Abstract
Introduction
Joint replacement surgery as a treatment for glenohumeral arthritis with glenoid bone loss is challenging. The aim of this study is to offer an anatomical orientation for glenoid reconstruction.
Methods
In this study, we measured size, inclination and version of the glenoid surface, as well as the distance between the articular line of the glenoid, base of the coracoid process, and acromion using computer tomographic (CT) imaging of 131 study participants aged 19–88 years in the period of 2010–2013.
Results
We measured a mean distance of 6.5 ± 0.2 mm from the glenoid articular line to the base of the coracoid process in the transverse CT plane. Body height has shown no significant impact on the glenoid morphology. We observed significant differences between males and females: The glenoid appeared to be located 5.2 ± 0.9 mm higher and the humeral head was 4.5 ± 0.7 mm larger in male subjects compared with females (r = .699; p < .01).
Conclusion
In our study, the base of the coracoid offers an anatomical reference during reconstruction of the glenoid in primary and revision shoulder arthroplasty. As only 2D-CT imaging allows for accurate assessment of glenoid bone defects, we consider conventional X-ray imaging insufficient for proper preoperative planning before shoulder arthroplasty.
Level of evidence
III.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In light of the reliable clinical outcomes over the last 25 years in Europe by treating glenohumeral arthritis and rotator cuff insufficiencies, the attention given to reverse shoulder arthroplasty (RTSA) has increased [8, 9, 27]. However, in spite of its great popularity, the RTSA is associated with a high rate of complications, including scapular notching, baseplate failure, periprosthetic fractures, instability and nerve lesions [1, 30]. Besides others, the sufficient function of the deltoid muscle by finding the optimal position for the baseplate has been proven to be a crucial factor for obtaining good clinical results [16, 22, 25].
Large glenoid defects are challenging and the orientation of the rotational center is often missing. Such defects are encountered in defect arthropathy, chronic shoulder instability and revision shoulder arthroplasty [14, 15, 26]. As shoulder arthroplasty continues to gain popularity, an increase in aforementioned revision procedures can be expected. Loosening and failure of the glenoid component is a common concern after RTSA [3, 5, 19, 20, 31]. A careful preoperative study of the glenoid morphology and accurate surgical planning are required [10, 12, 14, 29]. For this purpose, a CT scan is recommended prior to performing shoulder replacement surgery. Several radiological parameters have been described to identify glenoid bone defects preoperatively with the aim to choose the most appropriate implant and avoid potential intraoperative complications [14]. The use of computer-assisted navigated reverse shoulder arthroplasty contributes to significant alterations in screw purchase length, screw angulation and central cage perforation [7, 23]. In primary and revision reverse shoulder arthroplasty, adding lateralization seems to be indicated to reconstruct the rotational center. Lateralization can be achieved by extended glenosphere components or bony spacers [2, 4, 6]. However, the indication for a adding lateralization remains unclear and an anatomical orientation is often missing. Therefore, the aim of the presenting study is to establish an anatomical reference for glenoid reconstruction by means of CT analysis.
Materials and methods
Epidemiologic patient data
We collected 131 patients retrospectively with an age of 19–88 years: 77 patients were female (58.8%) and 54 were male (41.2%). The body height of our participants measured between a minimum of 150 and maximum of 190 cm (mean 170 cm, SD 0.09 cm), the height was acquired retrospectively using a standardized questionnaire. We selected our patients from 2010 to 2013; this timeframe included any patient receiving computed tomography (CT) for proximal fracture of the humerus, shoulder dislocation, or bruising. For the measurements of the humeral head, patients with the proximal humeral fractures were excluded. The patients with shoulder dislocation had a primary and traumatic dislocation, and there are no patients included with chronic instability. Patients with a history of shoulder arthrosis, as well as those with a fracture of the structures relevant to our study, such as the acromion, glenoid, and/or coracoid process, were excluded (n = 110). The CT images we used were obtained from the diagnostic scans conducted at the point of patient admission and filed under patient pseudonyms. A Siemens Emotion 16 with the following protocol made the CT scans: 2-mm axial and 3-mm coronal/sagittal 3-mm slices. All patients were positioned supine with the shoulder in neutral position. We conducted all measurements with the same program, namely OsiriX Imaging Software with 3D Curved multiplanar reconstruction.
Anatomic study
Using CT imaging, we measured the height, width, depth, inclination, and version of the glenoid in millimeters and degrees, as appropriate. The distance between the underlying bone of glenoid articular surface, base of the coracoid process, lateral edge of the acromion, and humeral head were measured in millimeters. We also measured the surface area of the humeral head in square millimeters wherever applicable. For the CT imaging, we used 3-mm slices coronal and sagittal, and 2-mm slices axial.
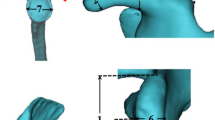
To identically position the respective parallel lines, we implemented the coordinate system 3D Curved MPR by defining x-, y- and z-axes. This allowed us to arrange the coordinates for all the measurements in both the coronal and transversal planes, as follows: using a best-fit circle the center of the glenoid was identified as zero point. The x-axis follows the zero point and passes through the scapular body and the y-axis is established along the borders of the glenoid parallel to the articular surface. Using a best-fit circle, the center of the glenoid was defined as midpoint of the articular surface in the coronal, transverse and sagittal planes. In the coronal plane, the z-axis was defined by the center of the glenoid and the body of the scapula. The center point of the glenoid was controlled by the sagittal plane (Fig. 1). The shape of the glenoid was classified in tear drop or oval shape in the sagittal plane.
3D MPR reconstruction by 2D scans, using a best-fit circle the center of the glenoid was identified as zero point; x-axis follows the zero point and goes through the scapular body and the y-axis is established along the borders of the glenoid parallel to the articular surface in the coronal (a), transverse (b) and sagittal planes (c)
The height of the glenoid was defined as the most cranial and caudal border of the glenoid in the coronal plane, while the most anterior and posterior border of the glenoid in the transversal plane represented the width (Figs. 2 and 3). Measuring the depth of the glenoid involves determining the distance between the lowest and highest points of the articular surface by the underlying bone.
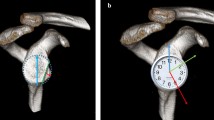
Degrees of version and inclination are determined by arranging the system of coordinates as follows: establishing the x-axis parallel to the scapula body in the transverse plane and the anterior–posterior plane, the angle is measured as the degrees between the y-axis and the parallel to the articular line of the glenoid (Fig. 4).
The inclination is measured in the anterior–posterior plane between the y-axis and a parallel to the articular line of the glenoid. We measured the distance from the articular surface of the glenoid to the base of the coracoid process in the anterior–posterior and transversal CT plane (Fig. 5) by running a line parallel to the y-axis through the base of the coracoid process and measuring its separation from the articular line of the glenoid in millimeters (GCF). The same steps were repeated in the transversal plane (GCS). Furthermore, we defined the distance between the articular surface of the glenoid and the lateral edge of the acromion and humeral head in millimeters (GA) by measuring the gap between the y-axis and a parallel line running through the lateral edge of the acromion and humeral head. In addition, we measured the distance between the coracoid base and lateral edge of the acromion in the anterior–posterior plane by aligning the y-axis with the base of the coracoid process and setting the zero point in the center of the glenoid articular surface. This allowed for measuring of the gap between the y-axis and the parallel line through the lateral edge of the acromion in millimeters (CA).
CT scans allowed, excluding the proximal humeral fractures, for approximation of the surface area of the humeral head, which we achieved by measuring the area in the transversal plane in square millimeters. A best-fit circle was used and matched to the greater tuberosity. All measurements were based on the three-dimensional coordinate system 3D Curved MPR corresponding to their 2D projections. The MPR is based on 2-mm axial slices in − 120.00-mm position. At all times, we oriented the coordinate system towards the center point of the glenoid articular surface.
Statistical analysis
The collected data were analyzed in the SPSS statistical program (Version 25.0.0.0, IBM, Armonk, NY, USA). Normal distributions between male and female subjects were monitored using the Kolmogorov–Smirnov test.
Correlations were identified via the Pearson test. The level of significance was set at a p value of < 0.05.
Due to the high number of tests conducted through the application of the Pearson test, we applied a Bonferroni correction to the level of significance. The interrater reliability was obtained via re-measurement of the CT images by a second observer. Both raters were blinded to the results of the other. Intra-rater-correlation was not measured.
Results
Radiographic outcome
We measured 131 shoulders to define the shape of the glenoid and 48.9% of the shoulders had the characteristic tear drop form, which means an oval glenoid shape with a slight indentation. The others just had an oval glenoid shape (Table 1). Table 2 presents the main radiographic measurements. The measurements of the glenoid articular surface revealed a mean height of 32.8 ± 3.7 mm (min. 23.7 mm, max. 44.6 mm) and a mean width of 26.4 ± 2.7 mm (min. 20.3 mm, max. 36.4 mm) with statistically significant gender-specific differences (r = 0.699; p < 0.05). The average measured height of the glenoid in our female participants amounted to 30.5 ± 2.2 mm, compared to 35.7 ± 3.1 mm in our male counterparts. Consequently, the glenoid appeared to be 3.67 ± 2.2 mm wider in men than in women (r = 0.662; p < 0.01).
We measured the mean glenoid depth as 3.2 ± 1.1 mm (min. 0 mm, max. 5.9 mm), with statistically significant measured differences between men and women (r = 0.408; p < 0.01): We found that the mean depth in women was 2.8 ± 0.9 mm, compared to 3.7 ± 0.9 mm in men. We could not observe any significant gender-specific differences in either angle inclination, with a mean value of 8.3 ± 6.08° (min. 0.08°, max. 25.9°) or degree of version, with a mean angle of 8.1 ± 6.3° (min. 0°, max. 26.3°).
The mean surface area of the humeral head measured 187.1 ± 29.9 mm2 in average (min. 128.3 mm2, max. 246 mm2), with significant differences between male and female subjects (r = 0.743; p < 0.01). Women were found to have a mean humeral head surface of 166.9 ± 20.2 mm2, whereas men were found to have a mean humeral head surface of 211.4 ± 18.8 mm2.
Regarding the space calculated between the glenoid articular surface and the base of the coracoid process, we conducted our measurements in two different spatial planes. We measured a mean distance of 6.5 ± 2.2 mm (min. 0 mm, max. 12 mm) in the transverse plane, compared to a mean separation by 16.9 ± 2.2 mm (min. 11.3 mm, max. 23.7 mm) in the frontal plane. Statistically significant differences between the sexes were not observed.
The mean distance between the glenoid articular surface and the lateral acromial edge measured 34.6 ± 5.4 mm (min. 18.7 mm, 45.6 mm), whereas the base of the coracoid process and lateral acromial edge were separated by 35.8 ± 6.0 mm (min. 17.6 mm, max. 47.8 mm).
The normal distribution of our values has shown no significant gender-specific difference. We did, however, measure significant differences in gender pertaining to the glenohumeral distance which is the gap between the greater tuberosity and glenoid articular surface. We observed a mean gap space of 49.7 ± 33 mm in female shoulders compared to 55.8 ± 3.0 mm in male shoulders. The overall average amounted to 52.9 ± 4.5 mm.
Statistical analysis
It can be seen as a general fact that the higher is the glenoid, the wider the glenoid (r = 0.646; p < 0.01) and the bigger the humeral head (r = 0.703; p < 0.01) and its separation from the glenoid articular surface (r = 0.684; p < 0.01). The distance between the humeral head and articular surface is the only parameter that correlates directly with the patient’s body height (r = 0.659; p < 0.01); other parameters do not correlate significantly with the patient’s body height. However, the patient’s gender is a deciding factor, and we illustrated that male subjects have a significantly larger humeral head and glenoid compared with female ones. Still, we could neither demonstrate a correlation of our measured angles with the gender of the patient, nor did we find evidence of the glenoid form affecting any of our measured parameters.
The degree of version correlates negatively with the distance between the base of the coracoid process and articular surface of the glenoid when viewed in the transverse plane (r = − 0.264; p < 0.01). Conversely, increasing inclination decreases the separation of the coracoid process base from the lateral edge of the acromion. Furthermore, we observed a positive correlation between the measured distance GA (r = 0.842; p < 0.01), size of the humeral head, and distance GH (r = 0.433; p < 0.01). GA and CA correlate negatively with the distance GCF. A high GA value corresponds with a high CA value (r = 0.642; p < 0.01). The measurement GA correlates positively with the height and width of the glenoid, along with the size of the head of the humeral and measured GH. In the first cycle of measurements, we already observed a sizeable variance in the degrees of version (36.91°), as well as inclination (40.11°), which is also reflected by the low interobserver reliability, with a Pearson correlation of –0.56.
Discussion
In case of glenoid bone loss, fixation and positioning of the baseplate of the glenoid component in the anatomical rotational center remains a challenge. Oftentimes, adding lateralization seems to be indicated; however, an anatomical reference to evaluate the needed amount is missing. There are only a few studies reporting on the clinical and radiological outcomes in this complex patient group [13, 15, 24, 26]. With the aging population and the growing number of reverse shoulder arthroplasties, the incidence of this shoulder pathology will increase. This underlines the importance for anatomical reference to reconstruct the anatomical rotational center.
Different causes can lead to glenoid defects: the most commonly encountered patterns are posterior erosion in glenohumeral osteoarthritis, superior erosion in rotator cuff tear arthropathy and anterior defects in a setting of chronic anterior dislocation. Furthermore, in the setting of revision shoulder arthroplasty central, peripheral and global defects can be encountered after component removal [14, 29]. Superior glenoid defects cause surgeons to place the baseplate too high in the glenoid, thereby exposing the arthroplasty to scapular notching and glenohumeral impingement [9]. Therefore, bone augmentation for glenoid defects in primary or revision shoulder arthroplasty are used daily [2, 4]. There are many studies describing the different types of augmentation [4, 13, 24]. But the optimal size of the bony augmentation corresponding to the glenoid defect on the CT scan is not well known. Our results suggest the distance between the base of the coracoid process and the articular surface of the glenoid as a reproducible parameter for describing glenoid anatomy in the setting of preoperatively planning. In case of a reduced measured distance, a lateralization of the rotational center should be discussed.
Therefore, the identification and careful evaluation of the glenoid bone defects seem to play a key role for a successful surgery. Failure to identify and correct posterior bone loss in the setting of a hypoplastic, biconcave or severely retroverted glenoid can result in an undesired retroversion of the baseplate. As a consequence, posterior scapular notching and posteromedial polyethylene wear can occur, associated with a reduction in the final external rotation [15, 17]. Recent studies have evaluated clinical results after bone grafting in revision surgery and reduced pain and increased mobility could be reported [2, 4, 15, 24].
In the case of a not appropriately reconstructed joint line, the center of rotation of the implant will be excessively medialized, resulting in a reduced deltoid tension with subsequently decreased flexion strength; furthermore, range of motion can be impaired and a higher risk for instability may develop [16, 17, 25].
For these reasons, numerous studies were designed to understand natural and pathological glenoid anatomy and to develop methods and techniques for appropriate component positioning to improve surgical results and prevent implant failure [2, 11, 28]. Of course, we are aware that implant systems exist especially designed to accommodate individuals using CT scans or the use of an intraoperative navigation in the fixation of the glenoid component. Actually, these systems are not commonly used and not very cost efficient. Nowadays, CT scans are frequently used to complete the radiological preoperative study prior to a shoulder replacement. It allows a precise characterization of glenoid morphology.
Our results suggest the base of the coracoid as an anatomical reference for glenoid reconstruction in the primary implantation and revision arthroplasty settings. With the coracoid process being unaffected by surgical procedures, the knowledge of this normal anatomical relations can be an adjunctive tool to guide the surgeon in challenging cases of glenoid bone defects. In case of a non-anatomical distance, it can be a helpful orientation for planning a bone graft. Another specific point of interest we discovered appears to be the patient’s gender, which is already well known [21]. We observed significant gender-specific differences in most of our measured parameters. The glenoid, for example, is significantly enlarged in male subjects, which in turn, proportionally influences the anatomical arrangement of the other structures in relation to each other. The gender-specific size of the glenoid is commonly reported. However, body height appears to have no influence on the size of the glenoid surface area, which means that tall individuals do not necessarily have a larger glenoid. However, the surface area of the humeral head seems to be directly proportional to the patient’s body height. This automatically leads to an increase of the distance between the articular surface of the glenoid and the greater tuberosity in tall patients. In these individuals, it would be important to investigate the influence of the lateralization of the glenoid component.
The limitations of the study are that the measurements were based on 3D multiplanar reconstruction corresponding on 2D scans. We are aware of numerous studies detailing how 3D measures and their corresponding 2D projections do not always yield the same conclusions in terms of anatomic reference. Moreover, the intra-rater reliability was not analyzed. With the intention to evaluate the accuracy of the CT-based measurements, a cadaveric study by our group will follow.
Conclusions
In conclusion, we can agree that pre- and intraoperative measuring techniques and their implications for the survival of reverse shoulder arthroplasty must be further established to meet the surgical demands of the current trend. Our results have established a distance of 6.5 ± 0.22 mm in the transverse CT plane between the articular surface of the glenoid and base of the coracoid process. The small variance in measurements as well as the high interobserver reliability (r = 0.72) further support the use of this reference point in preoperative planning.
References
Boileau P, Melis B, Duperron D, Moineau G, Rumian AP, Han Y (2013) Revision surgery of reverse shoulder arthroplasty. J Shoulder Elbow Surg 22(10):1359–1370. https://doi.org/10.1016/j.jse.2013.02.004
Pascal B, Nicolas M-S, Marc-Olivier G, Seeto BL, Chalmers PN, Nicolas H, Gilles W (2017) Angled BIO-RSA (bony-increased offset-reverse shoulder arthroplasty). A solution for the management of glenoid bone loss and erosion. J Shoulder Elbow Surg 26(12):S. 2133-2142. https://doi.org/10.1016/j.jse.2017.05.024
Deutsch A, Abboud JA, Kelly J, Mody M, Norris T, Ramsey ML et al (2007) Clinical results of revision shoulder arthroplasty for glenoid component loosening. J Shoulder Elbow Surg 16(6):706–716. https://doi.org/10.1016/j.jse.2007.01.007
Ernstbrunner L, Werthel J-D, Wagner E, Hatta T, Sperling JW, Cofield RH (2017) Glenoid bone grafting in primary reverse total shoulder arthroplasty. J Shoulder Elbow Surg 26(8):1441–1447. https://doi.org/10.1016/j.jse.2017.01.011
Farron A, Terrier A, Büchler P (2006) Risks of loosening of a prosthetic glenoid implanted in retroversion. J Shoulder Elbow Surg 15(4):521–526. https://doi.org/10.1016/j.jse.2005.10.003
Frankle MA, Teramoto A, Luo Z-P, Levy JC, Pupello D (2009) Glenoid morphology in reverse shoulder arthroplasty. Classification and surgical implications. J Shoulder Elbow Surg 18(6):S. 874-885. https://doi.org/10.1016/j.jse.2009.02.013
Gavaskar AS, Vijayraj K, Subramanian SM (2013) Intraoperative CT navigation for glenoid component fixation in reverse shoulder arthroplasty. Indian J Orthop 47:104–106
Grammont PM, Baulot E (2011) The classic. Delta shoulder prosthesis for rotator cuff rupture. Clin Orthop Related Res 469(9):S. 2424. https://doi.org/10.1007/s11999-011-1960-5
Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G (2006) Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Jt Surg Am 88(8):S. 1742-1747. https://doi.org/10.2106/JBJS.E.00851
Habermeyer P, Magosch P, Lichtenberg S (2006) Three dimensional glenoid deformity in patients with osteoarthritis. A radiographic analysis. J Bone Jt Surg Am 88:1301–1307
Henninger HB, Barg A, Anderson AE, Bachus KN, Burks RT, Tashjian RZ (2012) Effect of lateral offset center of rotation in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg 21(9):1128–1135. https://doi.org/10.1016/j.jse.2011.07.034
Hoenecke HR, Hermida JC, Flores-Hernandez C, D’Lima DD (2010) Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg 19(2):166–171. https://doi.org/10.1016/j.jse.2009.08.009
Jones RB, Wright TW, Roche CP (2015) Bone grafting the glenoid versus use of augmented glenoid baseplates with reverse shoulder arthroplasty. Bull Hosp Jt Dis 73(Suppl 1):S129–S135
Jean K (2012) Classifications of glenoid dysplasia, glenoid bone loss and glenoid loosening: a review of the literature. Eur J Orthop Surg Traumatol 23(3):301–310. https://doi.org/10.1007/s00590-012-1119-4
Klein SM, Dunning P, Mulieri P, Pupello D, Downes K, Frankle MA (2010) Effects of acquired glenoid bone defects on surgical technique and clinical outcomes in reverse shoulder arthroplasty. J Bone Jt Surg Am 92(5):1144–1154. https://doi.org/10.2106/JBJS.I.00778
Lädermann A, Williams MD, Melis B, Hoffmeyer P, Walch G (2009) Objective evaluation of lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg 18(4):588–595. https://doi.org/10.1016/j.jse.2009.03.012
Lévigne C, Boileau P, Favard L, Garaud P, Molé D, Sirveaux F, Walch G (2008) Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg 17(6):925–935. https://doi.org/10.1016/j.jse.2008.02.010
Lévigne C, Franceschi J (1999) Rheumatoid arthritis of the shoulder: radiological presentation and results of arthroplasty. In: Walch G, Boileau P (eds) Shoulder arthroplasty. Springer, Berlin, pp 221–230
Martin SD, Zurakowski D, Thornhill TS (2005) Uncemented glenoid component in total shoulder arthroplasty. Survivorship and outcomes. J Bone Jt Surg Am 87:1284–1292
Matsen FA III, Clinton J, Lynch J, Bertelsen A, Richardson ML (2008) Glenoid component failure in total shoulder arthroplasty. J Bone Jt Surg Am 90:885–896
Merrill A, Guzman K, Miller SL (2009) Gender differences in glenoid anatomy. An anatomic study. Surg Radiol Anat SRA 31(3):S. 183-189. https://doi.org/10.1007/s00276-008-0425-3
Müller AM, Born M, Jung C, Flury M, Kolling C, Schwyzer H-K, Audigé L (2018) Glenosphere size in reverse shoulder arthroplasty: is larger better for external rotation and abduction strength? J Shoulder Elbow Surg 27(1):44–52. https://doi.org/10.1016/j.jse.2017.06.002
Nashikkar PS, Scholes CJ, Haber MD (2019) Role of intraoperative navigation in the fixation of the glenoid component in reverse total shoulder arthroplasty: a clinical case-control study. J Bone Jt Surg Am 28(9):1685–1691. https://doi.org/10.1016/j.jse.2019.03.013
Neyton L, Boileau P, Nové-Josserand L, Edwards TB, Walch G (2007) Glenoid bone grafting with a reverse design prosthesis. J Shoulder Elbow Surg 16(3 Suppl):S71–S78. https://doi.org/10.1016/j.jse.2006.02.002
Schwartz DG, Kang SH, Lynch TS, Edwards S, Nuber G, Zhang L-Q, Saltzman M (2013) The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg 22(3):S. 357-364. https://doi.org/10.1016/j.jse.2012.02.002
Seidl AJ, Williams GR, Boileau P (2016) Challenges in reverse shoulder arthroplasty: addressing glenoid bone loss. Orthopedics. https://doi.org/10.3928/01477447-20160111-01
Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D (2004) Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Jt Surg Br 86(3):S. 388-395. https://doi.org/10.1302/0301-620x.86b3.14024
Verborgt O, De Smedt T, Vanhees M, Clockaerts S, Parizel PM, Van Glabbeek F (2011) Accuracy of placement of the glenoid component in reversed shoulder arthroplasty with and without navigation. J Shoulder Elbow Surg 20:21–26
Walch G, Badet R, Boulahia A, Khoury A (1999) Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty 14(6):756–760. https://doi.org/10.1016/s0883-5403(99)90232-2
Werner BS, Böhm D, Abdelkawi A, Gohlke F (2014) Glenoid bone grafting in reverse shoulder arthroplasty for long-standing anterior shoulder dislocation. J Shoulder Elbow Surg. https://doi.org/10.1016/j.jse.2014.02.017
Zumstein MA, Pinedo M, Old J, Boileau P (2011) Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty. A systematic review. J Shoulder Elbow Surg 20(1):S. 146-157. https://doi.org/10.1016/j.jse.2010.08.001
Funding
No grant or grant support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by our ethical committee, University of Bonn (AZ 125/13).
Informed consent
Informed consent was obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ott, N., Kieback, JD., Welle, K. et al. The base of coracoid process as a reference for glenoid reconstruction in primary or revision reverse shoulder arthroplasty: CT-based anatomical study. Arch Orthop Trauma Surg 142, 387–393 (2022). https://doi.org/10.1007/s00402-020-03642-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-020-03642-w