Abstract
Introduction
Magnetic resonance imaging (MRI) is the radiological modality of choice for diagnosing pathological fractures in situations of diagnostic uncertainty. With the increasing availability of MRI, we have observed a disturbing trend in utilising routine MRI scans to exclude pathological fractures in all patients with a history of cancer. The study objective was to determine if routine use of MRI scans in such patients is truly necessary and if other predictive factors can be utilised in lieu of the MRI scan.
Materials and methods
A 3-year retrospective study was conducted reviewing all extremity MRI scans performed for suspected pathological fractures and compared to X-rays. All patients presented with an extremity fracture, a known diagnosis of solid organ cancer and had an MRI to determine if the fracture was pathological. Subjects were followed up with serial X-rays up to 1 year.
Results
84 subjects were recruited. Comparing X-rays alone with MRI scans revealed 92% sensitivity and 98% specificity in detecting pathological fractures. Using X-rays in combination with an absent history of trauma increases the sensitivity to 100% but reduced the specificity to 91%. None of subjects in cancer remission had pathological fractures.
Conclusions
MRI is an imperative tool for operative planning in pathological fractures; however, we recommend against the routine use of MRI to diagnose pathological fractures in oncological patients. Patients with solid organ cancer remission, a positive history of significant trauma prior to sustaining the fracture, and the absence of pathological features on plain radiographs are strongly predictive against pathological fractures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metastatic carcinoma is the most common malignancy treated by orthopaedic surgeons [1]. Approximately 50% of all primary cancers tend to disseminate to the skeleton, the third most frequent site of metastases after the lung and liver [2]. Tumours which have a predilection for bony metastasis include prostate, breast, kidney, lung and thyroid in order of frequency [2]. Although the axial skeleton is the most common site for skeletal metastases, most pathological fractures arise in the long bone of the appendicular skeleton with the vast majority of cases affecting the femur [3]. 9–29% of patients who suffer from bony metastases develop pathological fractures [4]. Skeletal metastases and pathological fractures are seldom a cause of cancer mortality but result in considerable morbidity. As the treatment of primary tumours improves, longer survival has been reported after the diagnosis of bone metastases. A multidisciplinary team with proper orthopaedic care is crucial of many of these patients to improve their overall quality of life by minimising pain and maintaining function and independence. It is, therefore, crucial in the early setting to diagnose and differentiate pathological fractures from non-pathological fractures.
MRI is highly sensitive to the presence of skeletal metastases. Metastatic lesions usually are brighter than normal marrow on T2-weighted MRI scans and appear as focal areas of low signal intensity on T1-weighted MRI scans due to the significantly higher water content in metastatic lesions compared to bone marrow [5]. Furthermore, MRI can detect bony metastases that are not apparent on radioisotope bone scans [6].
With the increasing availability of MRI, we have observed a disturbing trend of utilising routine MRI scans to exclude pathological fractures in all patients with a history of cancer. The role of MRI scans in pathological fractures in the proximal femur has classically been for pre-operative planning and diagnosis in situations of diagnostic uncertainty. However, is there any value in extending the classical indication of MRI to include it as a routine investigation for diagnosing pathological fractures in all patients with cancer?
As such, the study objective was to determine if routine use of MRI scans in patients with cancer is truly necessary and if other predictive factors can be utilised in lieu of the MRI scan.
Materials and methods
We designed a retrospective 3-year cohort study. All subjects with a known solid organ malignancy who underwent an MRI to investigate for a pathological fracture of the extremity were included. This included any anatomical region including and distal to the shoulder and pelvis.
The inclusion criteria were as follows: subjects would have to (1) have a fracture of the extremity which includes any bone distal to the pelvis or shoulder girdle, (2) a history of solid organ malignancy and (3) an MRI to determine if the fracture was pathological or non-pathological. Subjects who had evidence of metastasis without a fracture, isolated spinal fracture or non-solid organ malignancies were excluded from this study. Non-solid organ malignancies including leukaemia and lymphoma were excluded as they have a different definition of remission and natural history.
Information relating to demographic data, a history of significant trauma, ambulatory status, and history of the malignancy including type, staging, grading, remission status and type of treatment were reviewed. X-ray films of each subject prior to MRI were also reviewed by two orthopaedic surgeons who were blinded to the diagnosis. Remission was defined as the absence of clinical, radiological and biochemical evidence of malignancy for 5 years. Pathological fractures were defined as fractures secondary to metastasis. Stress fractures and insufficiency fractures were not defined as pathological for the purpose of this study.
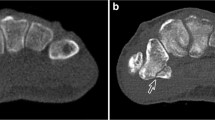
Features of pathological fractures on X-ray include a lucent fracture line with sclerosis, aggressive bone marrow pattern of destruction, mineralised matrix, endosteal scalloping, aggressive periosteal reaction, soft tissue mass, lytic or sclerotic lesions at the fracture site (Fig. 1) [3]. Any of the above features if noted on X-ray was deemed to be suspicious of a pathological fracture. Features of pathological fractures on MRI include muscle oedema, endosteal scalloping, periosteal signal abnormality and well-defined T1-bone marrow abnormality and the MRIs were read and interpreted by a consultant radiologist (Fig. 1) [3]. Subjects deemed to have non-pathological fractures were followed up with serial X-rays up to 1 year.
Statistical analyses were performed using SPSS version 19.0 software (SPSS Inc., Chicago, IL, USA). Cohen’s Kappa was used to determine if there was level of agreement between X-ray and MRI for detecting pathological fractures. Agreement was considered as poor if < 0.00, slight if between 0.00 and 0.20, fair if between 0.21 and 0.40, moderate if between 0.41 and 0.60, substantial if between 0.61 and 0.80 and almost perfect if > 0.80. Sensitivity and specificity of X-ray were also presented with MRI as the golden standard. The correlation between the status of cancer remission and pathological fractures was analysed using Chi-square test. A p value < 0.05 was considered to be statistically significant.
Results
Between 2012 and 2014, 84 subjects were recruited. 24 subjects had pathological fractures based on MRI findings (Fig. 2). The five most common cancers which were associated with pathological fractures were breast, lung, prostate, renal and colorectal cancer (Fig. 3). 17% of subjects with pathological fractures had cancer of unknown origin either because of a refusal of further investigation of the malignancy or demise without post-mortem.
When comparing X-rays to MRI in detecting pathological fractures, we found that X-rays alone were 92% sensitive and 98% specific. The strength of agreement (κ) was found to be 0.9114 (95% CI 0.813–1.000; p < 0.001) making X-rays alone statistically very reliable in determining pathological fracture when compared to MRI (Table 1).
In a subgroup analysis of subjects with no history of trauma prior to their fracture, we found that X-rays had an increased sensitivity of 100% but a reduced specificity to 91%. The Kappa analysis increased to 0.9222 in this subgroup analysis (Table 1).
Of the 20 subjects in remission, 19 of them had a non-pathological fracture (Table 2). One subject who was in remission and developed a pathological fracture on MRI was a patient with known fibrous histiocytoma 7 years prior to this study and subsequently underwent resection of his tumour with adjuvant radiotherapy. His X-rays and MRI revealed changes consistent with pathological fractures and he underwent surgical fixation of the fracture with intra-operative biopsy. Histological findings revealed no malignancy and his fracture was attributed to radiation necrosis and not tumour relapse. There were no pathological fractures in patients in cancer remission.
At 1-year follow-up for subjects with non-pathological fracture, 60% showed no evidence of pathological fractures or recurrence. 28.3% died, and 11.7% were lost during follow-up.
Discussion
Conventional radiographs vs MRI
This study reveals that X-rays alone are effective in detecting pathological fractures with 92% sensitivity and 98% specificity when compared to MRI. This is not a surprising finding as it is well established that close to 10% of pathological fractures are not confidently detected by plain radiographs alone [7, 8]. MRIs are highly sensitive to the presence of skeletal metastases [5]. A well-defined low-signal T1-weighted abnormality around a fracture is highly suggestive of an underlying tumour [7, 8]. Fatty marrow replacement, massive muscle oedema, a soft tissue mass and endosteal scalloping are also signs on MRI suggestive of pathological fractures [7, 8]. With greater anatomical delineation, it is not surprising that the MRI is better than conventional radiograph alone.
Conventional radiographs and trauma vs MRI
75% of pathological fractures occur with trivial or no trauma [9]. Hu et al. reported in a study of 139 patients that pathological fractures tend to occur either spontaneously (22.2%) or during functional activities including moving a chair or carrying a basin (52.8%) in the absence of significant trauma [9]. We have found that interpretation of conventional radiographs in subjects with fractures in the absence of trauma is as effective as MRI, with 100% sensitivity, in diagnosing pathological fractures.
The specificity of conventional radiography in this subgroup of subjects with no trauma was 91%. One possibility for the reduction in specificity is the fact that most of these subjects had severe osteoporosis with loss of trabecular pattern on hip radiographs and increased bony lucency making it more difficult to differentiate pathological and non-pathological fractures based on radiography [10]. It is in these situations of atraumatic fractures with diagnostic uncertainty that the threshold for an MRI should be lower.
Conventional radiograph and cancer remission vs MRI
A known history of malignancy also provides a valuable diagnostic clue. Recent weight loss, generalised malaise, appetite loss are red flags suggestive of active malignancy. For patients who have suspected metastases of unknown origin, however, the most common primary malignancies are in the lung or kidney [11]. It is imperative to determine whether the patient has active cancer or cancer in remission. This study suggests that subjects in solid organ cancer remission have a very low risk of sustaining tumour-related pathological fractures. In addition, 20 subjects were in cancer remission and none developed a pathological fracture secondary to tumour recurrence with bony metastasis. One subject who was in cancer remission and had a clear history of trauma was thought to have a pathological fracture of the femoral neck based on radiography alone. However, further evaluation on MRI revealed a non-pathological fracture.
Accuracy of MRI
MRI is thought to be 93–98% sensitive when compared to actual histological diagnosis of pathological fractures [7]. One subject in this study was thought to have a cancer-related pathological fracture on MRI but instead was finally diagnosed with radiation-related pathological stress fracture based on intra-operative histological findings. Final histology revealed no cancer recurrence at the fracture site. Wedin reported a 10% increased risk of stress fracture or non-union in patients with postoperative radiotherapy as we have seen in this case above [12]. A significant drawback of using MRI in pathological fractures is the fact that it can be difficult to distinguish changes in tumour from the effects of treatment, fracture and inflammation [5]. In one study, MRI scans were compared with histological specimens at 21 sites and only 7 of these contained tumour. 14 sites which were free of tumour had a significant false-positive scan on MRI (presumably due to the effects of treatment) [13]. Although this was not investigated in the study, it should be considered especially when using MRI to diagnose pathological fractures.
The present study has several strengths. The results were analysed using Cohen’s kappa strength of agreement instead of percentage agreement to compare qualitatively the agreement between MRI and X-ray which was high in this study. It also allowed sensitivity and specificity calculations of X-ray in comparison to MRI and a subgroup analysis to identify predictive factors that can be utilised in lieu of the MRI scan.
Several limitations are noted in our study. Retrospective data collection and analysis were performed which may have contributed to selection bias. Our study included a small sample size of 20 subjects with pathological fracture over a 3-year period. Subgroup analysis of subjects with specific cancer types would be of additional benefit but was not performed in view of the small sample size. Correlation of MRI findings with histological diagnosis would have enhanced the strength of this study. However, this was not performed as most subjects with a pathological fracture underwent reamed intramedullary fixation of a known metastatic disease and no open biopsy of the fracture site was performed. Bone reamings would not have been an accurate surrogate for an open biopsy. In the 1-year follow-up of subjects with non-pathological fracture, there were 11.7% lost to follow-up.
Conclusion
MRI is an imperative tool for operative planning in pathological fractures; however, we recommend against the routine use of MRI to diagnose pathological fractures in oncological patients. Patients with solid organ cancer remission, a positive history of significant trauma prior to sustaining the fracture, and the absence of pathological features on plain radiographs are strongly predictive against pathological fractures.
References
Heck RKJ (2013) Malignant tumors of bone. In: Canale ST, Beaty JH (eds) Campbell’s operative orthopaedics, 12th edn. Elsevier, Philadelphia
Hage WD, Aboulafia AJ, Aboulafia DM (2000) Incidence, location and diagnostic evaluation of metastatic bone disease. Orthop Clin N Am 31:515–528
Clain A (1965) Secondary malignant disease of bone. Br J Cancer 19:15–29
Tsuzuki S, Park SH, Eber MR et al (2016) Skeletal complications in cancer patients with bone metastases. Int J Urol 23:825–832
Rybak LD, Rosenthal DI (2001) Radiological imaging for the diagnosis of bone metastases. Q J Nucl Med 45:53–64
Kattapuram SV, Khurana JS, Scott JA et al (1990) Negative scintigraphy with positive magnetic resonance imaging in bone metastasis. Skelet Radiol 19:113–116
Fayad LM, Kawamoto S, Ihab R et al (2005) Distinction of long bone stress fractures from pathologic fractures on cross-sectional imaging: how successful are we? Am J Roentgenol 185:915–924
Fayad LM, Kamel IR, Kawamoto S et al (2005) Distinguishing stress fractures from pathological fracture: a multimodality approach. Skelet Radiol 34:245–259
Hu YC, Lun DX, Wang H (2012) Clinical features of neoplastic pathological fracture in long bones. Chin Med J (Engl) 125(17):3127–3132
Buckwalter JA, Brandser EA (1997) Stress and insufficiency fractures. Am Fam Physician 56:175–182
Toy PC, Heck RKJ (2013) General principles of tumors. In: Canale ST, Beaty JH (eds) Campbell’s operative orthopaedics, 12th edn. Elsevier, Philadelphia
Wedin R (2001) Surgical treatment for pathologic fracture. Acta Orthop Scand 72(4):1–29
Hanna SL, Fletcher BD, Fairclough DL, Jenkins JH 3rd, Le AH (1991) Magnetic resonance imaging of disseminated bone marrow disease in patients treated for malignancy. Skelet Radiol 20:79–84
Funding
There is no funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article received ethical approval and is in accordance with the ethical standards of the institutional research board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Seng, D.W.R., Kwek, E.B.K. Is routine MRI necessary to exclude pathological fractures in patients with an oncological history?. Arch Orthop Trauma Surg 138, 1633–1637 (2018). https://doi.org/10.1007/s00402-018-3012-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-018-3012-z