Abstract
Actinic cheilitis exhibits a potential of malignant transformation in 10–20 % of cases. The objective of this study was to compare the expression of MDM2 and SUMO-1 proteins between actinic cheilitis (AC) and squamous cell carcinoma (SCC) of the lip. The sample consisted of lower lip mucosa specimens obtained from cases with a clinical and histopathological diagnosis of AC (n = 26) and SCC (n = 25) and specimens of labial semi-mucosa (n = 15) without clinical alterations or inflammation. The tissue samples were stained with hematoxylin-eosin and anti-MDM2 and anti-SUMO-1 antibodies. Data were analyzed by the Kruskal–Wallis and Dunn’s tests (5 %). The median expression of MDM2 (kW = 36.8565; df = 3−1 = 2; p = 0.0001) and SUMO-1 (kW = 32.7080; df = 3−1 = 2; p = 0.0001) was similar in cases of AC and SCC of the lip, but differed significantly from that observed for normal labial semi-mucosa. Despite the limitations of the present study, immunohistochemistry demonstrated the overexpression of important proteins (MDM2 and SUMO-1) related to regulatory mechanisms of apoptosis in AC and SCC of the lip, but further studies are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Actinic cheilitis (AC) was first described by Ayres in 1923 [1] who observed lip lesions characterized by a process induced by solar radiation. The condition is more frequent in subjects older than 45 years, Caucasians, men, and nonsmokers [9]. The lower lip is the site most frequently affected (95 % of cases) since it receives more sun exposure due to its anatomical protrusion [26].
A significant increase in malignant lesions induced by ultraviolet (UV) radiation has been observed in tropical countries such as Brazil. This increase is a consequence of the fact that the economy of many of these countries is based on agriculture, farming, and fishing activities during which workers are constantly exposed to direct sunlight [3]. Most of the damage caused by UV light is due to changes that prevent the transcription of genetic information to mRNA, blocking the mechanism of DNA replication which, from a molecular viewpoint, leads to a reduction of mitotic activity and, from a clinical viewpoint, to alterations in the epithelium and lamina propria, compromising the photoprotective capacity of the lip [7].
Actinic cheilitis has the potential of malignant transformation and progression to squamous cell carcinoma (SCC) of the lip is observed in 10–20 % of cases [18]. Malignant transformation can occur within a period of 1–20 years if the condition is not diagnosed and treated adequately [8, 14, 19]. Lip SCCs account for 25–30 % of all oral cancers [11] and commonly affect the lip vermilion [5, 24]
During carcinogenesis, alterations occur in the mechanisms of cell proliferation and apoptosis and the altered expression of some proteins can be a strong indicator of the malignant transformation potential of a given lesion [28].
In regular cell development, the p53 protein is not required and it presents lower cellular levels, because of its short half-life; p53 protein maintains the integrity of the genome, cell cycle control, promotes apoptosis [17] and has negative effects on cell proliferation, by cell cycle block between the G1 and S phases [15]. In cell stress, its production is stimulated [17]. This regulation pattern changes in mutated p53, possibly with an increased proliferative activity. In oral carcinogenesis, overexpression of this protein is associated with rapid proliferation of neoplastic cells [15].
Protein p53 is regulated by post-transcriptional mechanisms, including its interaction with MDM2, which is an important negative regulator of p53, suppressing normal levels [10, 21]. Overexpression of MDM2 has been demonstrated in human cancers [23], including oral SCC [16]. The function of MDM2 is modulated physiologically by a post-transcriptional modifier, small ubiquitin-like modifier 1 (SUMO-1), in a process called sumoylation [21].
An increased expression of MDM2 is observed in lip SCC and AC specimens when compared to normal tissues [11]. To the best of our knowledge, no study has evaluated the expression of SUMO-1 in AC lesions. The objective of the present study was to analyze the expression of MDM2 and SUMO-1 proteins in epithelial cells of AC and lip SCC cases.
Methods
The study was approved by the Ethics Committee of Univ. Estadual Paulista (UNESP) (Permit No. 001/2011-PH/CEP).
The samples used in this study were obtained from paraffin-embedded specimens of vermilion mucosa of the lower lip. The following cases were selected from the archives of the Laboratory of Oral Pathology, São José dos Campos Dental School, UNESP: group 1 consisted of 26 cases of AC; group 2 consisted of 25 cases of SCC, and group 3 consisted of 15 cases of labial semi-mucosa without clinical alterations or inflammation. The last specimens were obtained from the periphery of biopsies performed to confirm the diagnosis of mucocele and fibrous hyperplasia. The demographic data were collected from anamnesis charts.
Sections (5 µm) were stained with hematoxylin-eosin to confirm the histological diagnosis using criteria for the histological grading of AC and the histopathological classification of malignancy of SCC, both proposed by the World Health Organization (WHO) [2].
For immunohistochemistry, the tissue blocks were cut into 3-µm sections and submitted to the streptavidin–biotin method using monoclonal antibodies against MDM2 (clone SMP14, dilution 1:50, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and SUMO-1 (clone D-11, dilution 1:50, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Antigen retrieval was performed in 1 mM EDTA for MDM2 and in 10 mM citric acid for SUMO-1. The sections were incubated with the primary antibodies for 18 h at 4 °C. Immunodetection was performed with the LSAB Visualization System (DAKO Corporation, Glostrup, Denmark) using 3,3′-diaminobenzidine as chromogen. The sections were counterstained with Mayer’s hematoxylin. Sections of osteosarcoma and normal labial semi-mucosa were used as positive controls for the expression of MDM2 [13] and SUMO-1 [4], respectively. Sections processed as described above but omitting the primary antibodies served as negative control.
Cells exhibiting nuclear staining were defined as positive for MDM2, and cytoplasmic and/or nuclear staining were defined as positive staining for SUMO-1. A total of 1,000 cells were counted at 400× magnification and the percentage of positive cells was calculated.
Data were analyzed by the Kruskal–Wallis and Dunn’s tests (5 %).
Results
Among the patients with a diagnosis of AC, 20 % were women and 80 % were men. Patient age ranged from 39 to 77 years, with a mean of 63.3 years. According to the WHO histological grading system [22], 13 (50 %) of the 26 AC cases were classified as mild epithelial dysplasia, 11 (42.3 %) as moderate dysplasia, and two (7.69 %) as severe dysplasia. The histopathological classification of malignancy proposed by the WHO was used for cases of SCC [22]. Seventeen (68 %) SCC cases were classified as well differentiated, four (16 %) as moderately differentiated, and four (16 %) as poorly differentiated.
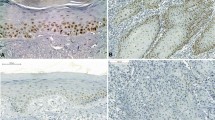
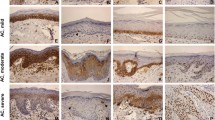
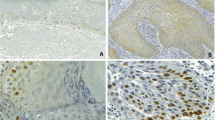
Light microscopy revealed the expression of MDM2 in AC and SCC specimens, with the observation of strong staining in all layers. No expression of MDM2 or SUMO-1 was observed in group 3. Figure 1 illustrates the pattern of immunohistochemical expression of MDM2 and SUMO-1.
For MDM2, the distributions of values differed (kw = 36.8565; df = 3−1 = 2; p = 0.0001). The same was observed for SUMO-1 (kw = 32.7080; df = 3−1 = 2; p = 0.0001) (Fig. 2).
Analysis by the Dunn’s test (5 %) showed similar median expression of MDM2 and SUMO-1 in cases of AC and SCC, whereas a significant difference was observed when compared to normal labial semi-mucosa.
Discussion
Changes in the expression of proteins related to the regulatory mechanism of apoptosis can be used as markers of malignant transformation of epithelial lesions such as AC. SCC is the most frequent malignant oral cancer and often arises from lesions with a potential of malignant transformation. According to Carinci et al. [7], the traditional methods used by pathologists are subjective and are not sufficiently sensitive to accurately predict which potentially malignant lesions will progress to carcinoma over time. Markers that can identify the progression of these lesions are therefore important.
The study of AC is relevant since it is one of the main conditions affecting the lip and a potential of malignant transformation has been reported in 10–20 % of cases. Exposure to sunlight or artificial UV radiation are the factors responsible for the onset and installation of the disease [11, 26]. Chronic exposure to UV radiation is also an important etiological factor for the development of lip carcinoma [3].
Immunohistochemical analysis revealed similar severe expression of MDM2 and SUMO-1 in all epithelial layers of AC and SCC lesions, whereas no expression of these proteins was observed in normal labial semi-mucosa. According to Katayama et al. [16], MDM2 and SUMO-1 are rarely detected in normal epithelial tissues.
MDM2 protein promotes p53 degradation by ubiquitination, suppressing cellular response to stress [21]. The balance between MDM2 autoubiquitination and p53 ubiquitination is physiologically modulated through a post-transcriptional modification by protein SUMO-1 [21]. Therefore, the removal of SUMO-1 from sumoylated MDM2 leads to MDM2 autoubiquitination, which results in p53 stabilization, leaving it available to perform its role in the neoplastic suppression [10]. Thus, the decrease in MDM2 sumoylation is related to high levels of p53 [6].
Exposure to UV radiation can cause DNA damage [27], with a consequent higher expression of p53, as demonstrated in immunohistochemical studies [12, 29]. The degradation of p53 protein, both the mutant and the wild type, can be promoted by MDM2 [21]. Thus, in a higher expression of p53, an increased expression of MDM2 is expected. Otherwise, by feedback control, high concentrations of p53 may activate the mdm2 gene and, consequently, increases the amount of MDM2 in the tissue [4]. In addition, DNA damage can also lead to mutations in the mdm2 gene, which results in loss of MDM2 protein ubiquitination [22], showing greater immunohistochemical expression [11]. However, for our knowledge there are no studies confirming MDM2 mutations on the molecular level in SCC.
Katayama et al. [16] reported high expression of MDM2 and SUMO-1 in oral SCC and epithelial dysplasia as observed in the present study. As mentioned earlier, MDM2 loses the capacity to be ubiquinated and sumoylated [22], with a consequent higher expression of SUMO-1 in these lesions. The authors concluded that the interaction between MDM2 and SUMO-1 may be associated with cell proliferation since sumoylated MDM2 is less susceptible to degradation, thus increasing its activity on p53 [16].
As observed in the present study, Freitas et al. [11] demonstrated overexpression of MDM2 in cases of AC with epithelial dysplasia. Martínez et al. [20] also found overexpression of MDM2 in cases of AC when compared to normal lip tissue. De Freitas et al. [11] suggested a possible role of the MDM2 biomarker in lip carcinogenesis, regardless of the stage of the disease. The present results agree with this suggestion since MDM2 expression was observed throughout the AC sample, irrespective of the degree of epithelial dysplasia.
Oral lichen planus immunohistochemical study showed absent or limited expression of SUMO-1 similar to normal mucosa, whereas this protein was overexpressed in cases of epithelial dysplasia and oral SCC [25]. Although, to the best of our knowledge, there are no studies in the literature investigating the expression of SUMO-1 in AC, the observation of similar expression of this protein in AC and SCC of the lip agrees with the findings of Oliveira Alves et al. [25]. In the present study, overexpression of SUMO-1 was detected throughout the AC sample, irrespective of the degree of epithelial dysplasia.
Notwithstanding, the objective of this study was to compare the expression of MDM2 and SUMO-1 proteins between AC and SCC of the lip. The research was conducted using paraffin-embedded tissue archival samples diagnosed as AC and SCC of the lip. Unfortunately, clinical follow-up of cases to evaluate possible malignant transformation was not possible.
Despite the limitations of the present study, immunohistochemistry demonstrated overexpression of MDM2 and SUMO-1 proteins related to regulatory mechanisms of apoptosis, in AC and SCC of the lip. The overexpression of MDM2 and SUMO-1 in AC samples, regardless the degree of epithelial dysplasia, may indicate a relation to carcinogenesis early stages by DNA-damage-induced UV radiation exposition.
References
Ayres S (1923) Chronic actinic cheilitis. JAMA 81:483–486
Barnes L, Eveson JW, Reichart P, Sidransky D (2005) World Health Organization classification of tumours. IARC Press, Lyon
Batista AC, Costa NL, Oton-Leite AF, Mendonça EF, de Alencar RC, Silva TA (2010) Distinctive clinical and microscopic features of squamous cell carcinoma of oral cavity and lip. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109:e74–e79
Berglund L, Björling E, Oksvold P, Fagerberg L, Asplund A, Szigyarto CA et al (2008) A genecentric Human Protein Atlas for expression profiles based on antibodies. Mol Cell Proteomics 7:2019–2027
Bork K, Hoide N, Korting GW, Burgdorf WHC, Young SK (1993) Diseases of the bucal mucosa and the lips. Malignant tumors. WB Saunders, Germany, pp 326–328
Buschmann T, Fuchs SY, Lee CG, Pan ZQ, Ronai Z (2000) SUMO-1 modification of Mdm2 prevents its self-ubiquitination and increases Mdm2 ability to ubiquitinate p53. Cell 101:753–762
Carinci F, Lo Muzio L, Piattelli A, Rubini C, Palmieri A, Stabellini G et al (2005) Genetic portrait of mild and severe lingual dysplasia. Oral Oncol 4:365–374
Cataldo E, Doku HC (1981) Solar cheilitis. J Dermatol Surg Oncol 7:989–995
Cavalcante AS, Anbinder AL, Carvalho YR (2008) Actinic cheilitis: clinical and histological features. J Oral Maxillofac Surg 66:498–503
Daujat S, Neel H, Piette J (2001) MDM2: life without p53. Trends Genet 17:459–464
de Freitas Mda C, Ramalho LM, Xavier FC, Moreira AL, Reis SR (2008) p53 and MDM2 protein expression in actinic cheilitis. J Appl Oral Sci 16:414–419
de Oliveira MG, Ramalho LM, Gaião L, Pozza DH, de Mello RA (2012) Retinoblastoma and p53 protein expression in pre-malignant oral lesions and oral squamous cell carcinoma. Mol Med Rep 6:163–166
Dong YB, Yang HL, Elliott MJ, McMasters KM (2003) Increased mdm-2 expression in a p53-independent manner blocks UV-induced cell cycle arrest and apoptosis in human osteosarcoma cells. Tumour Biol 24:130–139
dos Santos JN, de Sousa SO, Nunes FD, Sotto MN, de Araújo VC (2003) Altered cytokeratin expression in actinic cheilitis. J Cutan Pathol 30:237–241
Girod SC, Pfeiffer P, Ries J, Pape HD (1998) Proliferative activity and loss of function of tumour suppressor genes as ‘biomarkers’ in diagnosis and prognosis of benign and preneoplastic oral lesions and oral squamous cell carcinoma. Br J Oral Maxillofac Surg 36:252–260
Katayama A, Ogino T, Bandoh N, Takahara M, Kishibe K, Nonaka S et al (2007) Overexpression of small ubiquitin-related modifier-1 and sumoylated Mdm2 in oral squamous cell carcinoma: possible involvement in tumor proliferation and prognosis. Int J Oncol 31:517–524
Kim KI, Baek SH (2006) SUMOylation code in cancer development and metastasis. Mol Cells 22:247–253
Krunic AL, Garrod DR, Madani S, Buchanan MD, Clark RE (1998) Immunohistochemical staining for desmogleins 1 and 2 in keratinocytic neoplasms with squamous phenotype: actinic keratosis, keratoacanthoma and squamous cell carcinoma of the skin. Br J Cancer 77:1275–1279
Main JH, Pavone M (1994) Actinic cheilitis and carcinoma of the lip. J Can Dent Assoc 60:113–116
Martínez A, Brethauer U, Borlando J, Spencer ML, Rojas IG (2008) Epithelial expression of p53, mdm-2 and p21 in normal lip and actinic cheilitis. Oral Oncol 44:878–883
Meek DW, Knippschild U (2003) Posttranslational modification of MDM2. Mol Cancer Res 1:1017–1026
Melchior F, Hengst L (2000) Mdm2-SUMO1: is bigger better? Nat Cell Biol 2:E161–E163
Momand J, Wu HH, Dasgupta G (2000) MDM2–master regulator of the p53 tumor suppressor protein. Gene 242:15–29
Moore S, Johnson N, Pierce A, Wilson D (1999) The epidemiology of lip cancer: a review of global incidence and aetiology. Oral Dis 5:185–195
Oliveira Alves M, Balducci I, Rodarte Carvalho Y, Cabral L, Nunes F, Almeida J (2013) Evaluation of the expression of p53, MDM2, and SUMO-1 in oral lichen planus. Oral Dis 19:775–780
Ribeiro CF, Souza FH, Jordão JM, Haendchen LC, Mesquita L, Schmitt JV et al (2012) Photodynamic therapy in actinic cheilitis: clinical and anatomopathological evaluation of 19 patients. An Bras Dermatol 87:418–423
Sarasin A (1999) The molecular pathways of ultraviolet-induced carcinogenesis. Mutat Res 428:5–10
Sousa FA, Paradella TC, Carvalho YR, Rosa LE (2009) Immunohistochemical expression of PCNA, p53, bax and bcl-2 in oral lichen planus and epithelial dysplasia. J Oral Sci 51:117–121
Souza LR, Fonseca-Silva T, Pereira CS, Santos EP, Lima LC, Carvalho HA et al (2011) Immunohistochemical analysis of p53, APE1, hMSH2 and ERCC1 proteins in actinic cheilitis and lip squamous cell carcinoma. Histopathology 58(3):352–360
Acknowledgments
To National Council of Technological and Scientific Development, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support, and CIPAX (Medicina diagnóstica) for positive control tissue sections.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oliveira Alves, M.G., da Mota Delgado, A., Balducci, I. et al. Study of MDM2 and SUMO-1 expression in actinic cheilitis and lip cancer. Arch Dermatol Res 306, 837–841 (2014). https://doi.org/10.1007/s00403-014-1500-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-014-1500-8