Abstract
Purpose
To analyze changes in tendency of etiology and of antimicrobial resistance patterns to most common local and systemic antibiotics in chronic osteomyelitis of the tibia (COM-T) in a Level I trauma center over an 11-year period.
Methods
A retrospective review including all patients with COM-T who were surgically treated from January 2009 to December 2019. Patients were divided into two period groups: 2009–2014 and 2015–2019. Microbiologic etiology was analyzed. Bacterial resistance patterns evaluation was based on the Magiorakos et al. classification, including proportions of multidrug-resistant organisms (MDROs, acquired non-susceptibility to at least one agent in three or more antimicrobial categories), extensively drug-resistant (XDR) and pan drug-resistant (PDR) organisms encountered.
Results
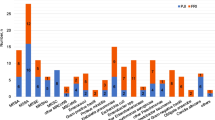
A total of 173 episodes of COM-T were identified. Monomicrobial infections represented 47.4% of all cases, while 28.3% had polymicrobial infections. Negative deep-bone cultures were identified in 24.3% of the patients. The most commonly isolated microorganisms were coagulase-negative Staphylococci (24.5%) and S. aureus (20.5%). No differences were found when comparing Gram-positive infections between periods (58.3% for 2009–2014 vs. 46.7% for 2015–2019; p = 0.10). Findings were similar for Gram-negative infections (37% vs. 33.7%; p = 0.62), although more polymicrobial infections were detected (24.7% vs. 33.3%, respectively; p = 0.359). MDROs were involved in 15% of the cases, with an upward trend when comparing both periods (12.8% vs. 23.6%; p = 0.07). The most-used combination of local antibiotics—glycopeptide (vancomycin) plus aminoglycoside (gentamicin or tobramycin)—was met with low rates of resistance in the most frequently isolated microorganisms.
Conclusion
According to the results of the present study, rates of Gram-positive and Gram-negative infections remained consistent during the two study periods, but with an upward trend in MDRO and polymicrobial infections detected. The local combination of a glycopeptide plus an aminoglycoside was effective in treating the most frequently isolated microorganisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic osteomyelitis (COM) is as old as humankind. Owing to its morbidity and sequelae, it continues to be one of the most feared complications in the trauma field. Nowadays, its prevalence is increasing [1], as its epidemiology in the lower extremity has shifted from hematogenous spread to a predominance of contiguous infection of surrounding tissues following an open fracture or implant-related infection [2]. The tibia, due to its characteristically poor soft tissue envelope, is the most affected bone [3].
Microorganisms have an innate ability to adapt to their environments, developing resistance to antibiotics and modifying their sensitivity profiles. Due to changes in etiology over the years, understanding of the microbiological spectrum of COM is of crucial importance to its management—particularly in choice of empiric antibiotic treatment, both local and systemic, during the early postoperative period. However, there is scant information in the literature regarding bacterial distribution, or the prevalence of multidrug-resistant organisms (MDROs), despite a concerning increase in cases in recent years [3,4,5], with only a few retrospective observational studies published [1, 3, 4, 6].
In an ideal scenario, therapy selection should be made based on the microorganism identified as causative. Nevertheless, antibiotic selection is usually based on periprosthetic joint infection (PJI) protocols, whose microbial cause has been more defined [7]. Percutaneous biopsies have frequently been used in pre-surgical microbiological diagnosis [8], but their efficiency is a matter of debate. First, it is difficult to be sure that a biopsy is performed in the infection’s precise location. Second, there are comorbidity and cost concerns. Some studies have shown sensitivities between 29 and 42% [9, 10]. Since our group [11] showed a sensitivity of 48.2% and specificity of 52%, pre-surgical percutaneous biopsy was not regularly performed. So, in actual practice, the causative microorganisms are typically unknown pre-surgery, and local antibiotic selection is empirical. This fact is especially important in cases of single-stage surgery [12] because the local antibiotic cannot be subsequently modified. Therefore, understanding the inherent spectrum is crucial in selecting an optimum local antibiotic.
The main purpose of the present study is to describe the etiology of COM of the tibia (COM-T) and to analyze any changes of tendency over the time. Objectives: (1) detect antimicrobial resistance patterns to the most common local antibiotics—vancomycin, gentamicin, tobramycin; (2) detect resistance profiles to other common systemic antibiotics, important to empirical treatment prior to receiving antibiogram results, and (3) analyze the incidence of MDROs.
Patients and methods
After obtaining Institutional Review Board approval, we conducted a retrospective review of our institutional database to identify all patients with surgically treated COM-T, from January 2009 to December 2019 in our Level I Trauma Center (which houses a national-referral Musculoskeletal Infection Unit). Inclusion criteria: adult patients (> 16 years old) with culture results available from their first surgical procedure and an established diagnosis of COM according to an internationally accepted definition [13]. For staged-procedure patients, only the first intervention was included for analysis. Patients who did not fit all inclusion criteria were excluded from the study, as were patients for whom all data was not available. The following data were recorded from our institutional database: sex, age, laterality, lesion localization, material or bone exposure, and osteomyelitis type per University of Texas Medical Branch (UTMB) classification, generally known as Cierny–Mader classification [14].
Definitions
-
An infection was diagnosed if at least one of the following criteria was fulfilled: (1) Presence of a sinus tract, (2) Bone or osteosynthesis material exposure, (3) Positive COM histology test, (4) Pus or intraoperative abscess, and (5) ≥ 2 positive cultures to the same pathogen.
-
Bacterial resistance patterns were based on the Magiorakos et al. classification [15]: Multidrug resistant (MDR): acquired non-susceptibility to at least one agent in three or more antimicrobial categories. Extensively drug resistant (XDR): non-susceptibility to at least one agent in all but two or fewer antimicrobial categories (i.e., bacterial isolates remain susceptible to only one or two categories). Pan drug resistant (PDR): non-susceptibility to all agents in all antimicrobial categories. This definition was created to describe acquired resistance profiles in Staphylococcus aureus, Enterococcus sp., Enterobacterales (other than Salmonella and Shigella), Pseudomonas aeruginosa and Acinetobacter sp. In this series, this definition was extrapolated to coagulase-negative Staphylococci and Pseudomonas sp.
Surgical sampling technique
Patients should have not received antibiotics for at least two weeks prior to the intervention. Before intravenous antibiotic administration, deep samples were taken according to protocols established in our unit to maximize sample performance. A special kit of instruments, consisting of six forceps and six 50 mL Falcon® conical tubes, was used to harvest samples. Instrument contact with the skin was avoided, and each forceps was used only once, to avoid bacterial cross-contamination. We obtained only deep specimens of dead bone (sequestrum), interface membrane or intramedullary pus during the first part of the surgery. Specimens were sent as soon as possible to the microbiology laboratory. We did not obtain samples from granulation tissue, nor from sinus or cutaneous ulcers. Samples were also always sent for histological examination.
Systemic antibiotic
Once surgical samples were collected for culture, individualized broad-spectrum empirical antibiotic coverage was begun, generally with an antimicrobial schedule that included a glycopeptide (Teicoplanin, due to its superior pharmacokinetic profile and lower risk of inducing nephrotoxicity, as compared to vancomycin) accompanied by either meropenem or a cephalosporin with anti-pseudomonas activity, depending on patient medical history and comorbidities.
Local antibiotic dead-space management
Local antibiotic strategy was chosen according to the type of COM and treatment selected. In single-stage treatments, antibiotic loaded resorbable bone substitutes were used to fill the dead space created after surgical debridement. In most of our cases the antibiotic used was a gentamicin–vancomycin combination. In cases of staged surgical strategy, we prefer to use high-dose antibiotic loaded polymethylmethacrylate (PMMA) during the first stage. PMMA is preferred in this setting as it provides high concentrations of local antibiotics [17,18,19], enhances stability, and is easily removed at subsequent stages.
Microbiological sample processing
The results of intraoperative culture samples were retrospectively analyzed. Biopsies were submitted in sterile containers, and—in some more recent cases—samples inoculated in blood culture bottles (BacT/Alert®, bioMérieux Inc., Marcy-l'Etoile, France) for submission. The microbiological processing protocol for these samples was maintained with little variation over time. It included tissue homogenization, inoculation into enriched solid and liquid media for aerobic and anaerobic bacterial growth, and extended incubation time. Results were considered negative if there was no visible growth after 14 days of incubation. Microorganisms isolated in culture were identified by manual biochemical tests, such as the analytical profile index, or automated biochemical tests on cards, with mass spectrometry added to microbial identification in the second half of the study (API®, VITEK®2 and VITEK®MS, respectively, all from bioMérieux Inc.). Antimicrobial susceptibility was continuously assessed by disc diffusion (Neo-Sensitabs™, ROSCO 190 Diagnostica A/S, Denmark), gradient diffusion (E-test®, bioMérieux Inc.) or microdilution (VITEK®2, bioMérieux Inc.) following the recommendations of EUCAST and CLSI guidelines.
Statistical analysis
Categorical variables were presented with their absolute values and percentages, whereas means and standard deviations (SD) were calculated for continuous variables. Groups were compared using the χ2 test or Fisher’s exact test for categorical variables, as appropriate. Continuous variables were evaluated with Student’s t test or Mann–Whitney U test. Binary logistic regression was conducted for possible associations between variables. All p values were two tailed; p values < 0.05 were considered as statistically significant. Statistical analysis was performed using the Stata v.14.0 software (StataCorp, College Station, TX).
Results
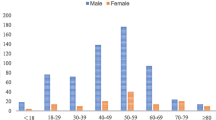
A total of 173 cases of COM-T in 171 patients were included. Patients were divided into two study periods: 101 patients treated between January 2009 and December 2014, and 72 patients treated between January 2015 and December 2019. Type III and IV in the Cierny and Mader classification (UTMB) [20] was observed in 92.5% (160/171) of the patients. Demographic, anatomical location, and classification details are summarized in Table 1.
Monomicrobial infection was present in 47.4% (82/173) of the cases: 48.5% (49/101) in 2009–14 and 47.2% (34/72) in 2015–2019. Polymicrobial infection comprised 28.3% (49/173) of the cases, being higher in the 2015–2019 period (24.7% versus 33.3%, respectively; p = 0.359). In 42 episodes (24.3%), a negative deep-bone culture was obtained: 27.7% (28/101) in the 2009–14 period and 19.4% (14/72) in the 2015–2019 period (p = 0.266).
In situ exposed bone or exposed metalwork (EMW) was present in 12.4% of the episodes. No statistically significant differences were found regarding sample negativity or identification of mono or polymicrobial infection in relation to EMW (p = 0.863).
Profile of identified organisms
A total of 200 positive cultures were obtained. The most commonly isolated microorganisms were coagulase-negative Staphylococci (CoNS) present in 24.5% of the cultures. Overall, Gram-negative bacilli (GNB) were isolated in 35.5% of the cultures, with Enterobacterales species in 20%—led by Enterobacter cloacae in 10% (20/200). All isolated pathogens are summarized in Table 2. No statistically significant differences were observed when comparing Gram-positive and Gram-negative bacterial infections between periods: 59.2% for 2009–14 vs. 50% between 2015 and 19 (p = 0.18) and 37% vs. 33.7% (p = 0.62), respectively.
Antibiotic sensitivity profile
Bacterial sensitivities to specific antibiotics are shown in Tables 3 and 4. The most-used combination of local antibiotics, glycopeptide (vancomycin) plus aminoglycoside (gentamicin or tobramycin), yielded low resistance rates in the most frequently occurring microorganisms. Our results show that the combination of vancomycin and aminoglycoside is effective against more than 90% of the most frequent microorganisms.
Multidrug-resistant organisms
MDROs were present in 15% of the cultures (30/200), with 10.1% (13/108) in the 2009–2014 group and 18.5% (17/92) for 2015–2019 (p = 0.07). Five patients presented samples with two different MDROs. Twenty of the thirty cases in which MDROs were detected (66.7%) corresponded with polymicrobial infections (Table 5). Thirteen out of 71 Gram-negative infections (18.3%) were caused by MDROs. There were seven cases of non-fermenting organisms—three cases of P. aeruginosa, two of A. baumanii and two of S. malthophilia, included for its inherent intrinsic resistance. MDROs were found in 15.8% (6/38) of Enterobacterales infections: two cases of E. cloacae (2/20, 10%), two of E. coli (2/4, 50%), one of P. mirabilis (1/6, 16.7%) and one case of M. morganii (1/1, 100%). Three cases were detected with the potential to over-express AmpC-type beta-lactamase chromosomal hyperproduction, one case of AmpC-type beta-lactamase plasmid hyperproduction and one case of extended-spectrum beta-lactamase producers (ESBL) mechanism. In Gram-positive bacterial infection, seventeen MDROs were identified (17/106, 16%). We detected five cases of Staphylococcus epidermidis (5/18, 27.7%), eight cases of CoNS other than S. epidermidis (NonSe-CoNS) (8/31, 25.8%) and four cases of methicillin-resistant staphylococci (MRS). No XDR or PDR pathogens were identified in this series of COM-T.
Discussion
In this series of 173 cases of COM-T, we found that most infections were caused by staphylococci, even though a small reduction of Gram-positive cocci—specifically related to CoNS—has been observed. An upward trend of MDROs has been observed over the last decade, being involved in 15% of study cases. As far as the authors know, this is the first study to specifically analyze the etiology of COM-T in a National Reference Infection Center.
Most cases in this study were monomicrobial infections, most frequently by Gram-positive cocci. The most commonly isolated species were CoNS (24.5%) and S. aureus (20.5%). Both presented a non-significant diminution in the 2005–2019 period. This trend has also been observed in other studies of periprosthetic infection [7, 21, 22]. An improvement in prophylaxis strategies, adding a glycopeptide to the cephalosporin, might explain this [23]. Regarding Gram-negative bacilli infections, recent PJI studies have reported large percentages, as high as 42% in one series [7, 21]. Our series found 35.5% GNB infections, with a non-significant statistical decrease from 37 to 33.7%.
Negative deep-bone cultures were observed in 24.3% of the cases, similar to other published studies [3, 4]. The small decrease in negative culture rate (27.7% in the 2009–2014 period and 19.4% in the 2015–2019 period; p = 0.266) can likely be explained by more severe control of the antibiotic-free period before surgery, and improvement of microbiological analyses and sample techniques. Conversely, there remains a lack of consensus on an internationally accepted definition of COM, so that different authors establish their own criteria for what is or is not osteomyelitis, making it difficult to know the true proportion of false negatives [24, 25].
Taking into account this problem and with the aim of improving the reproducibility of the results, an international consensus definition has been used for diagnosis of the infection, based on confirmatory criteria that are not exclusively microbiological. A negative culture can certainly mean a true negative result, and thus a misdiagnosis of infection. In cases of culture-negative results, molecular tests, based on 16S rRNA, are usually performed [26], but with inconsistent results; other molecular techniques such as PCR-multiplex or NGS could shed light in this field, but their true usefulness is still unknown [26]. If final microbiological examination is deemed negative, but with presence of other confirmatory infection criteria, a true culture-negative infection is established. In such patients a case-by-case antibiotic treatment is discussed by our dedicated multidisciplinary unit, based on the specific case’s features.
As described in other series [1, 6], an increase of polymicrobial infections has also been observed. This apparent increase in mixed flora is probably due to better detection methods rather than a real change in etiology. It seems logical to believe that EMW is associated with an increased risk of polymicrobial infection due to the breakdown of the dermal barrier. However, no statistical correlation was found between exposed bone or in situ EMW and negative, monomicrobial or polymicrobial infections. Sheehy et al. observed a relation between low-grade pathogens and history of EMW, and an association between negative culture and the absence of EMW history or fracture [6]. Dudareva et al. reported that metalwork was associated with MDROs infection [3].
Implication of MDROs is a concerning universal healthcare problem. With regard to PJIs, the majority of MDROs have been associated with an increase of antimicrobial-resistant Gram-negative infections. Following the classical Magiorakos criteria [15] and extrapolating this definition to CoNS and Pseudomona spp., we observed a 15% incidence of MDR organisms, with no statistical difference between periods. In our series, no XDR or PDR cases were observed. It is interesting to note that 66.7% of MDRO cultures were mixed-flora samples, and that five patients presented samples with two MDROs; all patients had undergone multiple surgeries and long hospitalization periods in the context of limb-threatening injuries.
The role of local antibiotics is of vital importance, as their concentration at the surgical site following implantation seems to be higher, and their use avoids some of the adverse effects of systemic antibiotics [27, 28]. Their selection is crucial, as it cannot be modified post-operatively in single-stage surgical patients. Our results suggest that the combination of a glycopeptide (vancomycin) with an aminoglycoside (gentamicin or tobramycin) could be an optimal choice, as it covers more than 90% of the most frequently seen microorganisms. Dudareva et al. found that this combination of antibiotics encountered only 7.2% of resistant microorganisms [3]. These results support the abandonment of routine percutaneous biopsies prior to surgery, since their effectiveness and actual sensitivity is not clear [8, 10, 11]. If this combination of local antibiotics is used, most microorganisms are covered in the early postoperative period; later, a targeted systemic antibiotic can be started based on the sensitivity profile of the isolated pathogen.
Regarding systemic treatment, the most broadly effective antibiotic following radical surgical debridement may be a glycopeptide with B-lactam, such as a cephalosporin or carbapenem. We advocate empirical treatment that addresses typical skin flora, plus a local antibiotic—then await microbiological sample results to decide on a targeted treatment. Judging from results in this study, we believe the best approach to COM-T does not necessarily include a broad-spectrum antibiotic such as a carbapenem for empirical treatment, because we did not observe a high incidence of ESBL producers. However, we recommend a multidisciplinary team decision and a case-specific choice of empirical antibiotic, taking in account patient medical history and comorbidities, previous surgeries in other centers, and prior antibiotic use. Antimicrobial therapy in COM-T is complex, and further studies are necessary to establish accurate etiological diagnosis and individualized therapy.
Several limitations should be acknowledged when reviewing the present study. First, it is retrospective, and so suffers from the inherent weaknesses of this study type. Second, our patient cohort could be considered small, though it is comparable to those of similar studies; this fact limited statistical power, and therefore the generalizability of results. Finally, all care was provided at a single, high-volume, specialized center, and it may be difficult to extrapolate our results to those of units which are smaller or located in other countries, with different patient characteristics. It is vital to establish the specific epidemiology of any specific center and to employ empirical treatment adequate to individual results. Studies employing prospective data retrieval, larger patient bases and more extensive follow-up are undoubtedly needed.
Conclusions
According to the results of the present study, rates of Gram-positive and Gram-negative infections remained consistent during the two study periods, but with an upward trend in MDRO and polymicrobial infections detected. The local combination of a glycopeptide plus an aminoglycoside was effective in treating the most frequently isolated microorganisms.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- COM:
-
Chronic osteomyelitis
- COM-T:
-
Chronic osteomyelitis of the tibia
- CoNS:
-
Coagulase-negative Staphylococci
- EMW:
-
Exposed metalwork
- ESBL:
-
Extended-spectrum beta-lactamase producers
- GNB:
-
Gram-negative bacilli
- MDROs:
-
Multidrug-resistant organisms
- PDR:
-
Pan drug resistant
- PMMA:
-
Polymethylmethacrylate
- PJI:
-
Periprosthetic joint infection
- XDR:
-
Extensively drug resistant
References
Kremers HM, Nwojo ME, Ransom JE et al (2015) Trends in the epidemiology of osteomyelitis: a population-based study, 1969 to 2009. J Bone Jt Surg 97(10):837–845
Trampuz A, Zimmerli W (2006) Diagnosis and treatment of infections associated with fracture-fixation devices. Injury 37(Suppl 2):S59–S66. https://doi.org/10.1016/j.injury.2006.04.010
Dudareva M, Hotchen AJ, Ferguson J et al (2019) The microbiology of chronic osteomyelitis: changes over ten years. J Infect 79(3):189–198. https://doi.org/10.1016/j.jinf.2019.07.006
Vemu L, Sudhaharan S, Mamidi N et al (2018) Need for appropriate specimen for microbiology diagnosis of chronic osteomyelitis. J Lab Physicians 10:21–25
Saltoglu N, Ergonul O, Tulek N et al (2018) Influence of multidrug resistant organisms on the outcome of diabetic foot infection. Int J Infect Dis 70:10–14
Sheehy SH, Atkins BA, Bejon P et al (2010) The microbiology of chronic osteomyelitis: prevalence of resistance to common empirical anti-microbial regimens. J Infect 60:338–343
Benito N, Franco M, Ribera A et al (2016) Time trends in the aetiology of prosthetic joint infections: a multicenter cohort study. Clin Microbiol Infect 22(8):732.e1-732.e7328. https://doi.org/10.1016/j.cmi.2016.05.004
Maffulli N, Papalia R, Zampogna B et al (2016) The management of osteomyelitis in the adult. Surgeon 14(6):345–360
White LM, Schweitzer ME, Deely DM, Gannon F (1995) Study of osteomyelitis: utility of combined histologic and microbiologic evaluation of percutaneous biopsy samples. Radiology 197(3):840–842
Wu JS, Gorbachova T, Morrison WB, Haims AH (2007) Imaging guided bone biopsy for osteomyelitis: are there factors associated with positive or negative cultures? AJR Am J Roentgenol 188(6):1529–1534
Corona PS (2016) Role of Pre-operative bone biopsy in the microbiological diagnosis of lower extremity chronic osteomyelitis. Bone Jt J 98-B(SUPP 23):18
Mifsud M, Ferguson JY, Stubbs DA, Ramsden AJ, McNally MA (2020) Simultaneous debridement, Ilizarov reconstruction and free muscle flaps in the management of complex tibial infection. J Bone Jt Infect 6(3):63–72. https://doi.org/10.5194/jbji-6-63-2020
Metsemakers WJ, Morgenstern M, McNally MA et al (2018) Fracture-related infection: a consensus on definition from an international expert group. Injury 49(3):505–510. https://doi.org/10.1016/j.injury.2017.08.040
Cierny G III, Mader JT, Pennick JJ (2003) A clinical staging system for adult osteomielitis. Clin Orthop Relat Res 414:7–24
Magiorakos A-P, Srinivasan A, Carey RB et al (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18(3):268–281. https://doi.org/10.1111/j.1469-0691.2011.03570.x
Perry CR, Pearson RL, Miller GA (1991) Accuracy of cultures of material from swabbing of the superficial aspect of the wound and needle biopsy in the preoperative assessment of osteomyelitis. J Bone Joint Surg Am 73(5):745–749
Corró S, Vicente M, Rodríguez-Pardo D et al (2020) Vancomycin-gentamicin prefabricated spacers in 2-stage revision arthroplasty for chronic hip and knee periprosthetic joint infection: insights into reimplantation microbiology and outcomes. J Arthroplasty 35(1):247–254
Stravinskas M, Horstmann P, Ferguson J et al (2016) Pharmacokinetics of gentamicin eluted from a regenerating bone graft substitute: in vitro and clinical release studies. Bone Jt Res 5(9):427–435
Stevens CM, Tetsworth KD, Calhoun JH et al (2005) An articulated antibiotic spacer for the management of infected total knee replacement: comparison of elution properties from simplex and palacos PMMA. J Orthop Res 23:27–33
Cierny G, Mader JT, Penninck JJ (2003) A clinical staging system for adult osteomyelitis. Clin Orthop 414:7–24
Peel TN, Cheng AC, Buising KL et al (2012) Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother 56(5):2386–2391. https://doi.org/10.1128/AAC.06246-11
Tai D, Patel R, Abdel MP et al (2021) Microbiology of hip and knee periprosthetic joint infections: a database study. Clin Microbiol Infect. https://doi.org/10.1016/j.cmi.2021.06.006
Huang SS, Plat R (2003) Risk of methicillin-resistant staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis 36(3):281–285
Walter G, Kemmerer M, Kappler C, Hoffmann R (2012) Treatment algorithms for chronic osteomyelitis. Dtsch Arzteblatt Int 109(14):257–264
Schmidt HGK, Tiemann AH, Braunschweig R, Diefenbeck M, Bühler M, Abitzsch D et al (2011) Definition of the diagnosis osteomyelitis-osteomyelitis diagnosis score (ODS). Z Orthop Unfallchirurgie 149(4):449–460
Sadowy E, Hryniewicz W (2020) Identification of Streptococcus pneumoniae and other Mitis streptococci: importance of molecular methods. Eur J Clin Microbiol Infect Dis 39(12):2247–2256. https://doi.org/10.1007/s10096-020-03991-9
Butini ME, Cabric S, Trampuz A, Di Luca M (2018) In vitro anti-biofilm activity of a biphasic gentamicin-loaded calcium sulfate/hydroxyapatite bone graft substitute. Colloids Surf B Biointerfaces 161:252–260
Post V, Wahl P, Richards RG, Moriarty TF (2017) Vancomycin displays time-dependent eradication of mature Staphylococcus aureus biofilms. J Orthop Res 35(2):381–388
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
The present study obtained the Institutional Review Board approval.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of all data present in the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carbonell-Rosell, C., Lakhani, K., Lung, M. et al. Etiology and antimicrobial resistance patterns in chronic osteomyelitis of the tibia: an 11-year clinical experience. Arch Orthop Trauma Surg 144, 773–781 (2024). https://doi.org/10.1007/s00402-023-05095-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-023-05095-3