Abstract
Introduction
The standard treatment for an acute compartment syndrome (ACS) of the lower leg is a four compartment fasciotomy. It can be performed through either one lateral or a lateral and medial incision. Selective fasciotomy, only opening the compartments with elevated pressure, is a less invasive procedure. The aim of this study was to describe a procedure of selective fasciotomy after pressure measurement and to determine its feasibility in a retrospective cohort study.
Methods
All patients with an ACS of the lower leg due to a proximal or tibia shaft fracture (AO 41 or 42) who received either a four compartment fasciotomy or a selective fasciotomy after pressure measurement between 2006 and 2016 were included. Every compartment with an intracompartment pressure of more than 30 mmHg was opened. The primary outcome was any missed compartment syndrome after selective fasciotomy. Secondary outcomes were reoperations for completing four compartment fasciotomy and persistent sensomotoric deficits.
Results
Fifty-one patients with a mean age of 43 years (6–76) were included. Of these, 41 (80%) had a selective fasciotomy. There was no missed compartment syndrome. One patient had a reoperation 8 h after primary selective fasciotomy due to ACS of the superficial and deep flexor compartment. The anterior compartment had to be released in all patients. In 67%, the release of 2 compartments was sufficient. Six patients had postoperative sensomotoric deficits at discharge with full recovery during follow-up.
Conclusion
Selective fasciotomy is feasible and seems to be safe. Future comparative studies will have to focus on possible benefits of this less invasive treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The acute compartment syndrome (ACS) after trauma of the lower leg is a rare but well-known diagnosis. The trauma-related incidence of ACS of the lower limb ranges between 1 and 53% depending on the type of injury [1,2,3,4]. The highest rates are reported following a fracture dislocation of the knee (53%) and the lowest rates after distal tibia fractures (1%) [1,2,3,4]. The incidence of ACS in tibial shaft fractures is 4.3%, representing 35–40% of all incidences of ACS [5, 6].
The treatment for ACS is immediate surgical fasciotomy. Numerous clinical series have shown the efficacy of early fasciotomy and the potential complication of late fasciotomy [7,8,9,10]. Even if a surgeon is unsure about the diagnosis, it is generally accepted that performing an unnecessary fasciotomy is better than performing a fasciotomy too late with its severe complications [7, 11]. However, this might result in overtreatment [12,13,14]. On the other hand, overtreatment after pressure measurement has also been reported [15].
Performing a four compartment fasciotomy has a couple of consequences and possible complications. Performing a less invasive (selective) procedure hypothetically might lower these complications. Therefore, we started the procedure of selective fasciotomy in which only the compartments with a raised intracompartment pressure > 30 mmHg are released.
The aim of this study was to describe the procedure of selective fasciotomy after pressure measurement in patients with an ACS after a tibial fracture and to determine the feasibility of this procedure in a retrospective cohort study.
Materials and methods
Study design
A retrospective cohort study was performed at a level 1 trauma centre. Our prospective trauma database was searched for patients with a compartment syndrome resulting from a lower leg fracture (AO-41 and AO-42) [16]. All patients with either a four compartment fasciotomy or selective fasciotomy after pressure measurement between 2006 and 2016 were eligible for inclusion. Exclusion criteria were: admission more than 24 h after trauma, pathological fractures, violation of the measurement protocol and patients who denied informed consent. Every patient file, discharge letter and follow-up letter were searched for information regarding the compartment syndrome. This study was approved by the Cantonal Ethic Committee Zürich (KEK-ZH-Nr. 2017-00782).
Diagnosis
After being admitted to our emergency department, all patients were seen by our surgical staff. On clinical suspicion of a lower leg fracture, X-rays were performed. The acute compartment syndrome was diagnosed either clinically or by pressure measurement. During the clinical examination, suspected patients were searched for the typical symptoms of an acute compartment syndrome, like excessive pain, pain on palpation, pain with passive stretch, tenseness for each lower leg compartment as well as neurological or vascular peripheral compromise. The latter being a very (too) late symptom.
All patients with a suspicious or manifest compartment syndrome in clinical examination in combination with the existing tibia fracture were moved urgently to the operation room. In cases of a clinically severe compartment syndrome involving all compartments, a classical four compartment fasciotomy was performed without pressure measurement. Subsequently the fracture was stabilized, either temporarily with an external fixator or definitively with an appropriate implant. In cases of less severe and/or only suspected compartment syndrome (pre- or intraoperatively), a perioperative compartment pressure measurement was performed followed by a fasciotomy if necessary and fracture stabilization (or vice versa).
Compartment pressure measurement
The pressure measurement was performed either in the emergency room using the Stryker® pressure monitor system (Fig. 1) or intraoperatively with a thick needle (20 gauge) connected to the arterial pressure device of the anaesthetist (Figs. 2, 3, 4).
Stryker system
To prepare for a pressure measurement, a Quick Pressure monitor set (Stryker®) was connected to the pressure monitor system that was pre-installed. This system was flushed with saline and calibrated at 0 mmHg with the measuring device at the level of the compartment.
After insertion of the needle in the compartment that was to be measured, the needle was flushed with 0.3 ml saline from the connected syringe to expel any skin or debris out of the needle. One has to wait until the measured value stops changing and is stabilized. This can take up to 1 min.
Every compartment was measured separately. The entry point for the anterior compartment was the middle of the lower leg, 1–2 cm laterally from the tibial crest. The peroneal compartment was measured in the lateral quarter of the middle lower leg. The posterior superficial and deep compartments were measured from the (posterior) medial side of the middle lower leg.
Intraoperative measurement with the arterial catheter method (Figs. 2, 3 and 4)
Patients were admitted to the operation theatre and disinfected and draped for lower leg osteosynthesis as normal. After team time-out, or at any later point during the operation on suspicion, the pressure measurement was performed. A sterile 20-G needle was connected to a sterile line to the arterial pressure measuring device of the anaesthetist. This system was flushed with saline and calibrated at 0 mmHg with the measuring device at the level of the compartment. After insertion of the needle in the compartment that was to be measured, the needle was flushed shortly to expel any skin or debris out of the needle. One has to wait until the measured value stops changing and is stabilized. This can take up to 1 min. The insertion point of the needle was the same as described above.
Operative procedure selective fasciotomy
The fasciotomy can be performed either before or after stabilization of the fracture, depending on the moment of diagnosis of the ACS. The fractures were stabilized using a minimal invasive approach either by external fixation, intramedullary nailing or minimal invasive plate osteosynthesis (MIPO). After fracture stabilization, the surgeon palpated the compartments again and, on suspicion, measured the intracompartment pressure (ICP).
An ICP of > 30 mmHg was considered an ACS. After measuring all four compartments, the compartment with the highest ICP was relieved by selective fasciotomy. The anterior and peroneal compartments were approached through an anterolateral approach and the posterior superficial and deep compartments through a medial approach via a second incision. The fasciotomy for the anterior and peroneal compartments was performed through a 6–15-cm-long lateral skin incision starting four fingers below the head of the fibula and two fingers medial of the fibula shaft. The fascia was opened with a short transverse incision to visualize the intermuscular septum. Then the fascia of the anterior and/or the peroneal compartment was incised distally and proximally, partly subcutaneously, with a pair of scissors. The superficial and the deep posterior compartments were incised through a second medial skin incision of 6–15 cm. The medial approach begins four fingers distally to the medial tibia plateau and two fingers posteriorly of the medial tibia shaft border. After skin incision, the fascia of the posterior superficial compartment was incised distally and proximally subcutaneously with a pair of scissors. The deep compartment was approached through the intermuscular septum. The fascia of the deep posterior compartment was incised in the same way as the other three compartments. After fasciotomy of the compartment with the highest pressure, the other three compartments were measured again. If the ICP was < 30 mmHg after this selective fasciotomy, no further fasciotomy of the other compartments was performed. If a second compartment still had an ICP > 30 mmHg, it was opened accordingly with new measurements of the other compartments afterwards.
Skin defects after (selective) fasciotomy were temporarily closed either by VAC-seal (ActiV.A.C.® Therapy System, KCI Medical GmbH, Rümlang Switzerland) or Epigard® (Biovision GmbH, Ilmenau, Germany) followed by secondary adaptation and/or closure. If secondary closure was not possible, a split skin graft was performed.
Postoperative treatment
Postoperative, patients were observed either at the surgical ward or the intensive care unit. The affected leg was positioned horizontally. Pain, muscle function, the neurovascular status and the tenderness on palpation were determined and registered every 2 h for the following 24 h according to protocol. If in doubt, the treating surgeon or surgeon on call performed a clinical assessment and a bedside ICP measurement using the Stryker pressure monitor system.
Primary outcome
The primary outcome was any missed compartment syndrome after selective fasciotomy. A missed compartment syndrome was defined as muscular necrosis.
Secondary outcomes
Secondary outcomes were: reoperations for completing four compartment fasciotomy following selective fasciotomy, sensory or motoric deficits, lesions of the superficial peroneal nerve, the distribution of the different compartments that were relieved and hospitalization time.
A reoperation for completing four compartment fasciotomy was defined as any secondary operation for further (four compartment) fasciotomy after primary selective fasciotomy. Sensoric or motoric deficits were defined as any sensoric or motoric deficits of the lower leg, persistent or diminishing during or after the hospital admission. Lesions of the superficial peroneal nerve were defined as clinical neurologic deficits in its innervation area combined with a pathologic EMG of this nerve.
Statistical analysis
Data were described using frequencies and percentages for dichotomous and categorical variables, mean and standard deviation (SD) for normally distributed continuous data. Continuous variables were compared using the Mann–Whitney U test because of small proportion analysis. Categorical data were compared using Pearson’s Chi square test or Fisher’s exact test for increased accuracy in small proportion analysis. Significance of statistical differences was attributed to p < 0.05. p values < 0.10 were considered borderline due to small numbers and, therefore, discussed. The analyses were performed with SPSS, version 22.0 (IBM Corp., Armonk, NY) for Windows.
Results
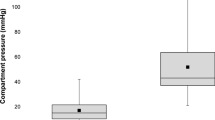
Fifty-one patients with 51 compartment syndromes were included (Fig. 5). Forty-one (80%) compartment syndromes were treated with selective fasciotomy, 10 (20%) with four compartment fasciotomy. The mean age was 43 years (SD 16.5, range 6–76). There was a near significant difference in age (p 0.052) between the group with selective fasciotomy and four compartment fasciotomy. Baseline characteristics are presented in Table 1.
In all selective fasciotomies (100%), the anterior compartment was involved and had to be opened. In 36 cases (88%), the peroneal compartment had to be opened as well. The superficial and deep posterior compartments had to be opened in only five cases (12%) and one case (2%), respectively. The approach in 35 cases was from lateral side. There was no approach from the medial side only. In six cases, the approach was from the medial and lateral side, still performing a selective fasciotomy. In these cases, the anterior compartment had to be opened from the lateral side and either the superficial or deep posterior compartment from the medial side (Table 1).
There was no missed compartment syndrome. One patient had a reoperation for completing a four compartment fasciotomy after initial selective fasciotomy. In the group of the selective fasciotomies, four patients had preoperatively developed sensomotoric deficits with prickling paresthesia in the foot. Six patients developed a diminishing sensomotoric deficit during the postoperative surveillance. After 1 year of follow-up, all patients with sensomotoric deficits had a complete recovery.
In the group of four compartment fasciotomies, one patient had a sensomotoric deficit preoperatively. This was completely reversible after 1 year of follow-up. There were no postoperative sensomotoric deficits in this group (Table 2). There was a trend towards a shorter hospitalization time (p 0.065) after selective fasciotomy.
Discussion
Selective fasciotomy of the lower leg can be an alternative to four compartment fasciotomy if combined with compartment pressure measurement. Our results demonstrate that selective fasciotomy for an acute compartment syndrome after lower leg fracture is feasible. In our series, using the described technique, this selective approach was successful in 80% of all patients without missing any compartment syndrome. Being at least equivalent to a four compartment fasciotomy in terms of safety a selective procedure might be considered beneficial for patients. We observed a wide variety in which compartment the ACS occurred. The anterior compartment was always involved, followed by the peroneal compartment in most cases. The posterior compartments were involved much more seldomly. In only one case did the initial selective fasciotomy have to be converted into a four compartment fasciotomy.
Using compartment pressure measurement intraoperatively on a routine basis enriches the personal experience of the individual surgeon in this technique and supports making the diagnosis in borderline cases. Moreover, pressure measurement is mandatory in all cases where clinical evaluation is not possible, for instance in anaesthetized patients or comatose patients on the intensive care unit. Routinely performed intraoperative compartment pressure measurement helps to identify compartment syndromes in patients who developed this pathology during the time interval between the clinical examination in the emergency department and the fracture stabilization in the operation room. During this delay, which can take several hours, a manifest compartment syndrome can easily arise and might be missed without intraoperative measurement.
Generally, the diagnosis of an ACS is made clinically. The typical clinical signs are excessive pain disproportionate to the severity of injury, paraesthesia, paralysis, palpable tightness and an increase of pain on passive stretch of the compartment involved [17]. However, the clinical presentation varies and not all the known signs are always present. Single clinical signs are known to have a low predictive value. Three or more of these clinical signs may raise the sensitivity. However, most of them, like paresis or paralysis, are late signs of the ACS [18, 19]. Making the diagnosis in patients who cannot give a clear history or participate in the clinical examination can be particularly difficult. This includes patients with additional neurological injuries, patients under general anaesthesia and patients in the intensive care unit [17].
Early diagnosis of ACS is important to avoid further impairments. Missing a compartment syndrome is devastating. A false-negative test or diagnosis is not acceptable. Therefore most surgeons, if in doubt, will perform a four compartment fasciotomy [11, 19]. Intracompartment pressure measurement has been described and suggested to aid in making the diagnosis of an ACS [20].
Several studies have presented different thresholds for making the diagnosis of an ACS measuring the ICP ranging from 30 to 45 mmHg [20, 21]. In our protocol, we have chosen 30 mmHg as the threshold. As discussed by Mubarak, a too low threshold may result in a false-positive test and causes a high rate of unnecessary fasciotomies and their related risks [20, 22]. Heppenstall et al. suggested the use of the CPP using the mean arterial blood pressure (MABP). They proved the importance of the perfusion pressure gradient between the arterial circulation and the compartment itself as the main factor in the establishment of muscle ischemia. In their experimental dog study, they discovered that the minimum perfusion pressure gradient between MABP and ICP (= CPP) is approximately 40 mmHg to meet the metabolic needs of the traumatized muscle [23, 24]. Taking all these studies into account, it is clear that there still is an ongoing debate about which cutoff value and which pressure has to be used. Other non-invasive techniques like biomarkers, magnetic resonance imaging, laser Doppler flowmetry or near-infrared spectroscopy are proposed but need further investigations to determine their value [17].
Many studies describe the four compartment fasciotomy as treatment for ACS [11, 19, 22, 25,26,27]. They showed the efficacy of early fasciotomy and the complications of late fasciotomy. The complication of an unnecessary fasciotomy compared to a missed and untreated ACS seems to be accepted. The potential disadvantages and complications of a fasciotomy are further surgery for delayed wound closure, pain and nerve injury, muscle weakness, chronic venous insufficiency and increased cost of care [7, 17, 19, 28].
The current golden-standard treatment of the lower leg ACS after fracture is the four compartment fasciotomy by dual- or single-incision technique [25, 29]. Bible et al. compared the single- vs. dual-incision technique with no difference in infection and nonunion rates [27]. As can be expected, they proved that there is an increased risk of infections and non-unions in lower leg fractures that needed a fasciotomy due to ACS. They assumed that the deep subcutaneous dissection to release all four compartments in the single-incision group keeps the risk of infection and non-unions on the same level. Blair et al. showed similar results in their retrospective cohort study [30]. Hypothetically, a less invasive technique like the selective fasciotomy could lower the incidence of these complications.
This study has several limitations that need to be addressed. First of all, the retrospective character of this study has its obvious drawbacks. Second, our sample size is too small for a statistical comparison between the selective and the four compartment fasciotomy groups. Furthermore, as some patients were primarily treated with a four compartment fasciotomy, there might have been a selection bias between these two groups due to severity of the injury. Additionally, our chosen ICP threshold of 30 mmHg for an ACS might have resulted in overtreatment.
To our knowledge, this is the first study to report on selective fasciotomy for ACS. We have shown that selective fasciotomy is feasible, and no compartment syndrome has been missed. The influence of selective fasciotomy for an ACS of the lower leg on the morbidity and complications is unclear and benefits are hypothetical. Further research should be performed comparing selective and four compartment fasciotomies to determine its value.
Conclusion
Selective fasciotomy in combination with pressure measurement for the ACS of the lower leg is safe and feasible. Future studies will have to define its value in the treatment of these injuries.
References
DeLee J, Stiehl J (1981) Open tibia fracture with compartment syndrome. Clin Orthop Relat Res 160:175–184
Blick SS, Brumback RJ, Poka A, Burgess AR, Ebraheim NA (1986) Compartment syndrome in open tibial fractures. J Bone Jt Surg Am 68(9):1348–1353. https://doi.org/10.2106/00004623-198668090-00007
Park S, Ahn J, Gee AO, Kuntz AF, Esterhai JL (2009) Compartment syndrome in tibial fractures. J Orthop Trauma 23(7):514–518. https://doi.org/10.1097/BOT.0b013e3181a2815a
Stark E, Stucken C, Trainer G, Tornetta P 3rd (2009) Compartment syndrome in Schatzker type VI plateau fractures and medial condylar fracture-dislocations treated with temporary external fixation. J Orthop Trauma 23(7):502–506. https://doi.org/10.1097/BOT.0b013e3181a18235
Elliott KG, Alan J (2003) Diagnosing acute compartment syndrome. J Bone Jt Surg Am 85(5):625–632
McQueen MM, Gaston P, Court-Brown CM (2000) Acute compartment syndrome. Who is at risk? J Bone Jt Surg Am 82(2):200–203
Hope MJ, McQueen MM (2004) Acute compartment syndrome in the absence of fracture. J Orthop Trauma 18(4):220–224
Rorabeck CH (1984) The treatment of compartment syndromes of the leg. J Bone Jt Surg Am 66(1):93–97
Matsen GSF (1976) Fasciotomy in the treatment of the acute compartment syndrome. J Bone Jt Surg Am 58(1):112–115
Lagerstrom CF, Reed RL II, Rowlands BJ, Fischer RP (1989) Early fasciotomy for acute clinically evident posttraumatic compartment syndrome. Am J Surg 158:36–39
Schmidt AH (2017) Acute compartment syndrome. Injury 48(Suppl 1):S22–S25. https://doi.org/10.1016/j.injury.2017.04.024
McQueen MM, Court-Brown CM (1996) Compartment monitoring in tibial fractures. The pressure threshold for decompression. J Bone Jt Surg Br 78(1):99–104
Ovre S, Hvaal K, Holm I, Strømsøe K, Nordsletten L, Skjeldal S (1998) Compartment pressure in nailed tibial fractures. A threshold of 30 mmHg for decompression gives 29% fasciotomies. Arch Orthop Trauma Surg 118(1–2):29–31
Schmidt AH (2013) Continuous Compartment Pressure Monitoring—Better Than Clinical Assessment?: commentary on an article by Margaret M. McQueen, MD, FRCSEd(Orth), et al. J Bone Jt Surg Am 95(8):e52
Janzing HMJ, Broos PLO (2001) Routine monitoring of compartment pressure in patients with tibial fractures: beware of overtreatment! Injury 32(5):415–421
Marsh JL, Slongo TF, Agel J, Broderick JS, Creevey W, DeCoster TA, Prokuski L, Sirkin MS, Ziran B, Henley B, Audigé L (2007) Fracture and dislocation classification compendium—2007: orthopaedic trauma association classification, database and outcomes committee. J Orthop Trauma 21(10 Suppl):S1–133
Shadgan B, Menon M, O'Brien PJ, Reid WD (2008) Diagnostic techniques in acute compartment syndrome of the leg. J Orthop Trauma 22(8):581–587. https://doi.org/10.1097/BOT.0b013e318183136d
Ulmer T (2002) The clinical diagnosis of compartment syndrome of the lower leg: are clinical findings predictive of the disorder? J Orthop Trauma 16(8):572–577
Schmidt AH (2016) Acute compartment syndrome. Orthop Clin N Am 47(3):517–525. https://doi.org/10.1016/j.ocl.2016.02.001
Whitesides TE, Haney TC, Morimoto K, Harada H (1975) Tissue pressure measurements as a determinant for the need of fasciotomy. Clin Orthop Relat Res 113:43–51
Mubarak SJ, Owen CA, Hargens AR, Garetto LP, Akeson WH (1978) Acute compartment syndromes: diagnosis and treatment with the aid of the wick catheter. J Bone Jt Surg Am 60(8):1091–1095
Matsen FA 3rd, Winquist RA, Krugmire RB Jr (1980) Diagnosis and management of compartmental syndromes. J Bone Jt Surg Am 62(2):286–291
Heppenstall RB, Shenton DW, Chance B, Hazelgrove J (1984) Compartment syndrome: a bioenergetic study using 31P-NMR spectroscopy. Orthop Res 67:330
Heppenstall RB, Sapega AA, Scott R, Shenton D, Park YS, Maris J, Chance B (1988) The compartment syndrome. An experimental and clinical study of muscular energy metabolism using phosphorus nuclear magnetic resonance spectroscopy. Clin Orthop Relat Res 226:138–155
Mubarak SJ, Owen CA (1977) Double-incision fasciotomy of the leg for decompression in compartment syndromes. J Bone Jt Surg Am 59(2):184–187
Masquelet AC (2010) Acute compartment syndrome of the leg: pressure measurement and fasciotomy. Orthop Traumatol Surg Res 96(8):913–917. https://doi.org/10.1016/j.otsr.2010.08.001
Bible JE, McClure DJ, Mir HR (2013) Analysis of single-incision versus dual-incision fasciotomy for tibial fractures with acute compartment syndrome. J Orthop Trauma 27(11):607–611. https://doi.org/10.1097/BOT.0b013e318291f284
Ritenour AE, Dorlac WC, Fang R, Woods T, Jenkins DH, Flaherty SF, Wade CE, Holcomb JB (2008) Complications after fasciotomy revision and delayed compartment release in combat patients. J Trauma 64(2 Suppl):S153–S161. https://doi.org/10.1097/TA.0b013e3181607750 152)
Maheshwari R, Taitsman LA, Barei DP (2008) Single-incision fasciotomy for compartmental syndrome of the leg in patients with diaphyseal tibial fractures. J Orthop Trauma 22(10):723–730. https://doi.org/10.1097/BOT.0b013e31818e43f9
Blair JA, Stoops TK, Doarn MC, Kemper D, Erdogan M, Griffing R, Sagi HC (2016) Infection and nonunion after fasciotomy for compartment syndrome associated with tibia fractures: a matched cohort comparison. J Orthop Trauma 30(7):392–396. https://doi.org/10.1097/Bot.0000000000000570
Acknowledgements
The authors thank Michelle Reynolds for the excellent copy-editing of this manuscript.
Funding
There was no external source of funding for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Basil Hatz, Herman Frima and Christoph Sommer declare that they have no conflict of interest.
Ethical approval
All the procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hatz, B.A., Frima, H. & Sommer, C. Selective fasciotomy for acute traumatic lower leg compartment syndrome: is it feasible?. Arch Orthop Trauma Surg 139, 1755–1762 (2019). https://doi.org/10.1007/s00402-019-03260-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-019-03260-1