Abstract
Background
This study aimed to evaluate whether a combination of platelet-rich plasma (PRP) and hyaluronic acid (HA) is more effective and safer than injection alone for treating KOA.
Materials and methods
MEDLINE (PubMed), the Cochrane Library, EMBASE, and Web of Science databases were systematically searched for articles published until January 2024, and gray literature and bibliographic references were searched. All published randomized controlled trials (RCTs) compared pain, functional outcomes, and adverse events (AEs) associated with PRP + HA therapy vs. PRP or HA treatments. Two independent researchers extracted the pertinent data and evaluated the methodological quality following the PRISMA guidelines. The primary outcomes were pain, functional outcomes, and AEs. A fixed-effects model was used for data analysis in cases with low heterogeneity (P > 0.10 and I2 < 50%). Otherwise, a random effects model was used.
Results
Ten RCTs involving 943 patients were included in the analysis. The statistical findings did not differ between the treatment of PRP + HA and PRP alone, while a discernible enhancement in treatment efficacy was observed when compared to HA monotherapy: the visual analog scale scores at 1- (mean difference[MD], −1.00; 95% CI: −1.37 − −0.62; P < .001), 6- (MD, −1.87; 95% CI: −3.46 − −0.28; P = .02), 12-months (MD, −2.07; 95% CI: −3.77 − −0.38; P = .02), and the Western Ontario and McMaster Universities Arthritis Index total scores at 12-months (MD, −8.82; 95% CI: −14.48 − −3.16; P = .002). The incidence of adverse events was notably lower with PRP + HA than with HA alone (OR, 0.37; 95% CI: 0.19 − 0.69; P = .00) or PRP alone (OR, 0.51; 95% CI, 0.30 − 0.87; P = .01).
Conclusions
PRP + HA therapy resulted in more pronounced pain and functional improvement in symptomatic KOA patients than HA treatments, and combination therapy may have higher clinical safety than PRP or HA monotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Knee osteoarthritis (KOA), characterized by the loss of articular cartilage, development of osteophytes, alterations in subchondral bone, and synovial hyperplasia [1], even finally leading to disability [2], affects approximately 250 million individuals worldwide [3]. However, due to the unclear pathological mechanisms of KOA [1, 4], there are no well-established pharmaceuticals or interventions capable of altering the progression of KOA [5,6,7]. The current treatment focus is primarily on pain management and functional enhancement [7].
Prior to the final stage of total knee arthroplasty (TKA), non-pharmacological interventions, such as dietary adjustments and exercise, were frequently recommended [8], alongside analgesics, corticosteroids, and nonsteroidal anti-inflammatory drugs (NSAIDs). Steroid injections are commonly used, but their efficacy diminishes after a month [9], and prolonged application may lead to adverse consequences for the existing cartilage [10]. Given the potential nephrotoxicity and gastrointestinal side effects associated with chronic NSAIDs and analgesic use [11, 12], there has been growing interest in advocating intra-articular injections of alternatives.
Hyaluronic acid (HA), a natural glycosaminoglycan found in the articular cavity [13], shows therapeutic potential owing to its viscoinductive properties and joint lubrication [14, 15], especially considering its reduced presence within the joints of individuals with osteoarthrosis [16]. Nevertheless, several studies [1, 3, 17, 18] strongly support the adoption of platelet-rich plasma (PRP) over the potentially limited efficacy of HA [3]. PRP is derived from centrifuged whole blood, resulting in a product with platelet concentrations higher than the baseline levels. They contain various growth factors and proteins that have been shown to enhance the regeneration of chondrocytes. PRP has emerged as a promising alternative biological treatment for pain alleviation and functional improvement in patients with KOA [2]. However, clinical evidence surrounding PRP remains contentious, with concerns raised about the potential impairment of PRP’s overall effects by leukocyte [2, 19].
An additional and relatively recent development in these therapeutic approaches involves the concurrent administration of PRP and HA. The theoretical objective is to synergize the effects of these two agents to maximize and prolong their efficacy [20,21,22,23]. Considering that previous reviews either incorporated non-randomized controlled trials (RCTs) or analyzed a limited number of RCTs [6, 24,25,26,27], and that a significant number of additional RCTs have been published in recent years [5, 20, 28, 29]. we consider it imperative to conduct an updated systematic review and meta-analysis to offer a comprehensive evaluation of the efficacy and safety of PRP + HA therapy regimens for individuals with osteoarthritis.
Methods
This meta-analysis has been reported in line with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [30]. This systematic review was registered with PROSPERO on June 5, 2023.
Search strategy
Two independent researchers conducted a comprehensive search to identify eligible RCTs in the MEDLINE (PubMed), Cochrane Library, EMBASE, and Web of Science databases. Data was retrieved on April 23, 2023, and updated on January 14, 2024 (Supplemental Files, sTable 1). Additionally, we screened the reference lists of the retrieved publications to identify additional RCTs. In cases of disagreement among researchers, a third author was consulted to resolve the disagreement and evaluate all articles.
We included RCTs that compared the efficacy and safety of PRP + HA therapy with individual components in patients with KOA of any stage. All single ingredient and combination therapy regimens were included, regardless of the dose or number of PRP or HA injections. Patients who used PRP + HA therapy were the experimental group, while those who used either HA or PRP therapy served as the comparison group. Trials that included at least one of the following outcome indicators (visual analog scale [VAS] scores, Western Ontario and McMaster Universities Arthritis Index [WOMAC]) total scores, Knee Injury and Osteoarthritis Outcome Scores, International Knee Documentation Committee (IKDC) scores, Lequesne index scores, and adverse events (AEs) were considered eligible, provided that data for participants with osteoarthritis could be extracted.
Study selection and data extraction
After removing duplicates, two researchers independently screened the titles and abstracts, followed by a full-text assessment of the relevant articles. Disagreements regarding the inclusion of the studies were resolved by consensus. Data were independently extracted by the same researchers using a predesigned data extraction form. This form, designed by the senior author, encompassed various pieces of information, including publication year, first author, study design, number of included patients, patient characteristics, interventions, Kellgren-Lawrence (K-L) grades of KOA, follow-up periods, and relevant scoring data of clinical efficacy and safety. Both researchers reviewed the articles and extracted the necessary data using a predetermined form. In case of any disagreements or discrepancies between the extractions, a third investigator was consulted to resolve these issues. Before concluding the study, the database was searched again to ensure that all relevant literature was included, thereby confirming the completeness of the included studies.
Risk-of-Bias Assessment
Two independent researchers assessed the risk of bias in the RCTs using the Cochrane Risk of Bias tool [31]. The risk of bias within each domain was categorized as low, high, or unclear (Supplemental Files, sFigures 1 and 2). Quality assessments of the studies were conducted independently by the same researchers, and any discrepancies were resolved through consensus. Funnel plots could not assess publication bias because none of the meta-analyses included more than 10 studies.
Statistical analysis
The mean difference (MD) served as the effect index for continuous variables, whereas the odds ratio (OR) was used as the effect index for dichotomous data. Additionally, 95% confidence intervals (CI) were calculated for all variables, and heterogeneity was evaluated using the Higgins I2 statistic. Statistical significance was set at P < 0.05. If the heterogeneity was low (P > 0.10 and I2 < 50%), a fixed-effects model was used for data analysis. Otherwise, a random effects model was used. Sensitivity analyses were performed to evaluate the robustness of the synthesized results.
Statistical analyses of the data and risk assessments were conducted using STATA (version 18.0; StataCorp LLC, College Station, Texas, USA) software and Review Manager version 5.4.1 (The Cochrane Collaboration).
Study quality assessment
The Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) criteria [32] were assessed by two researchers to evaluate the overall quality of evidence. The evidence quality dropped due to two reasons: high statistical variation (I2 > 50%) [33] and imprecise results (95% CI included the “0” line) (Supplemental Files, sTables 2 and 3).
Results
Literature screening process and results
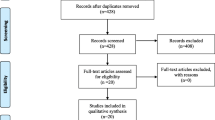
A total of 1904 relevant articles were identified through the searches of four medical databases. 78 articles were deemed potentially eligible after title and abstract reviews, and 68 were excluded after full-text evaluation. Finally, for the meta-analysis, 10 studies [4, 5, 10, 16, 20, 28, 29, 34,35,36] met the predetermined eligibility criteria (Fig. 1).
Study participants
These studies collectively encompassed a total patient sample size of 943, comprising six two-arm studies and four three-arm studies (PRP + HA: 345; PRP: 281; HA: 245), and the follow-up period was extended from 6 to 24 months (Table 1). After treatment, short-term efficacy ranged from 1 to 6 months, medium-term efficacy ranged from 6 to 12 months, and long-term efficacy extended beyond 12 months.
Intervention characteristics
Within the pool of included RCTs, PRP was administered at doses ranging from 3 to 8 ml, and HA was administered at doses of 2 to 3 ml. The treatment duration varied from 3 to 8 weeks, with injection intervals spanning 1–2 weeks. Notably, PRP and HA were administered together in all trials except for two studies [10, 29], which had dosing intervals. (Table 2).
Outcomes assessment
Comparison 1: PRP + HA vs. HA
VAS scores
Three studies documented VAS scores after treatment. At each follow-up time point, the results of subgroup analyses demonstrated a statistically significant difference in VAS scores at 1 month (MD, −1.00; 95% CI: −1.37 − −0.62; P < 0.001), 6-month (MD, −1.87; 95% CI: −3.46 − −0.28; P = 0.02), and 12-month (MD, −2.07; 95% CI: −3.77 − −0.38; P = 0.02) post-treatment (Fig. 2).
WOMAC total scores
Two studies recorded WOMAC total scores at 1-, 6-, and 12-months post-treatment. The pooled results indicated the absence of significant heterogeneity between two groups at 1 month (MD, −2.42; 95% CI: −13.44 − 8.60; P = 0.67) and 6-month (MD, −11.19; 95% CI: −22.72 − 0.34; P = 0.06), but exhibited a statistically significant difference at 12-month (MD, −8.82; 95% CI: −14.48 − −3.16; P = 0.002) (Fig. 3).
Comparison 2: PRP + HA vs. PRP
VAS scores
Three studies reported the VAS scores after treatment. The results at various follow-up time points revealed no significant differences at 1- and 6-months (Supplemental Files, sFigure 3).
WOMAC total scores
Four studies covered WOMAC total scores after treatment. The results of the subgroup analyses showed no significant heterogeneity at 1-, 3-, 6-, 12-, and 24-months (Supplemental Files, sFigure 4).
IKDC scores
Two studies reported the IKDC scores post-treatment. The pooled results suggested no significant heterogeneity in IKDC scores at 4–6 weeks and 6 months (Supplemental Files, sFigure 5).
Lequesne index scores
Two studies reported Lequesne index scores at 1- and 6-months post-treatment. The results showed no significant heterogeneity at 1- and 6-months (Supplemental Files, sFigure 6).
Adverse reactions
Eight studies reported AEs after the treatment. The most frequently reported complications were mild inflammatory reactions characterized by redness at the treatment site. The pooled results demonstrated that the rate of AEs in the PRP + HA group was significantly lower than that in the HA group (OR, 0.37; 95% CI, 0.19 − 0.69; P = 0.00) or PRP group (OR, 0.51; 95% CI, 0.30 − 0.87; P = 0.01) (Fig. 4).
Sensitivity analyses
We conducted sensitivity analyses of all subgroup studies using random-effects models, and the findings of this meta-analysis remained stable.
Discussion
Principal findings
In summary, the meta-analysis indicated a clear and statistically significant advantage for the PRP + HA therapy group over the HA-alone therapy group in terms of short-to medium-term pain relief and functional improvement. Compared with patients receiving PRP alone, the current statistical results showed no better or prolonged beneficial effects in the PRP + HA therapy group. The lack of significant differences may be attributed to the limited number of studies included in the analysis and the exclusion of non-RCTs. Finally, the combined therapy exhibited a safer profile than PRP- or HA-alone therapies. This information will assist orthopedic surgeons and patients with early-to mid-stage KOA or older patients unable to undergo surgery due to physical limitations in making informed choices about conservative treatments.
In this meta-analysis, we also increased the minimal clinically important difference (MCID) concept to assess the disparity between the PRP + HA and HA-alone groups: VAS scores [37] and WOMAC total scores [38]. Although the WOMAC total scores did not achieve the MCID (9 points) in this study, the PRP + HA group exhibited significant improvement over the HA group in VAS scores, with a decrease of 1.00 at 1 month, 1.87 at 6 months and 2.07 at 12 months; two of them were above the MCID in VAS scores (1.37). This indicates the clinical superiority of the PRP + HA treatment group in alleviating midterm pain compared to the HA group.
Standardization of combination therapy
Different preparations of PRP and HA can lead to variations in the treatment outcomes in patients with KOA. Currently, there are no standardized definitions for the preparation of PRP and HA, and various studies have used their own methods to prepare PRP, different concentrations of HA, varying injection doses, frequencies, and intervals for experimentation. For example, in an RCT conducted by Jacob et al. [16], the PRP injection dose was 2 ml, whereas in a trial conducted by Yu et al. [4], the PRP injection dose was 8 ml. HA is present in different concentrations and types [39,40,41], as reported in the relevant literature, and HA products injected into joints with a molecular weight of 3 × 103 kDa or higher, especially those derived from biological fermentation processes, have proven to be more effective and safer [40]. In the included studies, almost all utilized HA molecules with molecular weights lower than those mentioned above. This may have led to an increase in the number of AEs in the HA group, thereby affecting the reliability of the results. A systematic review suggested that repeated intra-articular HA injections are effective and safe for treating KOA [42], while multiple HA injections may increase the risk of infection during future TKA procedures [43, 44]. Therefore, conservative treatment aims not only to improve immediate symptoms but also to consider potential long-term complications that may occur in patients with KOA.
The effectiveness of different types of PRP [45], known as leukocyte-rich platelet-rich plasma (LR-PRP) and leukocyte-poor platelet-rich plasma (LP-PRP), for treating KOA has been debated with no clear agreement in the scientific community [46]. This prevailing understanding suggests that LP-PRP is linked to anti-inflammatory effects, whereas LR-PRP is associated with pro-inflammatory effects [19, 46]. Although a meta-analysis suggested that adverse reactions were prevalent in PRP treatments regardless of leukocyte concentration [47], recent studies comparing LR-PRP with LP-PRP for KOA treatment indicated that LP-PRP resulted in lower pain levels during intra-articular injections [2, 19, 48]. Of the 10 RCTs included in this meta-analysis, five used LR-PRP, two used LP-PRP, and three did not report relevant information. Hence, the varying occurrence of AEs in PRP groups across studies may have reduced the reliability of the combined results. Previous literature reports recommend that PRP treatment for patients with KOA between K-L grades 1 and 3 is typically administered in two to four sessions, spaced 2–4 weeks apart [9, 49]. This is consistent with the frequencies used in most of the included studies. Recent studies have shown that multiple PRP injections administered over several years are more effective than a single injection [50,51,52,53]. However, a recent meta-analysis found that multiple injections did not always provide significant pain relief or functional improvement [54]. This finding suggests that further research is required to confirm the optimal number of PRP injections. This difference resulted in considerable heterogeneity in the pooled results. In future studies, it will be essential to establish a standardized approach for combination therapy to improve reporting consistency.
Comparison with other studies
Previously, five meta-analyses investigated the effects of PRP + HA therapy: Zhang [6], Karasavvidis [25], Aw [24], Zhao [26] and Qiao [27]. The meta-analyses conducted by Zhang et al. [6], AW et al. [24], and Zhao et al. [26] demonstrated that PRP + HA therapy resulted in significantly greater improvement in VAS scores and WOMAC total and function scores compared to PRP-alone therapy in the short to medium term, while according to Qiao et al. [27], PRP-alone therapy outperformed PRP + HA therapy in WOMAC total and function scores. However, this meta-analysis revealed no significant differences in VAS scores at 1- or 6-months and in WOMAC total scores at 1-, 3-, 6-, 12-, and 24-months. The differences in these results may be related to the previous meta-analysis, which included non-RCTs [6, 24,25,26] and limited studies [27]. Furthermore, both Zhang et al. [6] and AW et al. [24] reported better IKDC scores in the PRP + HA therapy group than in the PRP-alone therapy group at the 6-month mark. In our analysis, we found no significant heterogeneities at 4–6 weeks and 6-month time points. The results reported by Karasavvidis et al. [25] and Qiao et al. [27] showed that superior improvement in pain and function with the combination therapy of PRP and HA compared to HA-alone therapy in terms of VAS scores at 3-, 6-, and 12-months. These findings align with the results of our meta-analysis of VAS scores at 6- and 12-months. Additionally, Zhao et al. [26] suggested that the combination therapy of PRP with HA did not show a significant difference in AEs compared to PRP- or HA-alone therapy. In addition, Qiao et al. [27] revealed that PRP-alone therapy was safer than PRP + HA therapy. In contrast, Zhang et al. [6] and AW et al. [24] demonstrated that dual therapy was generally safer than PRP-alone, which was consistent with our findings. Hence, this meta-analysis offers a comprehensive reassessment of the contentious findings from prior studies.
Limitations
This study included all recently published RCTs to eliminate potential selection bias introduced by non-RCTs. Therefore, the quality of evidence in the major subgroup analyses was moderate or above. In addition, we presented mean differences instead of standardized mean differences to make the results more accessible and understandable. Furthermore, this analysis provides valuable reference information on the short- and long-term efficacy of KOA treatment.
Nevertheless, the limited number of enrolled RCTs in this meta-analysis and the formulations of PRP and HA, were not consistent across trials, resulting in a restricted pool of studies for each outcome indicator. Notably, regarding WOMAC-related indicators, disparities in values have been observed in certain studies owing to varying threshold settings [36]. Furthermore, the relatively small sample sizes of certain RCTs could limit the statistical validity of the study [5, 28]. Lastly, owing to the limited number of RCTs included in the study, we were unable to conduct additional subgroup analyses related to different doses and frequencies of PRP interventions, as well as the K-L grades of KOA.
Conclusion
The findings of this study suggest that the combination of PRP and HA demonstrates promising clinical safety in patients with KOA. PRP + HA therapy exhibited a higher level of safety than the administration of PRP or HA alone. In symptomatic patients with KOA, the combined therapy of PRP and HA showed superior improvements in pain and function compared with patients treated solely with HA injections.
Data availability
Additional Information Explanation for why data not available: Prof. Zuo and Prof. Zhang had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
Chen L, Jin S, Yao Y, He S, He J (2023) Comparison of clinical efficiency between intra-articular injection of platelet-rich plasma and hyaluronic acid for osteoarthritis: a meta analysis of randomized controlled trials [Article]. Therapeutic Advances in Musculoskeletal Disease. https://doi.org/10.1177/1759720x231157043
Kim J-H, Park Y-B, Ha C-W (2023) Are leukocyte-poor or multiple injections of platelet-rich plasma more effective than hyaluronic acid for knee osteoarthritis? A systematic review and meta-analysis of randomized controlled trials [Review]. Arch Orthop Trauma Surg 143(7):3879–3897. https://doi.org/10.1007/s00402-022-04637-5
Wang Z, Wang R, Xiang S, Gu Y, Xu T, Jin H, Gu X, Tong P, Zhan H, Lv S (2022) Assessment of the effectiveness and satisfaction of platelet-rich plasma compared with hyaluronic acid in knee osteoarthritis at minimum 7-year follow-up: a post hoc analysis of a randomized controlled trial. Front Bioeng Biotechnol 10:1062371. https://doi.org/10.3389/fbioe.2022.1062371
Yu W, Xu P, Huang G, Liu L (2018) Clinical therapy of hyaluronic acid combined with platelet-rich plasma for the treatment of knee osteoarthritis [Article]. Exp Ther Med 16(3):2119–2125. https://doi.org/10.3892/etm.2018.6412
Xu Q, Pan Y, Li S, Yang F, Ma D, Su S-M, Zhang L-C, Feng J-B, Deng M-L (2022) CLINICAL STUDY OF PLATELET-RICH PLASMA COMBINED WITH SODIUM HYALURONATE INJECTION IN TREATING KNEE OSTEOARTHRITIS [Article]. Acta medica mediterranea 38(5):3421–3427. https://doi.org/10.19193/0393-6384_2022_5_505
Zhang Q, Liu T, Gu Y, Gao Y, Ni J (2022) Efficacy and safety of platelet-rich plasma combined with hyaluronic acid versus platelet-rich plasma alone for knee osteoarthritis: a systematic review and meta-analysis [Review]. J Orthop Surg Res 17(1):499. https://doi.org/10.1186/s13018-022-03398-6
Wang L, Wei L, Ma H, Wang M, Rastogi S (2022) Is platelet-rich plasma better than hyaluronic acid in the treatment of knee osteoarthritis? A meta-analysis of randomized controlled trials [Article]. Videosurgery and Other Miniinvasive Techniques 17(4):611–623. https://doi.org/10.5114/wiitm.2022.118777
Abdel Shaheed C, Awal W, Zhang G, Gilbert SE, Gallacher D, McLachlan A, Day RO, Ferreira GE, Jones CM, Ahedi H, Tamrakar M, Blyth FM, Stanaway F, Maher CG (2022) Efficacy, safety, and dose-dependence of the analgesic effects of opioid therapy for people with osteoarthritis: systematic review and meta-analysis. Med J Aust 216(6):305–311. https://doi.org/10.5694/mja2.51392
Chang K-V, Hung C-Y, Aliwarga F, Wang T-G, Han D-S, Chen W-S (2014) Comparative Effectiveness of Platelet-Rich Plasma Injections for Treating Knee Joint Cartilage Degenerative Pathology A Systematic Review and Meta Analysis [Article]. Arch Phys Med Rehab. https://doi.org/10.1016/j.apmr.2013.11.006
Lana JF, Weglein A, Sampson SE, Vicente EF, Huber SC, Souza CV, Ambach MA, Vincent H, Urban-Paffaro A, Onodera CM, Annichino-Bizzacchi JM, Santana MH, Belangero WD (2016) Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J Stem Cells Regen Med 12(2):69–78. https://doi.org/10.46582/jsrm.1202011
Migliorini F, Driessen A, Quack V, Sippel N, Cooper B, El Mansy Y, Tingart M, Eschweiler J (2021) Comparison between intra-articular infiltrations of placebo, steroids, hyaluronic and PRP for knee osteoarthritis: a Bayesian network meta-analysis [Article]. Arch Orthop Trauma Surg 141(9):1473–1490. https://doi.org/10.1007/s00402-020-03551-y
Wu Q, Luo X, Xiong Y, Liu G, Wang J, Chen X, Mi B (2020) Platelet-rich plasma versus hyaluronic acid in knee osteoarthritis: a meta-analysis with the consistent ratio of injection [Review]. J Orthopaed Su. https://doi.org/10.1177/2309499019887660
Li S, Xing F, Yan T, Zhang S, Chen F (2023) Multiple injections of platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of current evidence in randomized controlled trials [Review]. JPM. https://doi.org/10.3390/jpm13030429
Zhang HF, Wang CG, Li H, Huang YT, Li ZJ (2018) Intra-articular platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis. Drug Des Devel Ther 12:445–453. https://doi.org/10.2147/dddt.S156724
Han Y, Huang H, Pan J, Lin J, Zeng L, Liang G, Yang W, Liu J (2019) Meta-analysis comparing platelet-rich plasma vs hyaluronic acid injection in patients with knee osteoarthritis [Article]. Pain Med 20(7):1418–1429. https://doi.org/10.1093/pm/pnz011
Jacob G, Shetty V, Shetty S (2017) A study assessing intra-articular PRP vs PRP with HMW HA vs PRP with LMW HA in early knee osteoarthritis [Article]. J Arthrosc Jt Sur 4(2):65–71. https://doi.org/10.1016/j.jajs.2017.08.008
Belk JW, Lim JJ, Keeter C, McCulloch PC, Houck DA, McCarty EC, Frank RM, Kraeutler MJ (2023) Patients with knee osteoarthritis who receive platelet-rich plasma or bone marrow aspirate concentrate injections have better outcomes than patients who receive hyaluronic acid: systematic review and meta-analysis [Review]. Arthrosc J Arthrosc Relat Sur 39(7):1714–1734. https://doi.org/10.1016/j.arthro.2023.03.001
Singh H, Knapik DM, Polce EM, Eikani CK, Bjornstad AH, Gursoy S, Perry AK, Westrick JC, Yanke AB, Verma NN, Cole BJ, Chahla JA (2022) Relative Efficacy of Intra-articular Injections in the Treatment of Knee Osteoarthritis: A Systematic Review and Network Meta-analysis [Review]. Am J Sports Med. https://doi.org/10.1177/03635465211029659
Peng Y-N, Chen J-L, Hsu C-C, Chen CPC, Suputtitada A (2022) Intra-articular leukocyte-rich platelet-rich plasma versus intra-articular hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis of 14 randomized controlled trials [Review]. Pharmaceuticals. https://doi.org/10.3390/ph15080974
Ciapini G, Simonettii M, Giuntoli M, Varchetta G, De Franco S, Ipponi E, Scaglione M, Parchi PD (2023) Is the Combination of Platelet-Rich Plasma and Hyaluronic Acid the Best Injective Treatment for Grade II-III Knee Osteoarthritis? A Prospective Study [Article]. Adv orthop. https://doi.org/10.1155/2023/1868943
Chen WH, Lo WC, Hsu WC, Wei HJ, Liu HY, Lee CH, Tina Chen SY, Shieh YH, Williams DF, Deng WP (2014) Synergistic anabolic actions of hyaluronic acid and platelet-rich plasma on cartilage regeneration in osteoarthritis therapy. Biomaterials 35(36):9599–9607. https://doi.org/10.1016/j.biomaterials.2014.07.058
Chen WH, Lin CM, Huang CF, Hsu WC, Lee CH, Ou KL, Dubey NK, Deng WP (2016) Functional recovery in osteoarthritic chondrocytes through hyaluronic acid and platelet-rich plasma-inhibited infrapatellar fat pad adipocytes. Am J Sports Med 44(10):2696–2705. https://doi.org/10.1177/0363546516651822
Chiou CS, Wu CM, Dubey NK, Lo WC, Tsai FC, Tung TDX, Hung WC, Hsu WC, Chen WH, Deng WP (2018) Mechanistic insight into hyaluronic acid and platelet-rich plasma-mediated anti-inflammatory and anti-apoptotic activities in osteoarthritic mice. Aging (Albany NY). https://doi.org/10.18632/aging.101713
Aw AAL, Leeu JJ, Tao X, Razak HRBA (2021) Comparing the efficacy of dual Platelet-Rich Plasma (PRP) and Hyaluronic Acid (HA) therapy with PRP-alone therapy in the treatment of knee osteoarthritis: a systematic review and meta-analysis [Review]. J Exp Orthop. https://doi.org/10.1186/s40634-021-00415-1
Karasavvidis T, Totlis T, Gilat R, Cole BJ (2021) Platelet-rich plasma combined with hyaluronic acid improves pain and function compared with hyaluronic acid alone in knee osteoarthritis: a systematic review and meta-analysis [Review]. Arthrosc J Arthrosc Relat Sur. https://doi.org/10.1016/j.arthro.2020.11.052
Zhao J, Huang H, Liang G, Zeng LF, Yang W, Liu J (2020) Effects and safety of the combination of platelet-rich plasma (PRP) and hyaluronic acid (HA) in the treatment of knee osteoarthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord 21(1):224. https://doi.org/10.1186/s12891-020-03262-w
Qiao X, Yan L, Feng Y, Li X, Zhang K, Lv Z, Xu C, Zhao S, Liu F, Yang X, Tian Z (2023) Efficacy and safety of corticosteroids, hyaluronic acid, and PRP and combination therapy for knee osteoarthritis: a systematic review and network meta-analysis [Review]. BMC musculoskeletal disorders, Article. https://doi.org/10.1186/s12891-023-06925-6
Barac B, Damjanov N, Zekovic A (2019) The new treatment approach in knee osteoarthritis: efficacy of cellular matrix combination of platelet rich plasma with hyaluronic acid versus two different types of hyaluronic acid (ha) (prospective, randomized, double blind control study) [Meeting Abstract]. Ann Rheum Dis 78:500–500. https://doi.org/10.1136/annrheumdis-2019-eular.5018
Branch EA, Cook JJ, Cohen A, Plummer H, Emami A, Truett J, Anz AW (2023) Platelet-rich plasma is similar to platelet-rich plasma plus hyaluronic acid for the treatment of knee osteoarthritis at 2 years a randomized controlled trial [Article]. J Cartil Jt Preserv. https://doi.org/10.1016/j.jcjp.2023.100129
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O’Connell D, Oxman AD, Phillips B, Schünemann HJ, Edejer T, Varonen H, Vist GE, Williams JW Jr, Zaza S (2004) Grading quality of evidence and strength of recommendations. BMJ 328(7454):1490. https://doi.org/10.1136/bmj.328.7454.1490
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Glasziou P, Jaeschke R, Akl EA, Norris S, Vist G, Dahm P, Shukla VK, Higgins J, Falck-Ytter Y, Schünemann HJ (2011) GRADE Guidelines: 7 rating the quality of evidence--inconsistency. J Clin Epidemiol 64(12):1294–1302. https://doi.org/10.1016/j.jclinepi.2011.03.017
Papalia R, Zampogna B, Russo F, Torre G, De Salvatore S, Nobile C, Tirindelli MC, Grasso A, Vadala G, Denaro V (2019) The combined use of platelet rich plasma and hyaluronic acid: prospective results for the treatment of knee osteoarthritis [Article]. J biol regulat homeost agents 33(2):21–28
Sun SF, Lin GC, Hsu CW, Lin HS, Liou IHS, Wu SY (2021) Comparing efficacy of intraarticular single crosslinked Hyaluronan (HYAJOINT Plus) and platelet-rich plasma (PRP) versus PRP alone for treating knee osteoarthritis [Article]. Sci Rep 11(1):140. https://doi.org/10.1038/s41598-020-80333-x
Xu Z, He Z, Shu L, Li X, Ma M, Ye C (2021) Intra-Articular platelet-rich plasma combined with hyaluronic acid injection for knee osteoarthritis is superior to platelet-rich plasma or hyaluronic acid alone in inhibiting inflammation and improving pain and function [Article]. Arthrosc J Arthrosc Relat Sur 37(3):903–915. https://doi.org/10.1016/j.arthro.2020.10.013
Hawker GA, Mian S, Kendzerska T, French M (2011) Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken) 63(Suppl 11):S240-252. https://doi.org/10.1002/acr.20543
Saltzman BM, Leroux T, Meyer MA, Basques BA, Chahal J, Bach BR Jr, Yanke AB, Cole BJ (2017) The therapeutic effect of intra-articular normal saline injections for knee osteoarthritis a meta-analysis of evidence level 1 studies [Article]. Am J Sports Med 45(11):2647–2653. https://doi.org/10.1177/0363546516680607
Iturriaga V, Vásquez B, Bornhardt T, Del Sol M (2021) Effects of low and high molecular weight hyaluronic acid on the osteoarthritic temporomandibular joint in rabbit. Clin Oral Investig 25(7):4507–4518. https://doi.org/10.1007/s00784-020-03763-x
Altman RD, Bedi A, Karlsson J, Sancheti P, Schemitsch E (2016) Product differences in intra-articular hyaluronic acids for osteoarthritis of the Knee [Article]. Am J Sports Med 44(8):2158–2165. https://doi.org/10.1177/0363546515609599
Sun SF, Hsu CW, Lin HS, Liou IH, Chen YH, Hung CL (2017) Comparison of single intra-articular injection of novel hyaluronan (HYA-JOINT Plus) with synvisc-one for knee osteoarthritis: a randomized, controlled, double-blind trial of efficacy and safety. J Bone Joint Surg Am 99(6):462–471. https://doi.org/10.2106/jbjs.16.00469
Altman R, Hackel J, Niazi F, Shaw P, Nicholls M (2018) Efficacy and safety of repeated courses of hyaluronic acid injections for knee osteoarthritis: a systematic review. Semin Arthritis Rheum 48(2):168–175. https://doi.org/10.1016/j.semarthrit.2018.01.009
Cancienne JM, Werner BC, Luetkemeyer LM, Browne JA (2015) Does timing of previous intra-articular steroid injection affect the post-operative rate of infection in total knee arthroplasty? J Arthroplasty 30(11):1879–1882. https://doi.org/10.1016/j.arth.2015.05.027
Bedard NA, Pugely AJ, Elkins JM, Duchman KR, Westermann RW, Liu SS, Gao Y, Callaghan JJ (2017) The John N. insall award: do intraarticular injections increase the risk of infection after TKA? Clin Orthop Relat Res 475:45–52. https://doi.org/10.1007/s11999-016-4757-8
Le ADK, Enweze L, DeBaun MR, Dragoo JL (2018) Current Clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med 11(4):624–634. https://doi.org/10.1007/s12178-018-9527-7
Belk JW, Kraeutler MJ, Houck DA, Goodrich JA, Dragoo JL, McCarty EC (2021) Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis a systematic review and meta analysis of randomized controlled trials. Am j sports med. https://doi.org/10.1177/0363546520909397
Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ (2016) Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis [Article]. Am J Sports Med 44(3):792–800. https://doi.org/10.1177/0363546515580787
Zhao D, Pan JK, Yang WY, Han YH, Zeng LF, Liang GH, Liu J (2021) Intra-Articular injections of platelet-rich plasma, adipose mesenchymal stem cells, and bone marrow mesenchymal stem cells associated with better outcomes than hyaluronic acid and saline in knee osteoarthritis: a systematic review and network meta-analysis. Arthroscopy 37(7):2298-2314.e2210. https://doi.org/10.1016/j.arthro.2021.02.045
Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD (2016) Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review [Review]. Arthroscopy-the Journal of Arthroscopic and Related Surgery 32(3):495–505. https://doi.org/10.1016/j.arthro.2015.08.005
Smith PA (2016) Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: an fda-sanctioned, randomized, double-blind, placebo-controlled clinical trial [Article]. Am J Sports Med 44(4):884–891. https://doi.org/10.1177/0363546515624678
Cole BJ, Karas V, Hussey K, Pilz K, Fortier LA (2017) Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis [Article]. Am J Sports Med 45(2):339–346. https://doi.org/10.1177/0363546516665809
Duymus TM, Mutlu S, Dernek B, Komur B, Aydogmus S, Kesiktas FN (2017) Choice of intra-articular injection in treatment of knee osteoarthritis: platelet-rich plasma, hyaluronic acid or ozone options [Article]. Knee Surg Sports Traumatol Arthrosc 25(2):485–492. https://doi.org/10.1007/s00167-016-4110-5
Gormeli G, Gormeli CA, Ataoglu B, Colak C, Aslanturk O, Ertem K (2017) Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial [Article]. Knee Surg Sports Traumatol Arthrosc 25(3):958–965. https://doi.org/10.1007/s00167-015-3705-6
Han SB, Seo IW, Shin YS (2021) Intra-articular injections of hyaluronic acid or steroids associated with better outcomes than platelet-rich plasma, adipose mesenchymal stromal cells, or placebo in knee osteoarthritis: a network meta-analysis. Arthroscopy 37(1):292–306. https://doi.org/10.1016/j.arthro.2020.03.041
Acknowledgements
Not applicable.
Funding
The study was financially supported by the Science and Technology Development Program of Jilin Province (Nos. YDZJ202201ZYTS520, YDZJ202201ZYTS043, 20230508171RC), Program of Jilin Provincial Department of Finance (Nos.2023SCZ89, 2022SCZ03), The Bethune Program of Jilin University (No.2023B18), and The National Natural Science Foundation Youth Support Training Program of China-Japan Union Hospital, Jilin University (No.2022qnpy26).
Author information
Authors and Affiliations
Contributions
Concept and design: Zuo. Acquisition, analysis, or interpretation of data: Gao, Ma. Drafting of the manuscript: Gao, Ma. Critical revision of the manuscript for important intellectual content: Tang, Zhang, Zuo. Statistical analysis: Gao, Ma, Zhang. Obtained funding: Zuo. Administrative, technical, or material support: Zhang. Supervision: Tang, Zhang, Zuo.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, J., Ma, Y., Tang, J. et al. Efficacy and safety of platelet-rich plasma and hyaluronic acid combination therapy for knee osteoarthritis: a systematic review and meta-analysis. Arch Orthop Trauma Surg (2024). https://doi.org/10.1007/s00402-024-05442-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00402-024-05442-y







