Abstract
Introduction
Rotator cuff tears are common in the older population. Atrophy and fat infiltration develop un-evenly in torn supraspinatus (SSP) muscles leading to pre- and post-surgical complications. The purpose of the current study was twofold: first, to implement a volumetric and quantitative magnetic resonance imaging (MRI) approach to quantify the degree of muscle atrophy and fat infiltration within the SSP muscle and its four sub-regions (AS, PS, AD, and PD); second to compare 3-D MRI outcomes to the standard 2-D assessment and investigate their relationship with tear size.
Materials and methods
Fifteen cadaveric shoulders were obtained and MRI performed. Quantitative 3-D outcomes included SSP muscle volume, fossa volume, fat-free muscle volume, and fat fraction for the whole SSP muscle and its four sub-regions. 2-D and qualitative measurements included tear size, 2-D fat infiltration using the Goutallier classification, tangent sign, and occupation ratio.
Results
Linear regression outcomes with tear size were not significant for both cross-sectional area (r = − 0.494, p = 0.061) and occupation ratio (r = − 0.011, p = 0.969). Tear size negatively correlated with fat-free muscle volume for both AS and PS sub-regions (AS: r = − 0.78, p < 0.001; PS: r = − 0.68, p = 0.005, respectively) while showing no significant correlation with fat fraction outcomes. AD and PD sub-regions positively correlated with tear size and fat fraction outcomes (AD: r = 0.70, p = 0.017; PD: r = 0.52, p = 0.045, respectively), while no significant correlation was observed between tear size and fat-free muscle volumes.
Conclusion
Quantitative 3-D volumetric assessment of muscle degeneration resulted in better outcomes compared to the standard 2-D evaluation. The superficial supraspinatus muscle sub-regions primarily presented muscle atrophy, while the deep sub-regions were mainly affected by fat infiltration. 3-D assessments could be used pre-surgically to determine the best course of treatment and to estimate the muscles’ regenerative capacity and function.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rotator cuff (RC) tears are the most common causes of shoulder-related pain and disability, with a prevalence of 50–80%, especially in the middle-aged and elderly population, respectively [12, 23, 36]. While patients presenting with a RC tear may initially be asymptomatic, the tear will eventually increase in size over time, resulting in musculotendinous retraction, muscle atrophy, and fat infiltration within the RC muscles [2, 20,21,22, 29, 35, 36]. These abnormal tissue changes further confound the feasibility of RC repairs and healing. Thus, pre-surgical diagnostic imaging and analyses play a critical role in determining the patients’ best course of treatment [8].
The current clinical gold standard for assessing muscle atrophy and fat infiltration implements a qualitative and two-dimensional (2-D) classification system which evaluates the sagittal Y-view of the shoulder in a magnetic resonance (MR) image. The 2-D assessment, such as the Fuchs or Goutallier methods [3, 9], evaluates a single-image slice for pathological changes in the muscle. However, in addition to intra- and inter- rater variability, and non-volumetric outcomes, measurements are qualitative and do not accurately describe the amount of fat and fat-free muscle. Previous studies have shown a strong correlation between fat-free muscle volume and tear size [20], as well as fat fraction, fat volume, and tear size [18, 20, 25]. Quantitative and volumetric three-dimensional (3-D) estimations of fat-free muscle and fat fraction showed better reliability and reproducibility compared to outcomes from a 2-D single-image analyses [7, 11, 24, 33, 34]. In a previous study, our group has shown quantitative and volumetric measurements of fat fraction within the supraspinatus (SSP) muscle to be highly correlated with the extensibility, or deformation, of the musculotendinous unit [7]. These results indicate the importance of obtaining volumetric and quantitative measurements of muscle degeneration as prognostic factors for surgical and functional outcomes. Interestingly, a study by Meyer et al. found that atrophy and fat infiltration develops and progresses un-evenly in the torn SSP muscle, with the superficial region primarily resulting in muscle atrophy, and the deep region primarily affected by fat infiltration [22]. We have previously shown that the SSP muscle can be divided into four different sub-regions based on morphology and function (AS: anterior-superficial, AD: anterior-deep, PS: posterior-superficial, and PD: posterior-deep) [10, 37]. To better understand and to help estimate the residual function of the torn SSP muscle with increasing tear size, it is critical to evaluate each individual muscle sub-region for degenerative properties as these relate to degree of atrophy and intramuscular fat infiltration.
Other methods that are commonly used to measure muscle atrophy are the occupation ratio and tangent sign [28, 30, 32]. The process developed by Thomazeau et al. and Schaefer et al. demonstrated that the occupation ratio decreased in value as the size of the tear increased. When the muscle atrophies, it will fall below the tangent line going from the superior aspect of the scapula to the superior portion of the scapular spine, resulting in a positive tangent sign. However, if the muscle remains above the tangent line, it will result in a negative tangent sign. Rulewicz et al. showed that a positive tangent sign correlated with a larger rotator cuff tear [28]. Other studies have shown a strong correlation between the occupation ratio, tangent sign, and improved strength and mobility [28, 30]. Vidt et al. compared a 2-D occupation ratio with the 3-D volume of rotator cuff muscles, demonstrating that 2-D outcomes did not represent the 3-D volume of the rotator cuff muscles [34].
To better understand and to help estimate the residual function and healing potential of the torn SSP muscle with increasing tear size, it is critical to appropriately evaluate each individual muscle sub-region for degenerative properties as these relate to degree of atrophy and intramuscular fat infiltration. Therefore, the purpose of the current study was twofold: first, to implement a volumetric and quantitative MR imaging approach to quantify the degree of muscle atrophy and fat infiltration within the SSP muscle and each sub-region; second, to compare 3-D volumetric outcomes to the 2-D occupation ratio and investigate their relationship with tear size.
Materials and methods
Specimen preparation
Fifteen fresh-frozen cadaveric shoulders (11 males and 4 females; mean age, 71 years, range 25–94 years) were obtained after IRB approval. The specimens were kept frozen at – 20 °C and thawed overnight at room temperature in preparation for MR imaging. Post-imaging, the shoulders were dissected and classified based on tear size as intact (n = 6), small (n = 2), medium (n = 2), large (n = 3), and massive (n = 2) tendon tears, as established by Post et al. [26].
Magnetic resonance imaging
MR imaging examinations were performed on a 70 cm bore 3.0T clinical scanner (GE Healthcare, DV 25.0 R02 software) using a 3-channel shoulder surface coil (GE Healthcare: 3.0T HD Shoulder Array, model 2414331). The cadaveric shoulders included all bony and soft-tissue structures during imaging.
Three-dimensional (3-D) analyses
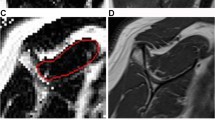
Muscle volume and volumetric intramuscular fat fraction outcomes were quantified using a 2-point Dixon MR imaging acquisition protocol. Specifications for the imaging protocol have been previously described by our group [6, 7, 33] and were as follows: 3-D, isotropic 2-point Dixon sequence (TR/TE, 3.9/1.2 ms; FOV, 30 cm; matrix, 200 × 200; slice thickness, 1.5 mm; flip angle, 10°; band- width, 142.86 kHz; NEX, 1). The imaging process resulted in fat-image and water-image DICOM data sets for each cadaveric shoulder. After the images were acquired and to isolate the target region volumes, the fat and water images were imported into Mimics imaging and editing software (Materialise, Plymouth, MI). The fat-image DICOM data sets were first imported into Mimics and used as the initial template for the segmentation process. The SSP muscle sub-regions were divided based on a previous study by Roh et al. [27] which describes the anterior region attached to the anterior tendon, and the posterior region attached to the posterior tendon. The anterior-superficial (AS) sub-region was defined as the muscle region located superior to the anterior internal tendon, while the anterior-deep (AD) sub-region consisted on the muscle inferior to the anterior internal tendon. The posterior-deep (PD) sub-region, laying below the posterior-superficial (PS) sub-region, was subdivided based on the most-inferior level of the anterior-deep sub-region (cleft) (Fig. 1).
a Internal tendon of the anterior region (yellow arrows), and cleft between the anterior and posterior regions (green arrow). b AS (anterior-superficial) sub-region located superior to the internal tendon (yellow circle); AD (anterior-deep) sub-region locates inferior to the internal tendon (red circle); PS and PD (posterior-superficial and deep) sub-regions are subdivided at the most-inferior level of the AD region (cleft) (blue and green circles, respectively)
Volumetric manual segmentations were performed for each of the four sub-regions in the fat-image data sets and these segmentations were copied into the water-image data sets to obtain a fat fraction outcome for each sub-region according to Eq. 1. From all muscle masks (whole SSP, AS, AD, PS, and PD), the muscle volumes were obtained and the fat-free muscle volumes were calculated by subtracting the fat infiltration volume (fat fraction (%) x muscle volume) from the muscle volume [20, 34]:
Muscle volume was calculated from oblique sagittal T1 weighted fast spin echo scans (FSE) (FSE-XL; (TR/TE, 700–900/min Full; FOV, 14 cm; echo train length, 4; matrix, 384 × 256; slice/space, 4/0; bandwidth, 32 kHz; NEX, 2. Images were imported into Mimics and a 3-D volume assessment performed. Briefly, the entire volume of the muscle was segmented from the Y-view and continuing proximally until the muscle could no longer be viewed on the images. A similar approach was implemented to calculate the fossa volume; the fossa was segmented from the Y-view continuing proximally until the muscle could no longer be viewed, and defined as the area surrounding the bone and the tangent line between superior aspect of the scapula and the superior portion of the scapular spine.
Outcome measurements from the 3-D analyses included muscle volume, fat-free muscle volume, fat fraction for the whole SSP muscle and its sub-regions (AS, AD, PS, and PD), and fossa volume.
Two-dimensional (2-D) single-image assessment
MR images used for 2-D analyses were obtained from the T1 scan specified above. Intramuscular fat infiltration of the whole SSP muscle was graded by an experienced musculoskeletal radiologist on a sagittal Y-view of the MR image using the Goutallier classification system as follows [3, 9]: stage 0 (normal muscle without any fatty streaks); stage 1 (muscle contains some fatty streaks); stage 2 (less fatty infiltration than muscle); stage 3 (as much fat as muscle); and stage 4 (more fat than muscle). The T1 images were then imported into Mimics to analyze the tangent sign, cross-sectional area (CSA) of the SSP muscle and SSP fossa. These measurements were obtained at the most lateral image, where the scapular spine and coracoid process are in contact with the body of the scapula [4, 8, 13, 19]. Then, the occupation ratio was calculated as the cross-sectional area of SSP muscle divided by the area of the scapular fossa [4, 13, 14, 32].
Outcome measurements from the 2-D analyses included 2D fat infiltration using the Goutallier classification system, tangent sign, and occupation ratio.
Statistical analyses
Statistical analyses were performed using the SPSS Statistical Package (Version 25, SPSS Inc, Chicago, IL). Outcome measurements included 3-D fat fraction (%), fat infiltration volume, and fat-free muscle volume for the whole SSP muscle and its sub-regions, as well as 2-D occupation ratio, tangent sign, and 2-D fatty build-up as classified using the Goutallier classification system. Intraclass correlation coefficient analyses (ICC2,1) was performed to investigate the inter-rater reliability of measurements. To evaluate reliability, fat-free muscle volume, fat fraction, and muscle volume were chosen as the primary outcome measurements from two independent observers. Spearman correlation analyses were performed to relate and compare fat-free muscle volume and fat fraction values with tear size. p < 0.05 was considered statistically significant.
Results
Three-dimensional analyses
Inter-rater reliability of measurements (ICC2,1) for fat-free muscle volume, fat fraction, and muscle volume outcomes were moderate to excellent (range 0.677–0.983), good to excellent (range 0.767–0.867), and excellent (range 0.949–0.985), respectively. Mean (SD) fat fraction outcomes for the whole SSP muscle, AS, AD, PS, and PD muscle sub-regions were 10.8% (0.8), 27.2% (8.2), 21.5% (11.8), 31.4% (14.2), and 22.3% (11.9), respectively. Fat-free muscle volumes for the respective regions were 13.7 cm3 (5.6), 3.9 cm3 (2.5), 7.2 cm3 (3.0), 0.9 cm3 (0.5), and 2.1 cm3 (0.8).
There were significant correlations for the whole SSP muscle when evaluating fat-free muscle volume and fat fraction vs. tear size (r = − 0.68, p = 0.005; r = 0.56, p = 0.030, respectively; Fig. 2). Muscle sub-region analyses resulted in a heterogeneous distribution of fat and muscle atrophy. Tear size resulted in a significant negative correlation with fat-free muscle volume for both AS and PS sub-regions (AS: r = − 0.78, p < 0.001; PS: r = − 0.68, p = 0.005) while showing no significant correlations with fat fraction outcomes (AS: r = 0.46, p = 0.112; PS: r = 0.38, p = 0.157). On the other hand, the AD and PD sub-regions showed a significant positive correlation between tear size and fat fraction outcomes (AD: r = 0.70, p = 0.017; PD: r = 0.52, p = 0.045), while no significant correlation was observed between tear size and fat-free muscle volumes (AD: r = − 0.37, p = 0.173; PD: r = − 0.26, p = 0.353, respectively; Fig. 3).
Mean (SD) values for muscle volume and muscle volume divided by fossa volume of the whole SSP muscle were 17.8 cm3 (6.3) and 1.27 (0.43), respectively. Fat-free muscle volume and fat-free muscle volume divided by fossa volume were 13.7 cm3 (5.6) and 0.95 (0.37), respectively. Tear size resulted in a significant negative correlation with the normalized 3-D outcomes; fat-free muscle volume/fossa, and muscle volume/fossa (r = − 0.78, p = 0.001; r = − 0.71, p = 0.003), respectively. Tear size also resulted in a significant negative correlation with the absolute 3-D outcomes; fat-free muscle volume and muscle volume (r = − 0.68, p = 0.005; r = − 0.55, p = 0.033), respectively (Fig. 4).
Two-dimensional single-image assessment
2-D fat infiltration of the whole SSP muscle classified by the Goutallier classification system resulted in 7 shoulders with stage 1, 5 with stage 2, 2 with stage 3, and 1 with stage 4. Fat infiltration outcomes of the whole SSP muscle were significantly and positively correlated with the qualitative Goutallier grades (r = 0.82, p < 0.001). Fat infiltration measures for specimens classified as stage 1 ranged from 12.9% to 22.2%, stage 2 ranged from 18.3% to 39.2%, and stages 3 and 4 ranged from 34.6% to 47.1%. Stages 3 and 4 were pooled together due to the small number of specimens in each group. Mean (SD) values of cross-sectional area (CSA) and occupation ratio of the SSP muscle were 363.3 mm2 (72.4) and 0.73 (0.27), respectively. Linear regression outcomes with tear size were not significant for both CSA (r = − 0.494, p = 0.061) and occupation ratio (r = − 0.011, p = 0.969) (Fig. 5). Finally, tangent sign outcomes resulted in the following: positive (1 large and 1 massive tears); negative (6 intact, 2 small, 2 medium, 2 large, and 1 massive).
Discussion
In the current study, we quantified volumetric fat-free muscle and fat fraction of the whole supraspinatus (SSP) muscle and of the four muscle sub-regions (AS: anterior-superficial, AD: anterior-deep, PS: posterior-superficial, and PD: posterior-deep). Similar to a previous study [34], quantitative 3-D volumetric assessments of muscle degeneration resulted in better outcomes when compared to the standard 2-D assessment. An important finding from the current study was the increased atrophy of both the anterior-superficial and posterior-superficial sub-regions, while the deep sub-regions were mostly affected by an increase in intramuscular fat infiltration.
It is important to understand the degenerative capacity and progression of muscle degeneration so that comprehensive muscle evaluations and adequate interpretations of volumetric and regional outcomes can be established in the setting of rotator cuff tears. After a tear, the SSP muscle expresses asymmetric atrophy accompanied by fibrosis and fatty infiltration within the muscle fibers [22]. When assessing muscle atrophy, the whole muscle is typically segmented or isolated during a 3-D assessment, and the entire cross-sectional area is outlined in a 2-D evaluation. However, due to the segmentation and outline process including the entire muscle, fibrotic tissue, and intramuscular fat, an overestimation of atrophy is observed. To overcome this overestimation in outcomes, fat-free muscle volume was calculated by subtracting fat infiltration volume from muscle volume, to differentiate the fat tissue from the muscle fibers [20, 34]. This fat-free muscle volume estimation approach has been previously shown to significantly correlate with muscle strength [34]. Specimens classified for 2-D fatty build-up using the Goutallier system were significantly and positively correlated with volumetric fat fraction outcomes, with a range for volumetric fat fraction for the whole SSP muscle of 34.6–47.1% for stages 3 and 4. While these values are below the < 50% threshold for stages 3–4 established by Goutallier, these findings are consistent with Nardo et al. [24] and Horiuchi et al. [11] showing overestimation of fat infiltration in a 2-D image analysis. These findings emphasize the importance of 3-D analyses as a more accurate approach.
Roh et al. advocated for the division of the SSP muscle into anterior and posterior regions based on the structural differences, and suggested that the anterior region functions as the primary contractile unit [27]. Kim et al. further subdivided the anterior and posterior regions into superficial, middle, and deep sub-regions [16], and supported the role of the anterior region as a contractile force production unit, while the posterior region is primarily involved in the adjustment of tension [1, 15]. On the assumption that both anterior- and posterior-deep sub-regions play a significant role in contractility and muscle force production, these sub-regions are highly susceptible to decreased contractility by intramuscular fat infiltration. Many investigators have reported a relationship between intramuscular fat infiltration and tear size [18, 20, 25], and the relationship between fatty build-up and preoperative or intraoperative contractile potential of the torn SSP muscle [5, 38]. The preoperative degree of intramuscular fat infiltration has been shown to be significantly correlated with post-operative muscle strength, with post-operative muscle strength of small-sized tears being significantly greater than that of patients with medium- and large-to-massive-sized tears [31]. Similarly, preoperative supraspinatus muscle atrophy and fat infiltration were significantly and negatively correlated with post-operative strength in external rotation and forward elevation [8]. While strength, function, and muscle contractility were not measured in the current study, these previous investigations support the results of the current study indicating quantitative and volumetric fat fraction measurements from the anterior- and posterior-deep sub-regions as a feasible approach and method to estimate the residual function of the torn SSP muscle.
There are several limitations in this study. First, the number of specimens in each tear size classification was small and the values obtained for atrophy and fat fraction might differ from those observed in-vivo. In two different investigations, Kim et al. found significant atrophy of the anterior- and posterior-superficial sub-regions in cadavers [17] and in patients using ultrasound imaging [15]. These studies further support the finding that the anterior and posterior-superficial sub-regions primarily atrophied. Second, quantitative 3-D analyses take longer than a 2-D approach, which might not make it appealing for a clinical implementation yet. Third, we have not measured muscle contractile properties. However, based on previous published literature reports, quantitative measurements in this study could be used as an estimation of function and strength. Finally, only the SSP muscle was analyzed and other rotator cuff muscles (infraspinatus, teres minor, and subscapularis) should be evaluated in the future. Overall, the results presented in this study suggest that quantitative fat-free muscle volume and fat fraction measurements using the 2-point Dixon MR imaging technique could be a suitable method to evaluate muscle atrophy, degeneration, and residual function of the SSP muscle.
Conclusion
The superficial supraspinatus muscle sub-regions primarily showed muscle atrophy, while the deep sub-regions were mainly affected by fat infiltration. Quantitative volumetric assessment of muscle degeneration could be used pre-surgically to determine the best course of treatment and to estimate the muscles’ regenerative capacity and function. Future studies implementing the methodologies from this study should be performed in-vivo.
References
Chung SW, Kim JY, Kim MH, Kim SH, Oh JH (2013) Arthroscopic repair of massive rotator cuff tears: outcome and analysis of factors associated with healing failure or poor postoperative function. Am J Sports Med 41(7):1674–1683
Fabis J, Danilewicz M, Omulecka A (2001) Rabbit supraspinatus tendon detachment: effects of size and time after tenotomy on morphometric changes in the muscle. Acta Orthop Scand 72(3):282–286
Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C (1999) Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg 8(6):599–605
Fukuta S, Tsutsui T, Amari R, Wada K, Sairyo K (2016) Tendon retraction with rotator cuff tear causes a decrease in cross-sectional area of the supraspinatus muscle on magnetic resonance imaging. J Shoulder Elbow Surg 25(7):1069–1075
Gerber C, Schneeberger AG, Hoppeler H, Meyer DC (2007) Correlation of atrophy and fatty infiltration on strength and integrity of rotator cuff repairs: a study in thirteen patients. J Shoulder Elbow Surg 16(6):691–696
Giambini H, Hatta T, Krzysztof GR et al (2017) Intramuscular fat infiltration evaluated by magnetic resonance imaging predicts the extensibility of the supraspinatus muscle. Muscle Nerve 57(1):129–135
Giambini H, Hatta T, Rezaei A, An KN (2018) Extensibility of the supraspinatus muscle can be predicted by combining shear wave elastography and magnetic resonance imaging-measured quantitative metrics of stiffness and volumetric fat infiltration: a cadaveric study. Clin Biomech (Bristol, Avon) 57:144–149
Gladstone JN, Bishop JY, Lo IK, Flatow EL (2007) Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med 35(5):719–728
Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC (1994) Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res 304:78–83
Hatta T, Giambini H, Uehara K et al (2015) Quantitative assessment of rotator cuff muscle elasticity: reliability and feasibility of shear wave elastography. J Biomech 48(14):3853–3858
Horiuchi S, Nozaki T, Tasaki A et al (2017) Reliability of MR quantification of rotator cuff muscle fatty degeneration using a 2-point Dixon technique in comparison with the Goutallier classification: validation study by multiple readers. Acad Radiol 24(11):1343–1351
Initiative USBaJ (2014) Burden of musculoskeletal diseases in the United States: prevalence, societal and economic cost(ed)^(eds), Rosemont, IL
Jo CH, Shin JS (2013) Changes in appearance of fatty infiltration and muscle atrophy of rotator cuff muscles on magnetic resonance imaging after rotator cuff repair: establishing new time-zero traits. Arthroscopy 29(3):449–458
Kikukawa K, Ide J, Kikuchi K, Morita M, Mizuta H, Ogata H (2014) Hypertrophic changes of the teres minor muscle in rotator cuff tears: quantitative evaluation by magnetic resonance imaging. J Shoulder Elbow Surg 23(12):1800–1805
Kim SY, Bleakney RR, Rindlisbacher T, Ravichandiran K, Rosser BW, Boynton E (2013) Musculotendinous architecture of pathological supraspinatus: a pilot in vivo ultrasonography study. Clin Anat 26(2):228–235
Kim SY, Boynton EL, Ravichandiran K, Fung LY, Bleakney R, Agur AM (2007) Three-dimensional study of the musculotendinous architecture of supraspinatus and its functional correlations. Clin Anat 20(6):648–655
Kim SY, Sachdeva R, Li Z, Lee D, Rosser BW (2015) Change in the pathologic supraspinatus: a three-dimensional model of fiber bundle architecture within anterior and posterior regions. Biomed Res Int 2015:564825
Lee S, Lucas RM, Lansdown DA et al (2015) Magnetic resonance rotator cuff fat fraction and its relationship with tendon tear severity and subject characteristics. J Shoulder Elbow Surg 24(9):1442–1451
Lippe J, Spang JT, Leger RR, Arciero RA, Mazzocca AD, Shea KP (2012) Inter-rater agreement of the Goutallier, Patte, and Warner classification scores using preoperative magnetic resonance imaging in patients with rotator cuff tears. Arthroscopy 28(2):154–159
Matsumura N, Oguro S, Okuda S et al (2017) Quantitative assessment of fatty infiltration and muscle volume of the rotator cuff muscles using 3-dimensional 2-point Dixon magnetic resonance imaging. J Shoulder Elbow Surg 26(10):e309–e318
Meyer DC, Hoppeler H, von Rechenberg B, Gerber C (2004) A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res 22(5):1004–1007
Meyer DC, Pirkl C, Pfirrmann CW, Zanetti M, Gerber C (2005) Asymmetric atrophy of the supraspinatus muscle following tendon tear. J Orthop Res 23(2):254–258
Minagawa H, Yamamoto N, Abe H et al (2013) Prevalence of symptomatic and asymptomatic rotator cuff tears in the general population: from mass-screening in one village. J Orthop 10(1):8–12
Nardo L, Karampinos DC, Lansdown DA et al (2014) Quantitative assessment of fat infiltration in the rotator cuff muscles using water-fat MRI. J Magn Reson Imaging 39(5):1178–1185
Nozaki T, Tasaki A, Horiuchi S et al (2015) Quantification of fatty degeneration within the supraspinatus muscle by using a 2-point Dixon method on 3-T MRI. AJR Am J Roentgenol 205(1):116–122
Post M, Silver R, Singh M (1983) Rotator cuff tear. Diagnosis and treatment. Clin Orthop Relat Res 173:78–91
Roh MS, Wang VM, April EW, Pollock RG, Bigliani LU, Flatow EL (2000) Anterior and posterior musculotendinous anatomy of the supraspinatus. J Shoulder Elbow Surg 9(5):436–440
Rulewicz GJ, Beaty S, Hawkins RJ, Kissenberth MJ (2013) Supraspinatus atrophy as a predictor of rotator cuff tear size: an MRI study utilizing the tangent sign. J Shoulder Elbow Surg 22(6):e6-10
Safran O, Schroeder J, Bloom R, Weil Y, Milgrom C (2011) Natural history of nonoperatively treated symptomatic rotator cuff tears in patients 60 years old or younger. Am J Sports Med 39(4):710–714
Schaefer O, Winterer J, Lohrmann C, Laubenberger J, Reichelt A, Langer M (2002) Magnetic resonance imaging for supraspinatus muscle atrophy after cuff repair. Clin Orthop Relat Res 403:93–99
Shin SJ, Chung J, Lee J, Ko YW (2016) Recovery of muscle strength after intact arthroscopic rotator cuff repair according to preoperative rotator cuff tear size. Am J Sports Med 44(4):972–980
Thomazeau H, Rolland Y, Lucas C, Duval JM, Langlais F (1996) Atrophy of the supraspinatus belly. Assessment by MRI in 55 patients with rotator cuff pathology. Acta Orthop Scand 67(3):264–268
Trevino JH 3rd, Gorny KR, Gomez-Cintron A, Zhao C, Giambini H (2019) A quantitative alternative to the Goutallier classification system using Lava Flex and Ideal MRI techniques: volumetric intramuscular fatty infiltration of the supraspinatus muscle, a cadaveric study. MAGMA 32(6):607–615
Vidt ME, Santago AC 2nd, Tuohy CJ et al (2016) Assessments of Fatty infiltration and muscle atrophy from a single magnetic resonance image slice are not predictive of 3-dimensional measurements. Arthroscopy 32(1):128–139
Williams MD, Ladermann A, Melis B, Barthelemy R, Walch G (2009) Fatty infiltration of the supraspinatus: a reliability study. J Shoulder Elbow Surg 18(4):581–587
Yamamoto A, Takagishi K, Osawa T et al (2010) Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg 19(1):116–120
Yuri T, Kuwahara Y, Fujii H, Kiyoshige Y (2017) Functions of the subregions of the supraspinatus muscle. Clin Anat 30(3):347–351
Yuri T, Mura N, Yuki I, Fujii H, Kiyoshige Y (2018) Contractile property measurement of the torn supraspinatus muscle using real-time tissue elastography. J Shoulder Elbow Surg 27(9):1700–1704
Acknowledgements
This study was supported by the University of Texas at San Antonio. A co-author, Takuma Yuri, is funded by a Grant-in-Aid for JSPS fellows (Grant number 19J10699). We would like to thank Dr. Hiromi Fujii.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Trevino III, J.H., Yuri, T., Hatta, T. et al. Three-dimensional quantitative measurements of atrophy and fat infiltration in sub-regions of the supraspinatus muscle show heterogeneous distributions: a cadaveric study. Arch Orthop Trauma Surg 142, 1395–1403 (2022). https://doi.org/10.1007/s00402-021-03765-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-021-03765-8