Abstract
Human leukocyte antigen (HLA)-B*58:01 allele is a significant risk factor for allopurinol-induced severe cutaneous adverse reactions (SCARs) which is potentially fatal. In some studies, chronic kidney disease (CKD) was also implicated to compound the risk of SCARs. We aim to investigate if pre-treatment HLA-B*58:01 screening can prevent allopurinol-induced SCARs in Chinese patients with CKD and its cost-effectiveness. We prospectively recruited Chinese CKD patients who required allopurinol during 2011–2015 and performed pre-treatment HLA testing (HLA screening group). Patients tested positive for HLA-B*58:01 were refrained from allopurinol while those tested negative were prescribed allopurinol. The incidence of SCARs in the HLA screening group was compared with the historical control in previous 5 years and the cost-effectiveness of HLA testing was analyzed. In the historical control (2006–2010), 3605 patients on allopurinol were screened, 22 out of 1027 (2.14%) CKD Chinese patients newly started on allopurinol developed SCARs, including 6 SJS/TEN. In the HLA screening group, 28 out of 192 patients (14.6%) tested HLA-B*58:01 positive were advised to avoid allopurinol; 156 out of 164 HLA-B*58:01-negative patients received allopurinol and none developed SCARs. The incidence rate of SCARs was significantly lower in the HLA screening group compared with controls (0% vs 2.14% respectively, p = 0.037*). The targeted HLA screening approach was associated with lower healthcare costs compared with no HLA screening (US$ 92,430 vs US$ 281,226). Pre-treatment HLA-B*58:01 screening is cost-effective to target on patients with CKD in Chinese to prevent allopurinol-induced SCARs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Steven–Johnson syndrome (SJS) and its related disease, toxic epidermal necrolysis (TEN) represent severe cutaneous adverse reactions (SCARs) that are potentially fatal. Withdrawal of the potential culprit drugs is by far the best approach to improve the outcome of patients with SJS/TEN.
Risk factors for SCARs are multi-factorial and genetic susceptibility such as human leucocyte antigen (HLA) specific allele, is one of the most important factors. Whether a patient will develop drug-induced SCARs is highly influenced by the expression of HLA. HLA are antigen presenting proteins on cell surfaces that are responsible for immune system regulation and are involved in the pathogenesis of drug hypersensitivity reactions [1]. Patients carrying the HLA-B*58:01 allele and received allopurinol were associated with 80-fold risk of development SJS/TEN compared with those without the allele [2]. A local study in Hong Kong also reported that allopurinol-induced SCARs patients carried at least one HLA-B*58:01 allele; however, there were also tolerant patients who carried the HLA-B*58:01 allele without developing the adverse reaction [3, 4].
HLA allele screening represents an important means to prevent drug-induced SCARs [1, 5]. In this context, the implementation of mandatory HLA allele screening prior to use of an anti-convulsant-carbamazepine in all public hospitals in Hong Kong since 2008 has helped to reduce incidence of carbamazepine-related SCARs and SJS/TEN [6, 7]. Theoretically, a similar approach can be adopted to minimize the risk of SCARs and SJS/TEN in patients who were to be commenced on allopurinol. However, one should recognize that the highest prevalence of HLA-B*15:02 allele was up to 20% in China and some Asian populations [6] and the odds ratio (OR) of developing of SJS/TEN in allele carrier was over 2500 compared to carbamazepine-tolerant patient without the allele [7]. The prevalence of HLA-B*58:01 varies from 8 to 20% in Chinese population [6,7,8,9] and the overall incidence of SJS/TEN is less common, thus the cost-effectiveness of screening HLA alleles in all patients who were to start allopurinol treatment remains undefined. It is postulated that such approach may be more cost-effective in high-risk patients, such as those with pre-existing CKD. Based on these backgrounds, this prospective study was set forth to investigate whether HLA screening can help prevent allopurinol-induced SCARs in CKD patients and the cost-effectiveness of this approach.
Materials and methods
Patients
This study was approved by the Institutional Review Board of the University of Hong Kong and Hospital authority, Hong Kong West Cluster (IRB: UW11-350). The study was conducted in full compliance with the ICH E6 guideline for Good Clinical Practice (ICH-GCP) and the principles of the Declaration of Helsinki. All subjects aged 18 years of age or above, attending Renal Clinics of two major tertiary hospitals in Hong Kong who have been diagnosed with CKD (including those with kidney transplantation) were screened. We defined chronic kidney disease based on the presence of either known kidney damage (including those with kidney transplantation) or presence of decreased kidney function (glomerular filtration rate (GFR) < 60 ml/min/1.73cm2) for 3 or more months [10]. We prospectively recruited patients who have not previously received allopurinol and who would normally have received it at the time of screening. The exclusion criteria were: (1) patients who were not ethnic Han Chinese; (2) patients who had previous haematopoietic stem cell transplantation; (3) patients with history of blood transfusion; and (4) patients with a documented history of allergic adverse reaction to allopurinol. We compared the clinical data and outcomes of our recruited patients with historical controls. Retrospective data as historical controls were collected from the hospital electronic patient records (ePR) and clinical data analysis and reporting system (CDARS) [11] in the same hospitals in previous years (2006–2010) to identify CKD patients with a diagnosis of allopurinol-induced SJS, TEN or major skin reactions secondary to drugs by International Classification of Disease, 9th revision, clinical modification (ICD-9-CM) code 695.1 and related conditions including 695.13 (SJS), 695.14 (SJS/TEN overlap), 695.15 (TEN). Diagnoses were confirmed based on clinical records, blood tests and skin biopsy results reviewed by qualified dermatologists.
Study procedures and follow-up
All recruited subjects were prescribed allopurinol at the time of screening visit, but were asked to defer taking the drug until they were informed of their HLA typing results. The results were reported to the clinicians in-charge within 1 week. Patients tested positive for HLA-B*58:01 were advised to refrain from allopurinol and given an alternative medication (febuxostat); whereas those tested negative were advised to commence allopurinol. Demographic and clinical data including age, gender, ethnicity, co-morbidities, allopurinol dosage, renal function, serum urate level at time of screening were documented. We followed up all subjects for at least first 2 months after drug initiation to monitor any symptoms of adverse reactions in regular visit and phone interview, with reference of one local study that the duration of exposure before symptom onset of SCARs ranged 10–56 days with mean 31.2 ± 15.4 [3]. Subjects were assessed by qualified dermatologist(s) immediately in the event that early symptoms of any cutaneous adverse reactions developed.
Genotyping of HLA
EDTA blood sample was collected from each subject. HLA-B*58:01 genotyping was conducted by the Division of Transplantation and Immunogenetics, Queen Mary Hospital, Hong Kong. Genomic DNA from EDTA blood samples was extracted using TBG EZbead blood DNA Extraction Kit (Texas BioGene Inc., Taiwan) according to the manufacturer's instructions. HLA-B genotypes were obtained using polymerase chain-reaction sequence-specific oligonucleotide probe methods using LifeCodes HLA-SSO Typing Kit (Gen-Probe, Stamford, CT) analysed by Luminex 200™ system (Luminex Corp., Austin, TX). HLA-B*58:01 positive was confirmed using sequence-specific primer or sequence based typing methods utilising the specific primers of SBTexcellerator® HLA typing Kit (Genome Diagnostics, Utrecht, the Netherlands).
Assessment of SCARs including SJS/TEN
The diagnosis of allopurinol-related drug eruption was then confirmed by qualified dermatologists based on clinical presentation with or without histological findings. The diagnostic criteria of severe cutaneous adverse reactions (SCARs), including erythema multiforme major (EMM), SJS and TEN, and drug hypersensitivity syndrome (DHS)/drug reactions with eosinophilia and systemic symptoms (DRESS) are based on the clinical morphology defined by Roujeau [12, 13]. We defined SJS as skin detachment of 10% of body-surface area, overlap SJS/TEN as skin detachment of 10–30%, and TEN as 30%. The criteria for drug hypersensitivity or DRESS are new onset of rash plus two of the following symptoms: eosinophilia, atypical circulating lymphocytes, leucocytosis, acute hepatocellular injury or worsening of renal function [14,15,16]. For those who did not fall into the above categories, morphological description and percentage of skin involvement were documented clearly by the attending dermatologist.
Identification of culprit drug
Culprit drug was identified based on the guideline, ALDEN (algorithm for assessment of drug causality in SJS/TEN) for identifying the causal medication [17]. Duration of latency was defined from the date of drug initiation to the date of symptoms onset.
Study outcomes and statistical analysis
The primary outcome was the incidence of allopurinol-induced SCARs. The secondary outcomes were allopurinol-induced SJS/TEN and cost-effectiveness of HLA screening approach. The sample size calculation was based on local prevalence of HLA-B*58:01 and study feasibility. With a prevalence of HLA-B*58:01 being 15% locally, ranging from 8 to 20% [2, 3, 6] in Han Chinese and the incidence of allopurinol-induced SCARs in CKD patients was 18% [18, 19], the estimated incidence of allopurinol-induced SCARs in HLA-B*58:01 CKD patients will be 2.7% (0.15 × 0.18). Therefore, 148 patients would achieve 80% power (alpha 0.05, with study: control enrolment ratio 1:3) to detect a reduction in the incidence rate from 2.7% to 0.03%. Categorical variables were expressed as frequencies (percentages), and analysed with Fisher’s exact or chi square test where appropriate. Continuous variables were expressed as mean (SD) or median (range), and analysed with independent t test or Mann–Whitney tests where appropriate. All statistical analyses were performed using SPSS (version 23.0, SPSS Inc. Chicago, IL, USA). All p values were two tailed and p value of <0.05 is considered as statistical significance.
Cost of HLA B*58:01 genotyping and hospitalization
The cost of genotyping HLA-B*58:01 in all patients prior to allopurinol commencement were compared with the cost of managing patients who developed SCARs. The cost of genotyping HLA-B*58:01 in all patients prior to allopurinol treatment was calculated as the nominal cost of genotyping HLA-B*58:01 per patients (US$ 90 per patient) multiplied by the total number of patients. The cost of managing patients who developed SCARs was calculated as the daily cost of hospitalization (US$ 3128 per day for intensive/high-dependency units and US$ 654 per day for general medical beds, respectively) multiplied by the duration of hospital stay. Taken that the cost of alternative uric acid lowering agent, febuxostat would be much higher than the cost of allopurinol, which was US$ 2.25 per 80 mg tablet per day versus US$ 0.234 per 200 mg tablet per day, we compared the annual cost of prescription of febuxostat to all patients without screening to that of the pre-treatment genotyping approach to evaluate the cost-effectiveness.
Results
Patient characteristics
Of the period 2006–2010, we screened 3605 patients who received allopurinol at the medical renal out-patient clinics in two tertiary hospitals (Queen Mary Hospital and Tung Wah Hospital) and included 1027 Chinese patients with CKD who were newly started on allopurinol as historic controls (Control group) (Table 1). We prospectively recruited 201 patients for HLA screening during the period of January 2011 to December 2015. After excluding the 9 subjects who were lost to follow-up or had withdrawn consent, 192 patients were included for data analysis (HLA screening group) (Fig. 1). A total of 1219 patients with CKD were included for analysis. In the HLA screening group, 28 patients (14.6%) were tested HLA-B*58:01 positive (Table 2) and were advised to avoid allopurinol. In the remaining 164 patients who were tested HLA-B*58:01-negative, 156 patients (95.1%) had received allopurinol. Eight patients did not take allopurinol as symptom was relieved by non-steroid anti-inflammatory drug (NSAID) or colchicine and continued on diet control alone.
Development of SCARs and SJS/TEN
In the HLA screening group, one patient who concurrently received NSAID reported urticarial eruption after 6 days of allopurinol treatment and the urticaria subsided after discontinuation of both agents. Three HLA-B*58:01-negative patients experienced exacerbation of gout during the initial phase of allopurinol treatment but improved as the treatment continued. No patients developed SCARs or SJS/TEN in both HLA-B*58:01 positive and negative groups (Table 3).
For the Control group, 22 patients developed allopurinol-induced SCARs, including 16 (72.7%) DRESS/DHS and 6 (27.2%) SJS/TEN (Table 3). Nineteen (86.4%) patients had stage 3 or above CKD. Allopurinol was discontinued in these 22 patients. For the 6 SJS/TEN patients, three patients underwent HLA-B*58:01 genotyping and all were positive (3/3, 100%), while the HLA-status of other three patients was not tested. Four DRESS/DHS patients underwent HLA-B*58:01 genotyping, of whom all were positive (4/4, 100%). The mean duration of hospital stay for DRESS/DHS cases was 5.5 ± 7.0 (range 3–22) days; SJS/TEN cases was 18.5 ± 10.3 (range 6–37) days; 66.7% (4 out of 6) required intensive care support for 10.5 ± 4.0 days (Table 3). Half of the patients (50%) required intravenous immunoglobulin therapy and 50% required systemic corticosteroid (prednisolone 1 mg/kg/day or equivalent). One patient died as a result of SJS/TEN. Three (50%) patients (2F:1M, age of 52–75 years, mean 60.3), had mild to moderate ocular complications of SJS/TEN (conjunctivitis, keratoconjunctivitis and corneal epithelial defect) and were managed conservatively during the acute stage with topical antibiotics (preservative free levofloxacin 0.5% eyedrops), topical steroids (prednisolone acetate 1% eye drops) and preservative free artificial tears, glass rod application on corneal adhesion. None of the patients required amniotic membrane transplantation during the acute stage. On long-term follow-up, one patient developed symblepharon of right lower eyelid with cornea scarring as a result of persistent ocular surface inflammation and eyelid margin keratinization, with subsequent visual impairment; and one patient developed right superior corneal pannus without symblepharon or significant limbal stem cell deficiency. There was no resulting visual impairment for the latter patient.
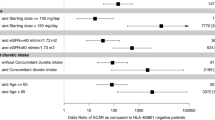
The HLA screening group showed a significantly lower incidence rate of allopurinol-induced SCARs compared with the Control group (0% vs 2.14%, p = 0.037*) (Table 4). The relative risk (RR) of developing allopurinol-induced SCARs in the HLA screening group were 0.12 (95% CI 0.0075–2.23, p = 0.14). For further sub-group analysis, in CKD stage 3 or above patients, the incidence of allopurinol-induced SCARs was even higher (3.07%, 19 out 619 patients with stage 3 or above CKD, compared to 0%, 0 out of 192 screening group subjects developed SCARs; p = 0.011*, with post hoc power > 80%; RR = 0.08, 95% CI 0.005–1.41, p = 0.08).
Cost-effectiveness of HLA screening
Using our historic controls as a cost-effectiveness analysis model, the cost of universal pre-treatment HLA screening in all controls was US$ 92,430 (i.e. US$ 90/test × 1027 patients). Based on our present data, the implementation of pre-treatment HLA screening would completely abrogate the occurrence of SCARs. Taken that a daily local hospital cost for Intensive care units and general wards was US$ 3128 and US$ 654, respectively, multiply the duration of hospital stays, the total cost of hospitalization of patients with SCARs was US$ 281,226; in particular, US$ 37,279 (in average) per SJS/TEN patient × 6 cases + US$ 3597 per DRESS/DHS patient × 16 cases, which was three-fold higher than performing HLA screening test. Thus, pre-treatment HLA screening can potentially reduce the health cost by 67.1% (US$ 92,430 with screening vs US$ 281,226 without screening).
Concerning the pharmaceutical cost, taken that the annual cost of febuxostat was US$ 821.25 per year (calculation: US$ 2.25 per 80 mg tablet febuxosat × 365 days), if we prescribed alternative uric acid lowering agents—febuxostat to patients without HLA screening, it would cost US$ 157,680 per year (calculation: US$ 821.25 × 192 patients), which would be significantly higher than that of the HLA screening/allopurinol approach, costing only US$ 54,282 [(US$ 90 per HLA test × 192 patients = US$ 17,280) + (US$ 0.234 per 200 mg daily dose of allopurinol × 365 days × 164 patients with negative HLA-B*58:01 status = US$ 14,007) + (US$ 2.25 per 80 mg daily dose of febuxostat × 365 days × 28 patients with positive HLA-B*58:01 status = US$ 22,995)]. Again, pre-treatment HLA screening approach can reduce the health cost by 65.5%.
Discussion
The incidence of SJS and TEN was estimated to be about 1–2 per million persons (PMP) per year and 0.4–1.2 PMP per year, respectively [20]. The mortality rate of SJS/TEN can be as high as 30–50% and 80% cases related to medications such antibiotics, anticonvulsants and uric acid lowering drugs [20, 21]. The pathogenetic mechanisms for drug-induced SCARs and SJS/TEN are highly complex and remain poorly understood. Drug-specific CD8 + lymphocytes have been detected in early blister fluid in SJS/TEN, while cytokines such as granzyme, perforin and natural killer cells directly causing damage to keratinocytes have also been implicated in the pathogenesis of drug-induced SCARs and SJS/TEN [22, 23]. Allopurinol is a xanthine oxidase inhibitor that is commonly used to treat hyperuricemia and gouty arthritis. It accounts for 5% of SCARs including SJS/TEN [24, 25]. It was suggested that HLA-B*58:01 carrier state was necessary but not sufficient for allopurinol-induced SCARs. Other co-factors such as renal insufficiency or viral infection have been implicated in the development of SCARs [4, 26, 27]. Studies have shown a relationship between allopurinol-induced SCARs and decreased creatinine clearance—patients with chronic kidney disease (CKD) had an increased risk of allopurinol-induced SCARs by fivefold [27, 28]. Allopurinol dosage adjustment have been suggested to reduce incidence of SCARs in renal insufficiency patients; however, studies failed to show the significant effect on reducing allopurinol hypersensitivity reactions [10, 29].
Our current data demonstrated that a HLA screening approach before the initiation of allopurinol can effectively prevent SCARs in CKD patients. Here we observed that the incidence rate of SCARs was significantly lower in HLA screening group compared with historic controls (0% vs 2.14%). Our results also suggested that a pre-treatment HLA screening approach reduced the risk of SCARs in CKD patients by 88% (RR = 0.12) although this did not reach statistical significance possibly due to the relatively small sample size and rarity of events. Notwithstanding, one should appreciate that no patient developed SCARs or SJS/TEN in the pre-treatment HLA screening group and this is extremely important because SJS/TEN is highly debilitating and potentially fatal. Moreover, SJS/TEN is not only associated with prolonged hospitalization (often in the intensive care unit (ICU)/burn units) and potential ocular sequalae and blindness, but also the use of costly therapies such as intravenous immunoglobulin (IVIg). The results were not surprising as HLA-B*58:01 showed very strong association with the development of SCARs and SJS/TEN, especially in patients of Han Chinese and Asian descents [30,31,32], and in some European countries [33,34,35]. Aside from SJS/TEN, there has been a number of reports on a potential association between HLA-B*58:01 and SCARs, although different studies have shown considerable variations in the magnitude [29, 30, 36]. The risk of allopurinol-induced SCARs was substantially elevated in HLA-B*58:01 carriers, showing > 150-fold risk higher than allopurinol-tolerant controls with negative status [36, 37]. In a Taiwan cohort study on allopurinol-induced SCARs, HLA-B*58:01 was not only strongly associated with the occurrence of SCARs but its status also correlated with disease severity [28]. HLA-B*58:01 carriers had 5 times higher risk in developing SCARs than in maculopapular exanthema (OR 44 and 8.5, respectively). Moreover, the gene dosage effect of HLA-B*58:01 also influenced the development of allopurinol-induced SCARs. HLA-B*58:01-homozygous patients have 4.8 times higher risk than heterozygotic carrier, with OR of 72 and 15, respectively. Furthermore, patients with CKD are shown to be more susceptible to allopurinol-induced SCARs, posing 85-fold risk of allopurinol-induced SCARs in HLA-B*58:01carriers than those with normal renal function (OR 1269 vs 15, respectively) [28]. Based on these observations and our present data, it is reasonable to advocate HLA screening in patients who were to commence allopurinol, especially in high-risk subjects such as those with pre-existing CKD.
While it was intuitive that universal pre-treatment HLA screening can help prevent SCARs and SJS/TEN patients receiving allopurinol, the financial implications and cost-effectiveness remains valid concerns in the implementation of such program. In this study, we explored whether focusing on high-risk patients (i.e. patients with CKD) might enhance the cost-effectiveness of pre-treatment HLA testing. Indeed our results showed that targeted pre-treatment HLA screening is associated with much lower overall healthcare costs than not instigating HLA testing. While not performing HLA screening may save costs for genetic test and only a small number of patients will develop SCARs or SJS/TEN, one should be cognizant that patients who developed SJS/TEN would incur substantial healthcare burden as a result of prolonged stay in ICU/burn units and expensive salvage therapies for this condition. Patients with SCARs also required assessment/care by qualified dermatologists and additional medications. Our results were consistent with recent studies from Taiwan [38] and Korea [39, 40] which also showed the cost-effectiveness of HLA-B*58:01 screening in preventing allopurinol-induced SCARs in CKD patients. Furthermore, our calculations have not taken into account the long-term disability, disfigurement and mortality related to SJS/TEN. In this study, we also presented the drug costs if we were to commence febuxostat in all patients without HLA screening. Although this approach has the advantage to initiate immediately at the clinic setting, the annual drug costs are significantly higher than the HLA screening/allopurinol approach and thus have considerable impacts on drug budgets. While our data demonstrate that pre-treatment HLA-B*58:01 allele screening is cost-effective in Han Chinese CKD patients to prevent allopurinol-related SCARs, one should appreciate that these cost-effectiveness analysis data may not always be extrapolated to other populations/localities due to the differences in allele frequency of HLA-B*58:01, costs and availability of HLA testing and other medical facilities. As substantial difference in the genetic make-up and mixing of different minor ethnicities exist between Northern and Southern Chinese as well as in other countries, further studies are required to validate our findings in other populations. To prevent SCARs, while clinicians should consider HLA-B*58:01 pre-screening in Han Chinese adults with CKD and that of Asian countries such as Korean or Thai descent before initiating allopurinol therapy, for populations such as Caucasians, with gene frequency of only 1%, this test is not likely to be cost-effective, and the net benefit is not known [41, 42].The relatively small sample size remains an important limitation of this study, which may jeopardize the power to detect the rare occurrence of SJS/TEN. However, there are considerable resource implications and difficulty in conducting large-scale prospective study to address this clinical question and comparison with historic controls as in our study appeared to be a feasible and reasonable study design. Other limitations of using historic controls include the difference in general medical care and treatment approaches that may affect overall patient outcomes and cost-effectiveness analysis.
Conclusion
Pre-treatment HLA-B*58:01 screening can prevent allopurinol-induced SCARs in Chinese CKD patients and is cost-effective.
References
Chung WH, Hung SI, Chen YT (2007) Human leukocyte antigens and drug hypersensitivity. Curr Opin Allergy Clin Immunol 7:317–323. https://doi.org/10.1097/ACI.0b013e3282370c5f
Hung SI, Chung WH, Liou LB et al (2005) HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci USA 102:4134–4139. https://doi.org/10.1073/pnas.0409500102
Chiu ML, Hu M, Ng MH et al (2012) Association between HLA-B*58:01 allele and severe cutaneous adverse reactions with allopurinol in Han Chinese in Hong Kong. Br J Dermatol 167:44–49. https://doi.org/10.1111/j.1365-2133.2012.10894.x
Goncalo M (2018) HLA-B*58:01 is not the only risk factor associated with allopurinol-induced severe cutaneous adverse drug reactions. Ann Transl Med 6:S7. https://doi.org/10.21037/atm.2018.08.42
Klein J, Sato A (2000) The HLA system. First of two parts. N Engl J Med 343:702–709. https://doi.org/10.1056/NEJM200009073431006
Kwok J, Guo M, Yang W et al (2016) HLA-A, -B, -C, and -DRB1 genotyping and haplotype frequencies for a Hong Kong Chinese population of 7595 individuals. Hum Immunol 77:1111–1112. https://doi.org/10.1016/j.humimm.2016.10.005
Chen Z, Liew D, Kwan P (2014) Real-world efficiency of pharmacogenetic screening for carbamazepine-induced severe cutaneous adverse reactions. PLoS ONE 9:e96990. https://doi.org/10.1371/journal.pone.0096990
Ko TM, Tsai CY, Chen SY et al (2015) Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study. BMJ 351:h4848. https://doi.org/10.1136/bmj.h4848
Cheng H, Yan D, Zuo X et al (2018) A retrospective investigation of HLA-B*5801 in hyperuricemia patients in a Han population of China. Pharmacogenet Genomics 28:117–124. https://doi.org/10.1097/FPC.0000000000000334
Chapter 1: Definition and classification of CKD (2013). Kidney Int Suppl (2011) 3:19–62. https://doi.org/10.1038/kisup.2012.64
Cheung NT, Fung V, Wong WN et al (2007) Principles-based medical informatics for success–how Hong Kong built one of the world’s largest integrated longitudinal electronic patient records. Stud Health Technol Inform 129:307–310
Bastuji-Garin S, Rzany B, Stern RS et al (1993) Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol 129:92–96
Roujeau JC (1997) Stevens-Johnson syndrome and toxic epidermal necrolysis are severity variants of the same disease which differs from erythema multiforme. J Dermatol 24:726–729. https://doi.org/10.1111/j.1346-8138.1997.tb02524.x
Arellano F, Sacristan JA (1993) Allopurinol hypersensitivity syndrome: a review. Ann Pharmacother 27:337–343. https://doi.org/10.1177/106002809302700317
Roujeau JC, Stern RS (1994) Severe adverse cutaneous reactions to drugs. N Engl J Med 331:1272–1285. https://doi.org/10.1056/NEJM199411103311906
Choudhary S, McLeod M, Torchia D, Romanelli P (2013) Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome. J Clin Aesthet Dermatol 6:31–37
Sassolas B, Haddad C, Mockenhaupt M et al (2010) ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther 88:60–68. https://doi.org/10.1038/clpt.2009.252
Jung JW, Song WJ, Kim YS et al (2011) HLA-B58 can help the clinical decision on starting allopurinol in patients with chronic renal insufficiency. Nephrol Dial Transplant 26:3567–3572. https://doi.org/10.1093/ndt/gfr060
Huang HY, Luo XQ, Chan LS et al (2011) Cutaneous adverse drug reactions in a hospital-based Chinese population. Clin Exp Dermatol 36:135–141. https://doi.org/10.1111/j.1365-2230.2010.03922.x
Rzany B, Mockenhaupt M, Baur S et al (1996) Epidemiology of erythema exsudativum multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis in Germany (1990–1992): structure and results of a population-based registry. J Clin Epidemiol 49:769–773. https://doi.org/10.1016/0895-4356(96)00035-2
Roujeau JC, Kelly JP, Naldi L et al (1995) Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med 333:1600–1607. https://doi.org/10.1056/NEJM199512143332404
Harris V, Jackson C, Cooper A (2016) Review of toxic epidermal necrolysis. Int J Mol Sci 17:2135. https://doi.org/10.3390/ijms17122135
Su SC, Chung WH (2013) Update on pathobiology in Stevens-Johnson syndrome and toxic epidermal necrolysis. Dermatol Sin 31:175–180
Atzori L, Pinna AL, Mantovani L et al (2012) Cutaneous adverse drug reactions to allopurinol: 10 year observational survey of the dermatology department–Cagliari University (Italy). J Eur Acad Dermatol Venereol 26:1424–1430. https://doi.org/10.1111/j.1468-3083.2011.04313.x
Lam MP, Yeung CK, Cheung BM (2013) Pharmacogenetics of allopurinol–making an old drug safer. J Clin Pharmacol 53:675–679. https://doi.org/10.1002/jcph.67
Suzuki Y, Inagi R, Aono T et al (1998) Human herpesvirus 6 infection as a risk factor for the development of severe drug-induced hypersensitivity syndrome. Arch Dermatol 134:1108–1112. https://doi.org/10.1001/archderm.134.9.1108
Vazquez-Mellado J, Morales EM, Pacheco-Tena C, Burgos-Vargas R (2001) Relation between adverse events associated with allopurinol and renal function in patients with gout. Ann Rheum Dis 60:981–983. https://doi.org/10.1136/ard.60.10.981
Ng CY, Yeh YT, Wang CW et al (2016) Impact of the HLA-B(*)58:01 allele and renal impairment on allopurinol-induced cutaneous adverse reactions. J Invest Dermatol 136:1373–1381. https://doi.org/10.1016/j.jid.2016.02.808
Cao ZH, Wei ZY, Zhu QY et al (2012) HLA-B*58:01 allele is associated with augmented risk for both mild and severe cutaneous adverse reactions induced by allopurinol in Han Chinese. Pharmacogenomics 13:1193–1201. https://doi.org/10.2217/pgs.12.89
Somkrua R, Eickman EE, Saokaew S et al (2011) Association of HLA-B*5801 allele and allopurinol-induced Stevens Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. BMC Med Genet 12:118. https://doi.org/10.1186/1471-2350-12-118
Yang F, Yang Y, Zhu Q et al (2015) Research on susceptible genes and immunological pathogenesis of cutaneous adverse drug reactions in Chinese hans. J Investig Dermatol Symp Proc 17:29–31. https://doi.org/10.1038/jidsymp.2015.6
Park HJ, Kim YJ, Kim DH et al (2016) HLA allele frequencies in 5802 Koreans: varied allele types associated with SJS/TEN according to culprit drugs. Yonsei Med J 57:118–126. https://doi.org/10.3349/ymj.2016.57.1.118
Goncalo M, Coutinho I, Teixeira V et al (2013) HLA-B*58:01 is a risk factor for allopurinol-induced DRESS and Stevens-Johnson syndrome/toxic epidermal necrolysis in a Portuguese population. Br J Dermatol 169:660–665. https://doi.org/10.1111/bjd.12389
Lonjou C, Borot N, Sekula P et al (2008) A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics 18:99–107. https://doi.org/10.1097/FPC.0b013e3282f3ef9c
Genin E, Schumacher M, Roujeau JC et al (2011) Genome-wide association study of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe. Orphanet J Rare Dis 6:52. https://doi.org/10.1186/1750-1172-6-52
Wu R, Cheng YJ, Zhu LL et al (2016) Impact of HLA-B*58:01 allele and allopurinol-induced cutaneous adverse drug reactions: evidence from 21 pharmacogenetic studies. Oncotarget 7:81870–81879. https://doi.org/10.18632/oncotarget.13250
Kang HR, Jee YK, Kim YS et al (2011) Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet Genomics 21:303–307. https://doi.org/10.1097/FPC.0b013e32834282b8
Ke CH, Chung WH, Wen YH et al (2017) Cost-effectiveness analysis for genotyping before allopurinol treatment to prevent severe cutaneous adverse drug reactions. J Rheumatol 44:835–843. https://doi.org/10.3899/jrheum.151476
Park DJ, Kang JH, Lee JW et al (2015) Cost-effectiveness analysis of HLA-B5801 genotyping in the treatment of gout patients with chronic renal insufficiency in Korea. Arthritis Care Res (Hoboken) 67:280–287. https://doi.org/10.1002/acr.22409
Park HW, Kim DK, Kim SH et al (2019) Efficacy of the HLA-B(*)58:01 screening test in preventing allopurinol-induced severe cutaneous adverse reactions in patients with chronic renal insufficiency-a prospective study. J Allergy Clin Immunol Pract 7:1271–1276. https://doi.org/10.1016/j.jaip.2018.12.012
Khanna D, Fitzgerald JD, Khanna PP et al (2012) 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 64:1431–1446. https://doi.org/10.1002/acr.21772
Saito Y, Stamp LK, Caudle KE et al (2016) Clinical Pharmacogenetics implementation consortium (CPIC) guidelines for human leukocyte antigen B (HLA-B) genotype and allopurinol dosing: 2015 update. Clin Pharmacol Ther 99:36–37. https://doi.org/10.1002/cpt.161
Acknowledgements
The authors would like to extend the appreciation to Dr. Kendrick Co Shih who reviewed and advised on SJS/TEN cases with ocular complications in the study.
Funding
This project was funded by the S.K. Yee Medical Foundation (Project number 212218).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no competing conflict of interest from all authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wong, C.SM., Yeung, CK., Chan, CY. et al. HLA-B*58:01 screening to prevent allopurinol-induced severe cutaneous adverse reactions in Chinese patients with chronic kidney disease. Arch Dermatol Res 314, 651–659 (2022). https://doi.org/10.1007/s00403-021-02258-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-021-02258-3