Abstract
Background
Iatrogenic nerve lesions during surgical interventions are avoidable complications that may cause severe functional impairment. Hereby, awareness of physicians and knowledge of structures and interventions at risk is of utmost importance for prevention. As current literature is scarce, we evaluated all patients treated surgically due to peripheral nerve injuries in our specialized nerve center for the presence of iatrogenic nerve lesions.
Methods
We evaluated a total of 5026 patients with peripheral nerve injuries treated over a time period of 8 years in our facility for the prevalence of iatrogenic nerve injuries, their clinical presentations, time to treatment, mechanisms and intraoperative findings on nerve continuity.
Results
A total of 360 (6.1%) patients had an iatrogenic cause resulting in 380 injured nerves. 76.6% of these lesions affected the main branch of the injured nerve, which were mainly the radial (30.5%), peroneal (13.7%) and median nerve (10.3%). After a mean delay of 237 ± 344 days, patients presented 23.2% with a motor and 27.9% with a mixed sensory and motor deficit. 72.6% of lesions were in-continuity lesions. Main interventions at risk are displayed for every nerve, frequently concerning osteosyntheses but also patient positioning and anesthesiologic interventions.
Discussion
Awareness of major surgical complications such as iatrogenic nerve injuries is important for surgeons. An often-seen trivialization or “watch and wait” strategy results in a huge delay for starting an adequate therapy. The high number of in-continuity lesions mainly in close proximity to osteosyntheses makes diagnosis and treatment planning a delicate challenge, especially due to the varying clinical presentations we found. Diagnostics and therapy should therefore be performed as early as possible in specialized centers capable of performing nerve repair as well as salvage therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients suffering from acute trauma with the necessity of a surgical intervention endure distress and potential impairment depending on the severity of the trauma. When this trauma is aggravated by an iatrogenic nerve injury, potential impairment may increase further with lifelong consequences and additional surgeries.
Therefore, knowledge about procedures and anatomical structures at risk is of utmost importance to increase awareness during interventions.
Kretschmer et al. (2001) illustrated, that 17.4% of traumatic nerve lesions have an iatrogenic origin [1]. Mainly, these injuries occur during orthopedic surgery [1]. Compression, mechanical stress such as traction from hooks, heat damage from monopolar cautering, direct cuts or the misidentification of anatomical structures cause these injuries [2, 3].
Recognition, acceptance and adequate dealing with iatrogenic nerve injuries is a highly sensitive topic and influenced by psychological factors of the treating surgeons [1, 4]. Lack of expertise, experience and trivialization of the resulting injuries are seen frequently [5].
Other investigations showed that only 21% of injuries were recognized in the early period after surgery [6] and only 35% were treated within the first 6 months after occurrence [1].
Research on this topic from specialized trauma centers can help to shed light on the relevance of the topic and raise awareness among surgeons.
We therefore aimed to analyze all iatrogenic nerve lesions treated in our major trauma center in a retrospective evaluation to provide detailed data on mechanisms, procedures at risk and clinical presentation.
Methods
All patients with peripheral nerve injuries surgically treated at our specialized nerve center during the time period from January 2012 to July 2020 were assessed retrospectively.
Out of this collective all patients with peripheral nerve injury were identified using the digital hospital information system and ICD Classification System. Data acquisition was performed by two independent reviewers (MA, KSZ) in a pseudonymized manner.
An anonymized database was created using Microsoft Excel.
The database was screened for all patients who had undergone at least one previous surgery, regardless of whether the previous surgery was in domo or ex domo. Patients were included in this study when the nerve lesion was present after intervention but not at initial trauma surgery and the corresponding nerve lesion was defined as iatrogenic nerve lesion.
Local ethical board approval was obtained from the Landesärztekammer Rhld.-Pf., Mainz; (EK Nr: 2021-16091).
After full data acquisition, SPSS Statistics Version 27 (IBM, USA) was used for statistical interpretation. Data were tested for normal distribution. Outcome parameters were age at surgery, time between surgery causing the nerve lesion and surgical treatment of the iatrogenic nerve lesion, affected nerve, presence of motor deficit, presence of sensory deficit, intervention at risk and nerve continuity.
Descriptive statistics were performed and supported by the mean, median or mode if appropriate and standard deviation.
Results
Demographics
We analyzed a total of 5026 patients treated for peripheral nerve lesions in our center. Out of this population, an iatrogenic cause was found in 350 patients. This corresponds to a prevalence of 6.1% among our population of patients surgically treated for peripheral nerve lesions.
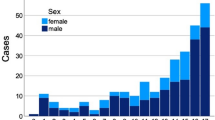
Of these 350 patients were 163 male and 187 female with a mean age of 52 (18) years.
In total, these 350 patients had 380 iatrogenic nerve injuries. Patients were sent by treating physicians from all over Germany.
Time of injury/surgery
Time between the causing surgery and treatment in our facility was 237 ± 344 days (median: 109 d).
Affected nerves
291 (76.6%) nerves had injuries to their main branch, 89 (23.4%) affected smaller (superficial) branches. Most commonly affected was the radial nerve (116; 30.5%), followed by the peroneal nerve (52; 13.7%). The median nerve was affected in 39 cases (10.3%) and the ulnar nerve in 36 cases (9.5%). Prevalence of all nerve injuries are displayed in Fig. 1.
Combined nerve injuries mainly affected the combination of median and ulnar as well as peroneal and tibial nerve.
Clinical presentation
Patients presented at our specialized nerve center in 163 cases (42.9%) with a solitary sensory deficit. In 106 cases (27.9%) we found a mixed motor and sensory deficit and in 88 cases (23.2%) a motor deficit. 23 cases (6.1%) had no such documented clinical presentation due to direct intraoperative repair or missing records.
Interventions at risk
Of 380 nerve injuries, 360 (94.7%) were due to surgical procedures. Eleven (2.9%) were caused by patient positioning for surgery and nine (2.4%) due to anesthesiologic interventions.
Incorrect patient positioning affected mainly the radial nerve (45.5%) followed by brachial plexus (18.1%) and median (9.1%), ulnar (9.1%), peroneal (9.1%) and femoral nerve (9.1%).
Anesthesiologic interventions (mainly catheter placement) affected the femoral nerve (66.6%) followed by peroneal (11.1%), tibial (11.1%) and lateral femoral cutaneous nerve (11.1%).
Table 1 shows those surgeries that are most likely to result in iatrogenic lesions, differentiated for each nerve. The radial nerve, as the most affected nerve, is mainly injured during humeral osteosynthesis (40.6%) followed by extensor tendon compartment splitting (12.8%) and forearm osteosynthesis (10.8%). Implantation or explantation of hip prostheses is the leading cause of injuries to the sciatic (63.2%) and femoral (60.0%) nerves. Finger nerve injuries are mainly resulting from M. Dupuytren surgeries (36.1%) and trigger finger release (25.0%) (Fig. 2).
Main intervention causing multiple nerve injuries was carpal tunnel release (20% of cause).
Nerve continuity
Detailed record evaluation of intraoperative or (if available) neuro-imaging records revealed that, overall, 72.6% of the lesions had a preserved nerve continuity. In contrast, 20.3% had a complete and 3.9% had a partial transection of the nerve. Hereby finger nerves were identified to be more likely transected (72%) than other nerves. A detailed analysis of nerve continuity can be found in Table 2.
Discussion
Detailed knowledge and awareness of structures and interventions at risk are essential for the prevention of iatrogenic nerve injuries. Even though iatrogenic injuries are not the leading cause of nerve injuries, they are a severe, long lasting, but also mostly avoidable complication.
In particular, the location of the surgery and the surgical procedure conditions influence the occurrence of nerve injuries.
Our collective of 380 nerve injuries revealed the radial nerve as mainly affected (30.5%).
Hereby, especially osteosyntheses of the humerus (40.6%) and of the forearm (10.8%) make up more than 50% of these injuries. This can be explained by the anatomic course of the radial nerve in close proximity to the humerus and the superficial branch at the wrist endangered during the reduction of distal radius fractures and surgical release of the first extensor tendon compartment (Fig. 3).
As 78% of radial nerve lesions were found to have preserved continuity, traction and crush injury due to surgical instruments can be considered as the main cause. The estimated number of unreported cases must be even higher, as most of the lesions were in-continuity with a high potential of recovery within 3–6 months, which might not have been referred to our specialized center.
Same concept seems to apply for the peroneal nerve, as we found more than 70% of iatrogenic lesions in combination with osteosynthesis of the lower leg, again with 80% preserved continuity.
For soft tissue procedures without osteosynthesis, we found carpal tunnel release as the cause of 23.7% of median and 11.4% of ulnar nerve lesions. It is also the leading intervention at risk for a combined iatrogenic nerve injury. As this procedure is often performed by practitioners in an outpatient clinic special attention should be taken. Thus the nerve injury of carpal tunnel release often affects the thenar branch and the loss of opposition of the thumb is sometimes not recognized by patients, the estimated number of unreported cases is even higher [7, 8]. Especially the presence of ulnar nerve lesions during carpal tunnel release operations indicates this procedure as of underrated risk and advocates doubt for surgical procedures with high visualization potential of the nerve during surgery [7] (Fig. 4).
In general, we found 72.6% of nerve injuries to be in-continuity lesions. These mainly crush-type or traction-type injuries have a higher regeneration potential than discontinuity lesions. One could argue, that the vast majority is in-continuity and a traditional “watch and wait” strategy can be chosen by the surgeon. This seems to be clinical routine in some practice when considering the delay of 237 days between injury and treatment at our specialized nerve center. This is for several reasons problematic. It is widely known, that the distal target of the nerve, the muscle, has a limited regeneration time window of maximum 18 months (equals about 547d). This is in direct conflict with nerve regeneration time of about 1mm/day. If treatment of e.g. a high radial nerve injury is delayed with a mean of half of possible regeneration time and 18.1% of these patients do not have preserved anatomy, direct nerve repair is impossible and patients will suffer from life-long impairment. We furthermore found in 76.6% of all injuries the main branch affected, resulting in maximum impairment distal to the injury site.
We, therefore, advocate early referral to a specialized center for patients with potential nerve injuries to assess the right diagnostics and treatment strategies and keep the management in “one hand”. Especially in-continuity lesions in close proximity to internal fixation require delicate individual treatment strategies. Special diagnostics such as MR neurography, neurosonography and clinical experience in nerve surgery as well as salvage tendon transfers are here important to optimize functional outcome [9, 10]. New studies reveal a high potential for detailed nerve imaging even in close proximity to internal fixation plates which could be further beneficial in the future [10].
In a study on iatrogenic nerve lesions with 340 patients, Antoniadis et al. (2016) showed in their collective, that the median nerve was the most affected nerve and mainly was affected during carpal tunnel release (41 out of 58 cases) [4]. Hereby the primary focus of the center may bias patient selection as our unit is more trauma associated than other e.g. oncologic centers. Patient referral is hereby also dependent on external doctors referring their iatrogenic injuries to a center of their knowledge, therefore analysis among various centers can vary. Future multicenter analysis should reveal further details and reduce potential referral bias.
Iatrogenic nerve injuries result rarely out of incorrect positioning during anesthesia [5]. This is also in accordance with our findings revealing 2.9% of injuries being caused by positioning and 2.4% due to anesthesiologic interventions such as catheter placement. Hereby again, the radial and peroneal nerve are highly at risk due to their superficial anatomy.
Independent of the mechanism of injury, patients suffer from sensory or motor impairment as well as pain [11, 12]. In a small population of 58 patients, Lefebvre et al. (2020) investigated the presence of sensory and motor deficit after injury. Around 60% of these patients showed a mixed motor and sensory deficit [11]. This is in contrast to our findings which revealed only 27.9% with a mixed deficit. Patients presented at our service with a solitary sensory deficit (42.9%) and only 23.2% with a motor-only deficit. This is important to know, especially for routine postoperative check-ups of the patients, where care should be taken not only to motor but also sensory impairment. Injuries to sensory branches can result in neuroma formation which appears in about 1–10% of nerve injuries [13, 14]. Again, especially the superficial branch of the radial nerve is at risk for neuroma development due to its superficial course and presence in the grasping zone of the hand [15].
Bage and Power (2021) argue that it is a utopian belief to be able to completely avoid iatrogenic nerve injuries [12]. Nevertheless, specific expertise and understanding of iatrogenic nerve injuries are necessary for prevention. Further studies are desirable to reveal a certainly higher estimated number of unreported cases of neurapraxia due to restitutio before clinical presentation in specialized centers. Hereby iatrogenic nerve lesions are a potential complication that can occur during intervention, nevertheless the crucial skill is the awareness of a potential complication and early referral to specialized centers, rather than a “watch and wait” strategy.
Diagnostics
In the event of suspected potential nerve injury, an expeditious and comprehensive clinical assessment within 24 h of trauma is recommended. Accurate documentation of sensory and motor deficits in specific target areas and muscles is crucial for recognizing and interpreting potential regeneration. For instance, in proximal Grade 2 injuries according to Seddon's classification, the height and progression of regeneration can be monitored through functional reinnervation of muscle groups distal to the injury. Classification based on strength grades and sensory grades aids in this process[16].
Closed injuries pose particular challenges in assessing their extent and the likelihood of spontaneous functional recovery. It should be noted that rare cases may involve damage to the same nerve at two different levels. Surgical intervention may be necessary during the course of treatment if regeneration is not occurring. In this context, the clinical examination of the patient and regular reevaluations by consistent and experienced examiners play pivotal roles in improving outcomes through timely intervention, if necessary.
In addition to the clinical examination, further diagnostic measures should be undertaken. Neurophysiological examinations such as nerve conduction studies (NCS) and electromyography (EMG) are crucial for distinguishing between different degrees of nerve injury. However, these examinations are meaningful only starting from 7 days after the injury, as nerve conduction velocity decreases during this period, enabling differentiation between partial and complete conduction blocks. Parameters such as nerve conduction velocity, motor latency, and amplitude are employed for the initial assessment of the damage. Slowed nerve conduction velocities indicate segmental demyelination as seen in neurapraxia. Normal or mildly reduced nerve conduction velocities combined with reduced amplitude suggest axonal damage (axonotmesis). Absent nerve conduction velocities and amplitudes are typically observed in cases of neurotmesis. Electromyography (EMG) can also be diagnostically valuable, revealing pathological spontaneous activities (fibrillations) in cases of axonal damage but not in neurapraxia. Neurotmesis, similar to nerve conduction studies, does not elicit a response in EMG. It is worth noting that the meaningfulness of EMG examination requires a minimum of 14 days after the injury, as Wallerian degeneration must have sufficiently progressed to display denervation signs. Moreover, EMG provides an opportunity to demonstrate reinnervation tendencies and facilitate early physiotherapeutic exercises with the patient using biofeedback [17].
Alongside clinical examination, imaging techniques such as neurosonography and magnetic resonance neurography (MRN) should be considered. Recent advancements in imaging technology have significantly improved the accurate classification and conservative treatment of many injuries without the need for open exploration. Neurosonography, utilizing high-resolution probes ranging from 12–24 MHz, enables high-resolution visualization of nerve injuries. Special algorithms allow the depiction of individual fascicles of superficially located nerves without excessive background noise, necessitating an experienced examiner. MRN, a promising technique for visualizing peripheral nerve injuries, employs the latest 3 Tesla MRI coil technology to enhance the signal-to-noise ratio and ensure high spatial resolution. This multidimensional imaging technique enables the visualization of deeper structures [10, 16, 17].
However, the limited availability of MRN, with only a few centers offering this specialized imaging, is a significant drawback. The procedure, duration (usually 45–60 min), and contraindications are similar to those of a regular MRI. Certain injury patterns may pose challenges to performing MRN, as patients typically need to be positioned with the arm maximally elevated. In general, the presence of titanium osteosynthesis material is not an absolute contraindication for MRI. Nevertheless, metal artifacts may partially obscure nerve pathology, necessitating consultation with an experienced neuroradiologist to determine the indication [9, 10].
In summary, a timely treatment plan considering all findings should be developed to assess the options between conservative and surgical management. In cases of inconclusive or unclear findings and prognosis, surgical exploration becomes unavoidable.
Surgical procedures range from neurolysis in the case of scarred tissue up to nerve grafting and peripheral nerve transfers to regain function. This is especially relevant in high nerve injuries to assure distal function, e.g. opposition reconstruction in high median nerve injury [18].
Conclusion
In this analysis, we identified an iatrogenic cause of a peripheral nerve injury in 6.1% of all patients treated with nerve injury. Hereby, the radial nerve was by far the most affected nerve. Interventions at risk are mainly osteosyntheses in close proximity to nerves. As the time between injury and treatment at our specialized center had a mean of 237 days, many patients do not qualify for early nerve repair, especially in 18.1% of the cases with intraoperative confirmed discontinuity of the nerve. As sometimes a “neglect” in treating physicians is seen, we performed this study to increase awareness among all specialties for the potential presence of iatrogenic nerve injuries and advocate early referral to specialized centers.
References
Kretschmer T, Antoniadis G, Braun V et al (2001) Evaluation of iatrogenic lesions in 722 surgically treated cases of peripheral nerve trauma. J Neurosurg 94:905–912. https://doi.org/10.3171/jns.2001.94.6.0905
Weber RV, Mackinnon SE (2007) Median nerve mistaken for palmaris longus tendon: restoration of function with sensory nerve transfers. HAND 2:1–4. https://doi.org/10.1007/s11552-006-9011-5
McGeorge D, Sturzenegger M, Buchler U (1992) Tibial nerve mistakenly used as a tendon graft. Reports of three cases. J Bone Joint Surg Br 74:365–366. https://doi.org/10.1302/0301-620X.74B3.1587878
Antoniadis G, Kretschmer T, Pedro MT et al (2014) Iatrogenic nerve injuries. Dtsch Ärztebl Int. https://doi.org/10.3238/arztebl.2014.0273
Stöhr M (1996) Iatrogene Nervenläsionen: Injektion, Operation, Lagerung, Strahlentherapie ; 33 Tabellen, 2., vollst. überarb. Aufl. Thieme, Stuttgart New York
Khan R, Birch R (2001) Iatropathic injuries of peripheral nerves. J Bone Joint Surg Br 83:1145–1148. https://doi.org/10.1302/0301-620X.83B8.0831145
Harhaus L, Daeschler SC, Aman M, et al (2022) [Differential therapeutic approaches in treatment of carpal tunnel syndrome]. Handchir Mikrochir Plast Chir Organ Deutschsprachigen Arbeitsgemeinschaft Handchir Organ Deutschsprachigen Arbeitsgemeinschaft Mikrochir Peripher Nerven Gefasse Organ V 54:236–243. https://doi.org/10.1055/a-1839-8297
Aman M, Boecker AH, Thielen M et al (2021) Single incision thenar muscle reconstruction using the free functional pronator quadratus flap. BMC Surg 21:310. https://doi.org/10.1186/s12893-021-01308-x
Boecker AH, Lukhaup L, Aman M et al (2022) Evaluation of MR-neurography in diagnosis and treatment in peripheral nerve surgery of the upper extremity: a matched cohort study. Microsurgery 42:160–169. https://doi.org/10.1002/micr.30846
Aman M, Schwarz D, Stolle A et al (2022) Modern MRI diagnostics of upper-extremity-related nerve injuries-a prospective multi-center study protocol for diagnostics and follow up of peripheral nerve injuries. J Pers Med 12:1548. https://doi.org/10.3390/jpm12101548
Lefebvre R, Russo F, Navo P, Stevanovic M (2020) Characteristics of iatrogenic nerve injury from orthopedic surgery correlate with time to subspecialty presentation. Plast Reconstr Surg Glob Open 8:e2678. https://doi.org/10.1097/GOX.0000000000002678
Bage T, Power DM (2021) Iatrogenic peripheral nerve injury: a guide to management for the orthopaedic limb surgeon. EFORT Open Rev 6:607–617. https://doi.org/10.1302/2058-5241.6.200123
Dahlin LB, Wiberg M (2017) Nerve injuries of the upper extremity and hand. EFORT Open Rev 2:158–170. https://doi.org/10.1302/2058-5241.2.160071
van der Avoort DJJC, Hovius SER, Selles RW et al (2013) The incidence of symptomatic neuroma in amputation and neurorrhaphy patients. J Plast Reconstr Aesthetic Surg JPRAS 66:1330–1334. https://doi.org/10.1016/j.bjps.2013.06.019
Watson J, Gonzalez M, Romero A, Kerns J (2010) Neuromas of the hand and upper extremity. J Hand Surg 35:499–510. https://doi.org/10.1016/j.jhsa.2009.12.019
Radtke C, Vogt PM (2014) Nerve injuries and posttraumatic therapy. Unfallchirurg 117:539–555. https://doi.org/10.1007/s00113-014-2574-7
Amrami KK, Khanna A, Frick MA, Spinner RJ (2023) Imaging peripheral nerve injuries of the lower extremities: what surgeons need to know. Semin Ultrasound CT MR 44:347–363. https://doi.org/10.1053/j.sult.2023.04.001
Aman M, Böcker A, Kneser U, Harhaus L (2021) Selective nerve transfers for thenar branch reconstruction. Oper Orthop Traumatol 33:384–391. https://doi.org/10.1007/s00064-020-00689-1
Funding
The authors received no financial support for the research, authorship and publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has a potential conflict of interest with respect to the research, authorship, mentioned products or devices and publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aman, M., Zimmermann, K.S., Pennekamp, A. et al. Mechanisms, interventions at risk and clinical presentation of iatrogenic nerve lesions in trauma patients. Arch Orthop Trauma Surg 143, 7245–7253 (2023). https://doi.org/10.1007/s00402-023-05009-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-023-05009-3