Abstract
Introduction
Fracture-related infection (FRI) represents a challenging clinical scenario. Limited evidence exists regarding treatment failure after initial management of FRI. The objective of our investigation was to determine incidence and risk factors for treatment failure in FRI.
Materials and methods
We conducted a retrospective review of patients treated for FRI between 2011 and 2015 at three level 1 trauma centers. One hundred and thirty-four patients treated for FRI were identified. Demographic and clinical variables were extracted from the medical record. Treatment failure was defined as the need for repeat debridement or surgical revision seven or more days after the presumed final procedure for infection treatment. Univariate comparisons were conducted between patients who experienced treatment failure and those who did not. Multivariable logistic regression was conducted to identify independent associations with treatment failure.
Results
Of the 134 FRI patients, 51 (38.1%) experienced treatment failure. Patients who failed were more likely to have had an open injury (31% versus 17%; p = 0.05), to have undergone implant removal (p = 0.03), and additional index I&D procedures (3.3 versus 1.6; p < 0.001). Most culture results identified a single organism (62%), while 15% were culture negative. Treatment failure was more common in culture-negative infections (p = 0.08). Methicillin-resistant Staphylococcus aureus (MRSA) was the most common organism associated with treatment failure (29%; p = 0.08). Multivariate regression demonstrated a statistically significant association between treatment failure and two or more irrigation and debridement (I&D) procedures (OR 13.22, 95% CI 4.77–36.62, p < 0.001) and culture-negative infection (OR 4.74, 95% CI 1.26–17.83, p = 0.02).
Conclusions
The rate of treatment failure following FRI continues to be high. Important risk factors associated with treatment failure include open fracture, implant removal, and multiple I&D procedures. While MRSA remains common, culture-negative infection represents a novel risk factor for failure, suggesting aggressive treatment of clinically diagnosed cases remains critical even without positive culture data.
Level of evidence
Retrospective cohort study; Level III.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fracture-related infection (FRI) continues to be challenging for both patients and orthopaedic surgeons, with potentially devastating effects on patient function and long-term outcomes [1,2,3]. A significant proportion of patients are at risk as the incidence of infection following fracture fixation ranges from 1.8% in closed, low-energy fractures to 27% in high energy, open fractures [4]. Infection in the setting of orthopaedic implants can be especially difficult to treat, often requiring extended-course antibiotics, repeat surgical procedures, or even amputation [5,6,7].
Many concepts regarding the treatment of implant-related infection in fracture patients have been adapted from treatment algorithms for periprosthetic joint infection (PJI) in arthroplasty [1, 8] due to the comparatively scarce evidence specific to musculoskeletal trauma patients. There are some similarities in treatment approaches for these two groups of patients, including the potential need for aggressive debridement, antibiotic administration, and the decision between implant retention or explant followed by eventual replacement of the implant [5, 9, 10]. While some crossover exists between PJI treatment approaches and those involving fracture patients, many differences in the treatment of these two patient populations exist. Operative fixation in musculoskeletal trauma involves the added complexity of damaged overlying soft tissue and the potential for concurrent vascular structure compromise—factors that likely contribute to the higher rate of infection in orthopedic trauma surgery [11]. Additionally, microbial infiltration can weaken and disrupt normal bone healing [12]. Ultimately, the most important distinction is the presence of a fracture which compromises biomechanical stability. Failure to provide adequate stability at the zone of injury leads to a cycle of ongoing soft tissue damage, disruption of neovascularization, bone resorption, and persistent infection [8, 13]. Balancing the management of implant-related infection in proximity to actively healing bone and soft tissue escalates the difficulty that surgeons face when treating these patients.
While rates and predictors of treatment failure of PJI have been well described in arthroplasty [3, 14,15,16], evidence regarding the incidence and risk factors for treatment failure of FRI is more limited. For this reason, our goal was to determine the incidence of and identify risk factors for treatment failure in FRI based on the experience at three level 1 trauma centers.
Patients and methods
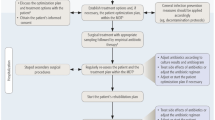
We conducted retrospective review of patients who underwent surgical intervention for FRI at three adult level 1 trauma centers. Institutional review board approval was obtained at each of the three centers. Subjects were identified from a trauma registry or billing database from January 2011 to December 2015. Patients met inclusion criteria if they were 16 years of age or older and underwent operative irrigation and debridement (I&D) for presumed FRI at any time after having undergone surgical fixation of an open or closed fracture. A minimum of 6 months of follow-up was required for inclusion. Patients were excluded if the presumed infection was associated with hip or knee prothesis or spine instrumentation, and if infection occurred without preceding fracture fixation. Patients were also excluded if an I&D was performed for the treatment of a post-operative hematoma. Only patients who underwent I&D for an infection diagnosed clinically by the following criteria were included.
We defined infection as erythema, purulent drainage, and/or exposed hardware presenting after definitive wound closure that required return to the operating room for I&D at the discretion of the treating surgeon. It should be noted that not all patients may have met confirmatory criteria by the newly defined FRI consensus definition [17]. Cultures were obtained at the time of index I&D in all patients. Antibiotic therapy was initiated in consultation with an infectious disease specialist, with the antibiotic regimen tailored according to culture sensitivity results. If the intraoperative cultures failed to identify a pathogen, then empiric antibiotics were selected per infectious disease recommendations. The specific antibiotic used and the total duration that extended antibiotic therapy was given were both determined in consultation with the infectious disease specialists.
The standard protocol at all three institutions was to repeat operative I&D every 2–3 days as deemed necessary by the surgical team. Based on the standard practice at these institutions, after 7 days had elapsed from the last planned surgical debridement, operative treatment of the infection was considered complete. The primary outcome was treatment failure, defined as the need for secondary surgical debridement or implant revision or removal due to recurrent infection occurring at least 7 days following the presumed final procedure during the initial phase of management.
Patient demographics and injury characteristics were extracted from the medical record. Operative reports were used to determine the number of I&D procedures and how implants were handled—retention or removal following the initial diagnosis of infection was also documented. Outcomes were documented through the last available follow-up visit for each patient.
Statistical analysis was performed using Stata software (College Station, Texas, USA). We summarized baseline characteristics by treatment success using means and standard deviations (SD) for continuous variables and proportions for categorical variables. A Student’s t test or Kruskal–Wallis test were performed as appropriate to compare continuous variables based on conditions of normality. A χ2 test was used to compare categorical variables. In both cases, statistical significance was determined by a p value ≤ 0.05. Multivariable logistic regression was conducted using hypothesis-driven variables with a p value < 0.20 according to our univariate analyses to identify independent associations with treatment failure. Based on our number of treatment failures (n = 51), we were able to include five variables in the regression model—Charlson Comorbidity Index (CCI), whether or not a fracture was open at presentation, how the implant was handled, the number of initial I&D procedures, and the culture result.
Results
We identified 134 patients with FRI that met study inclusion criteria (Table 1). The study population consisted of 61 females and 73 males with an average age of 54.5 years (SD 15.7 years) and an average CCI of 2.6 (SD 2.3). Most sustained fractures of the lower extremity (92%). Thirty of the fractures were classified as open injuries at presentation. Twenty-eight percent of patients were treated at the rural level 1 trauma center while the remainder were treated at two urban level 1 trauma centers.
A total of 51 patients (38.1%) failed the initial course of treatment (Table 1). Patients in the treatment failure group were approximately 4.1 years older on average, with a mean age of 57.0 (p = 0.14). The mean Charlson Comorbidity Index (CCI) score for patients among those who failed was 2.9 compared to 2.5 in the treatment success group was 2.5 (p = 0.11). Patients who failed were more likely to have had an open injury (31% versus 17%; p = 0.05), to have undergone implant removal as part of their initial treatment (p = 0.03), and to undergo additional index I&D procedures (3.3 versus 1.6; p < 0.001).
The majority of culture results identified a single organism (62%), whereas only 15% were culture-negative (Table 1). Treatment failure was slightly more common in those with cultures that failed to identify an organism (p = 0.08). Staphylococcal species were the most commonly identified microorganisms (Table 2). In cases where an organism was identified, methicillin-resistant Staphylococcus aureus (MRSA) was the most common organism responsible for treatment failure (29%; p = 0.08), while methicillin-sensitive Staphylococcus aureus (MSSA) was more common in the treatment success group (36%; p = 0.04). Additional details on culture results and microorganisms that were identified can be found in Table 2.
Multivariate regression analysis demonstrated a statistically significant association with two or more I&Ds at index diagnosis of infection and treatment failure (odds ratio (OR) 13.22, 95% CI 4.77–36.62, p < 0.001). Culture-negative infection was also significantly associated with treatment failure (OR 4.74, 95% CI 1.26–17.83, p = 0.02). Treatment failure was also associated with elevated CCI, open fracture, and implant removal, though none achieved statistical significance (Table 3).
Discussion
FRI remains a challenging problem encountered in the care of the traumatized orthopaedic patient. Our investigation of 134 patients with FRI after surgical intervention demonstrated an unacceptably high rate of treatment failure at nearly 40%. Unfortunately, our rate of treatment failure is comparable to multiple prior studies that demonstrated excessive failure rates, ranging from 26 to 39%. [18,19,20,21,22,23,24] Among these studies, Gitajn et al. identified a 26% treatment failure rate (103/391 patients) while comparing culture-positive and culture-negative infections [21]. Berkes et al. and Rightmire et al. assessed treatment of FRI in the setting of implant retention, citing treatment failure rates of 29% (36/123 patients) and 32% (22/69 patients), respectively. [20, 24] Ovaska et al. assessed outcomes related to FRI after open reduction internal fixation of ankle fractures with 27% (26/97 patients) experiencing treatment failure [23]. Despite substantial heterogeneity amongst these investigations, most clinicians would likely agree that the incidence of treatment failure in this clinical scenario remains too high, and the potential to improve the management of FRI exists.
In our cohort, treatment failure was associated with open fracture, implant removal, culture-negative infection, and having undergone two or more I&Ds at initial diagnosis and treatment. Multivariate analyses reaffirmed the association of culture-negative infection and multiple I&D procedures with treatment failure. Gitajn et al. were among the first to highlight culture-negative infections as important clinical entities encountered in FRI. In contrast to our findings, they did not identify a meaningful difference in the rate of treatment failure among culture-positive and culture-negative infections [21]. The strength of our association may be tempered by the relatively low number of culture-negative infections in our study population, especially as the body of literature in arthroplasty has also demonstrated equivalent or better outcomes in culture-negative infection [25, 26]. When cultures were positive, MSSA was protective, whereas MRSA was the most common organism identified in treatment failure. A high incidence of MRSA infection overall and in treatment failure has been frequently documented in FRI [20, 21, 24, 27]. There are several factors that contribute to MRSA being a particular concern in trauma patients. There is a high prevalence of colonization in this population, and surgeons may have a limited ability to optimize the host prior to fracture surgery [28]. Additionally, perioperative cefazolin fails to provide sufficient coverage for MRSA [27]. Ultimately, our data support continued vigilance when treating MRSA-associated FRI but also suggests aggressive treatment is also important in the management of clinically diagnosed infections even in the absence of positive cultures.
Patients who develop FRI often undergo single or multiple I&Ds depending on a variety of clinical factors and primarily surgeon preference [29]. I&D of devitalized tissue has been described as critical in the eradication of infections associated with implants [30]. Previous studies have suggested that serial debridement is necessary to continually disrupt biofilm formation [31]. However, our finding that multiple I&D procedures was associated with treatment failure is supported by multiple studies [18, 23]. In our study, this may be a marker for more severely infected or more purulent wounds as these are the ones that often undergo additional repeat I&D procedures. It is also likely that the surgical protocol employed in our study period contributed to this finding. Regardless, our data clearly suggest a negative effect of recurrent debridement procedures, and instead, support recent recommendations that focus on a thorough initial debridement and early closure or soft tissue coverage, rather than repeated violation of the surgical wound and the fracture milieu, increasing the risk for contamination and delayed healing [10, 32]. This delicate balance between adequate debridement of infected tissue and avoiding disturbance of fracture healing and the tenuous soft tissue envelope adds complexity to the treatment of FRI.
Our univariate analysis suggested open fracture and implant removal may portend treatment failure; however, statistical significance was not reached in the regression model for these variables. This may be attributable to the limited number of open injuries in our study population, as open fractures are widely understood to be significant risk factors for infection [33], and other investigators have identified an associations between open fracture and treatment failure in FRI [18, 24]. Implant removal, particularly when removed prior to fracture union, is another variable that has been well-documented as detrimental in the management of FRI [18, 20, 23, 24]. The overwhelming majority of patients who did not experience treatment failure had implants maintained or removed only after fracture union in our study. Maintaining fracture stability not only fosters a favorable mechanical environment for osseus union, but it is critical to allow the soft tissues to rest and avoids ongoing damage [13]. Any foreign body effect attributed to the presence of an implant is essentially outweighed by the benefits of stability [8]. Balancing the concern for biofilm formation and the subsequent development of chronic infection or osteomyelitis with fracture stability is yet another challenging component of the management of FRI.
We must acknowledge several limitations of our study. First, this is a retrospective cohort study and is subject to the many weaknesses inherent to this form of study design. Second, a major limitation includes the lack of standardization in the diagnosis of infection, as the clinical judgement of the treating physician was allowed, consistent with multiple prior studies [34]. Recently, consensus recommendations on the diagnosis and management of FRI were published by a panel of experts subsequent to our investigation [8, 35, 36]. Use of this new information would have added strength to our work. Third, our dataset may neglect important patient factors that could increase the risk of treatment failure. As an example, prior authors highlighted the impact of smoking status on the risk of failure [20, 23, 24], while our dataset only included age, sex, CCI, and diabetes status. Fourth, FRI represents a complex clinical scenario with likely substantial heterogeneity among the cases included in our study. In a practical sense, this enabled the inclusion of a larger number of cases, albeit at the expense of standardization of treatment pathways. Although the broad treatment concepts were constant, patients received prompt surgical I&D followed by an extended antibiotic regimen formulated in consultation with infectious disease physicians. Additionally, the surgical protocol for multiple debridements employed during the study period may be considered somewhat outdated but was consistent with standards at the time. The protocol itself is likely a component of the strong association between repeat I&D and treatment failure. Fifth, the inclusion of patients with as few as 6 months of follow-up is not in accordance with recent expert recommendations [8]. Though the majority of the patients included in our study had a year or more of follow-up, our methodology may neglect to capture all significant treatment failure events. Sixth, we failed to include time to onset of symptoms and implant type in our dataset. Both variables may impact the association between implant removal and treatment failure in our study. Prior authors have identified these factors as important determinants of failure with duration of infection and implant surface area implicated in biofilm formation [1, 24, 37, 38]. There are several important strengths to our study. Multiple sites were included, both urban and rural level one trauma centers, providing increased demographic diversity and extending the generalizability and external validity of this study. Further, we were able to identify a large study population, particularly of patients experiencing treatment failure. To our knowledge, this represents one of the largest studies on treatment failure in FRI, and our investigation adds to the limited, but expanding, body of knowledge surrounding this topic.
Conclusion
In conclusion, we witnessed a rate of failure after treatment of FRI of 38.1% which lends further credence to the challenging nature of this clinical problem. Our investigation identified important risk factors for failure including open fracture, implant removal, and multiple debridements, which are in agreement with the work of prior authors. We are the first to highlight culture-negative infection as a potential risk factor for failure, suggesting aggressive treatment of clinically diagnosed cases remains critical even without positive culture data.
References
Metsemakers WJ, Kuehl R, Moriarty TF et al (2018) Infection after fracture fixation: current surgical and microbiological concepts. Injury 49(3):511–522
Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ (2002) The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol 23(4):183–189
Shah RP, Plummer DR, Moric M, Sporer SM, Levine BR, Della Valle CJ (2016) Diagnosing infection in the setting of periprosthetic fractures. J Arthroplasty 31(9 Suppl):140–143
Birt MC, Anderson DW, Bruce Toby E, Wang J (2017) Osteomyelitis: recent advances in pathophysiology and therapeutic strategies. J Orthop 14(1):45–52
Darouiche RO (2004) Treatment of infections associated with surgical implants. N Engl J Med 350(14):1422–1429
Jakobsen TH, Eickhardt SR, Gheorghe AG et al (2018) Implants induce a new niche for microbiomes. APMIS 126(8):685–692
Zimmerli W (2014) Clinical presentation and treatment of orthopaedic implant-associated infection. J Intern Med 276(2):111–119
Metsemakers WJ, Morgenstern M, Senneville E et al (2020) General treatment principles for fracture-related infection: recommendations from an international expert group. Arch Orthop Trauma Surg 140(8):1013–1027
Tintle SM, Forsberg JA, Potter BK, Islinger RB, Andersen RC (2009) Prosthesis retention, serial debridement, and antibiotic bead use for the treatment of infection following total joint arthroplasty. Orthopedics 32(2):87
Moojen DJ, Zwiers JH, Scholtes VA, Verheyen CC, Poolman RW (2014) Similar success rates for single and multiple debridement surgery for acute hip arthroplasty infection. Acta Orthop 85(4):383–388
Greene LR (2012) Guide to the elimination of orthopedic surgery surgical site infections: an executive summary of the Association for Professionals in Infection Control and Epidemiology elimination guide. Am J Infect Control 40(4):384–386
Bilgili F, Balci HI, Karaytug K et al (2015) Can normal fracture healing be achieved when the implant is retained on the basis of infection? An experimental animal model. Clin Orthop Relat Res 473(10):3190–3196
Foster AL, Moriarty TF, Zalavras C et al (2021) The influence of biomechanical stability on bone healing and fracture-related infection: the legacy of Stephan Perren. Injury 52(1):43–52
Tornero E, Morata L, Martinez-Pastor JC et al (2015) KLIC-score for predicting early failure in prosthetic joint infections treated with debridement, implant retention and antibiotics. Clin Microbiol Infect 21(8):786.e9-786.e17
Kurtz SM, Lau EC, Son MS, Chang ET, Zimmerli W, Parvizi J (2018) Are we winning or losing the battle with periprosthetic joint infection: trends in periprosthetic joint infection and mortality risk for the medicare population. J Arthroplasty 33(10):3238–3245
Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J (2012) Economic burden of periprosthetic joint infection in the United States. J Arthroplasty 27(8 Suppl):61-65.e1
Metsemakers WJ, Morgenstern M, McNally MA et al (2018) Fracture-related infection: a consensus on definition from an international expert group. Injury 49(3):505–510
Hellebrekers P, Leenen LP, Hoekstra M, Hietbrink F (2017) Effect of a standardized treatment regime for infection after osteosynthesis. J Orthop Surg Res 12(1):41
Hellebrekers P, Verhofstad MHJ, Leenen LPH, Varol H, van Lieshout EMM, Hietbrink F (2019) The effect of early broad-spectrum versus delayed narrow-spectrum antibiotic therapy on the primary cure rate of acute infection after osteosynthesis. Eur J Trauma Emerg Surg. https://doi.org/10.1007/s00068-019-01182-6
Rightmire E, Zurakowski D, Vrahas M (2008) Acute infections after fracture repair: management with hardware in place. Clin Orthop Relat Res 466(2):466–472
Gitajn IL, Heng M, Weaver MJ, Ehrlichman LK, Harris MB (2016) Culture-negative infection after operative fixation of fractures. J Orthop Trauma 30(10):538–544
Spitzmuller R, Gumbel D, Guthoff C et al (2019) Duration of antibiotic treatment and risk of recurrence after surgical management of orthopaedic device infections: a multicenter case-control study. BMC Musculoskelet Disord 20(1):184
Ovaska MT, Makinen TJ, Madanat R, Vahlberg T, Hirvensalo E, Lindahl J (2013) Predictors of poor outcomes following deep infection after internal fixation of ankle fractures. Injury 44(7):1002–1006
Berkes M, Obremskey WT, Scannell B et al (2010) Maintenance of hardware after early postoperative infection following fracture internal fixation. J Bone Joint Surg Am 92(4):823–828
Choi HR, Kwon YM, Freiberg AA, Nelson SB, Malchau H (2013) Periprosthetic joint infection with negative culture results: clinical characteristics and treatment outcome. J Arthroplasty 28(6):899–903
Kim YH, Kulkarni SS, Park JW, Kim JS, Oh HK, Rastogi D (2015) Comparison of infection control rates and clinical outcomes in culture-positive and culture-negative infected total-knee arthroplasty. J Orthop 12(Suppl 1):S37-43
Torbert JT, Joshi M, Moraff A et al (2015) Current bacterial speciation and antibiotic resistance in deep infections after operative fixation of fractures. J Orthop Trauma 29(1):7–17
Metsemakers WJ, Onsea J, Neutjens E et al (2017) Prevention of fracture-related infection: a multidisciplinary care package. Int Orthop 41(12):2457–2469
Blanchette KA, Wenke JC (2018) Current therapies in treatment and prevention of fracture wound biofilms: why a multifaceted approach is essential for resolving persistent infections. J Bone Jt Infect 3(2):50–67
Halawi MJ, Morwood MP (2015) Acute management of open fractures: an evidence-based review. Orthopedics 38(11):e1025–e1033
Estes CS, Beauchamp CP, Clarke HD, Spangehl MJ (2010) A two-stage retention debridement protocol for acute periprosthetic joint infections. Clin Orthop Relat Res 468(8):2029–2038
Park SH, Silva M, Bahk WJ, McKellop H, Lieberman JR (2002) Effect of repeated irrigation and debridement on fracture healing in an animal model. J Orthop Res 20(6):1197–1204
Gustilo RB, Anderson JT (1976) Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am 58(4):453–458
Metsemakers WJ, Kortram K, Morgenstern M et al (2018) Definition of infection after fracture fixation: a systematic review of randomized controlled trials to evaluate current practice. Injury 49(3):497–504
Depypere M, Kuehl R, Metsemakers WJ et al (2020) Recommendations for systemic antimicrobial therapy in fracture-related infection: a consensus from an International Expert Group. J Orthop Trauma 34(1):30–41
Govaert GAM, Kuehl R, Atkins BL et al (2020) Diagnosing fracture-related infection: current concepts and recommendations. J Orthop Trauma 34(1):8–17
Al-Mayahi M, Betz M, Muller DA et al (2013) Remission rate of implant-related infections following revision surgery after fractures. Int Orthop 37(11):2253–2258
Morgenstern M, Kuehl R, Zalavras CG et al (2021) The influence of duration of infection on outcome of debridement and implant retention in fracture-related infection. Bone Joint J. 103-B(2):213–221
Funding
The work of this study was not supported by external funding sources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
This study underwent institutional review board (IRB) approval.
Informed consent
The requirement of informed consent was waived in this retrospective, minimal-risk study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yong, T.M., Rackard, F.A., Dutton, L.K. et al. Analyzing risk factors for treatment failure in fracture-related infection. Arch Orthop Trauma Surg 143, 1387–1392 (2023). https://doi.org/10.1007/s00402-021-04277-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-021-04277-1