oversaw data collection, IRB approval, and study conduct. SX wrote manuscript and analyzed results. JRW performed statistical analysis and provided critical revisions of the manuscript. PV supported data collection, made critical edits to the manuscript, and scored all raw data outputs.
Corresponding author
Ethics declarations
Competing interests
Shuai Xu has stock options and is a consultant of Geologie - the manufacturer of one of the products studied here. Jessica Walter has a spouse with stock options and a consultant of Geologie – the manufacturer of one of the products studied here.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gern, A., Walter, J., Xu, S. et al. A randomized controlled double-blinded split-face prospective clinical trial to assess the efficacy, safety, and tolerability of a novel 3-step routine compared to benzoyl peroxide for the treatment of mild to moderate acne vulgaris. Arch Dermatol Res 316, 230 (2024). https://doi.org/10.1007/s00403-024-02874-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00403-024-02874-9