Abstract
Introduction
Numerous publications are dealing with acute Achilles tendon rupture. To our knowledge, no systematic trial has been published analyzing the incidence, risk factors and the potential clinical impact of postoperative tendon calcifications (PTC) after percutaneous Achilles tendon repair. Therefore, the aim of this study was to analyze these relevant aspects.
Materials and methods
Between March 2003 and November 2010, a total of 126 patients with an acute, complete Achilles tendon rupture were treated with a percutaneous technique according to Ma and Griffith at a single university-based trauma department. The follow-up included a detailed clinical and sonographic examination. To assess the functional outcome and possible impact of PTC after percutaneous Achilles tendon repair, the Thermann and AOFAS scores were used. 81 patients (65 men and 16 women) with a median age of 46 years (range 24–76) were available for a follow-up examination. The median time of follow-up was 64 months (range 15–110 months).
Results
PTC occurred in nine out of 81 patients (11.1%). All patients with PTC were male with a median age of 52 years (range 26–76 years). In the group of patients with PTC, the median overall Thermann score was 94 (range 68–100) and the median overall AOFAS score was 97 (range 85–100). In the group of patients without PTC, the median overall Thermann score was 88.5 (range 60–100) and the median overall AOFAS score was 97 (range 85–100). No significant differences were detected between the group of patients with PTC and the group of patients without PTC and the clinical outcome according to the Thermann (p = 0.21) and the AOFAS scores (p = 0.37). None of the patients with PTC sustained a re-rupture. The overall re-rupture rate was 4.9%. PTC was no risk factor for wound and neurological complications.
Conclusion
The incidence of PTC after percutaneous Achilles tendon repair was 11.1%. Male gender and advanced age seem to be risk factors for PTC. In this study, PTC had no negative impact on the postoperative clinical outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rupture of the Achilles tendon is a common injury in active individuals. In general, it occurs in male patients between the third and fifth decade participating in recreational sports. The treatment of acute Achilles tendon rupture is still discussed controversially [1,2,3,4,5,6,7,8,9,10]. With operative treatment, lower re-rupture rates can be achieved compared to conservative treatment, but it is associated with higher risks of other complications such as wound infections [3, 5, 6].
Since Ma and Griffith [11] first described a percutaneous surgical technique for the Achilles tendon repair in 1977 several study groups have investigated the benefits of it and modified the original technique. In contrast to open repair of the Achilles tendon, percutaneous procedures have a lower complication rate. However, the main complications are higher re-rupture rates compared to open procedures and sural nerve injury [12,13,14].
In a previous study, Bleakney et al. [15] described postoperative tendon calcification (PTC) in 14% after percutaneous Achilles tendon repair. However, the authors did not analyze the correlation between PTC and the postoperative functional outcome. Kraus et al. [16] assessed the effect of PTC after open repair of the Achilles tendon in 36 patients and demonstrated that patients with PTC had a poor functional outcome.
To our knowledge, no study has yet investigated the effect of PTC after percutaneous Achilles tendon repair on the functional outcome. The objective of the present study was to assess the incidence of PTC and their relationship to the postoperative clinical outcome. Furthermore, this systematic analysis included a search for potential risk factors that lead to PTC after percutaneous Achilles tendon repair.
Materials and methods
Between March 2003 and November 2010, a total of 126 patients (108 men, 18 women) with a complete Achilles tendon rupture have been treated at a single university-based trauma department with a percutaneous technique according to Ma and Griffith. The study population was selected on the basis of all medical charts and documentation. All patients were enrolled consecutively.
The following patient exclusion criteria (see Table 1) were used: partial Achilles tendon lesion, previous Achilles tendon disease, Achilles tendon calcification in pre-operative ultrasound examination and missing documented pre-operative ultrasound examination.
The initial clinical routine diagnosis of complete Achilles tendon rupture was made on the basis of history and clinical examination (Thompson sign, hyperdorsiflexion sign, palpable tendon gap). The diagnosis was confirmed by sonographic examination (Siemens SonoLine 13.5 MHz linear head) in all patients in order to determine tendon approximation via plantar flexion. A lateral radiograph of the ankle was performed to exclude coincident bony avulsion injuries and calcifications.
In the complete study population (n = 126), the majority of ruptures were sustained during sporting activities (n = 92, 73%) and a small proportion occurred during other activities (n = 34, 27%). The median time between trauma and operation was 5 days (range 0–17 days). The median duration of the operation was 18 min (range 6–39 min, missing data in four cases). The median time of hospitalization was 4 days (range 0–16, missing data in one case).
81 patients were available for follow-up examination including 65 men and 16 women with a median age of 46 years at the time of injury (range 24–76 years). The median time of follow-up was 64 months (range 15–110 months).
Operative treatment
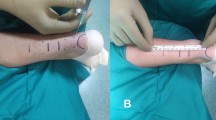
All operations were performed in the presence of a senior attending surgeon or by themselves in a percutaneous technique according to Ma and Griffith [11]. The operation was done under general anesthesia in prone position without a tourniquet. Two 2 cm longitudinal, dorsi-medial and dorsi-lateral incisions were made at a distance of approximately 3–4 cm cranial of the palpable defect that marks the tendon rupture while the lateral incision had a strong topographic relation to the sural nerve. The lower leg fascia was dissected without opening the peritendon. A medial and a lateral incision marginal above the footprint of the Achilles tendon were performed to reconstruct the ruptured Achilles tendon using a PDS cord (0.7 mm, Ethicon GmbH, Norderstedt, Germany).
Rehabilitation program
The patient’s foot was immobilized in a functional boot with a 15° heel wedge (VACOped, OPED, Oberlaindern, Germany) for 4 weeks. Afterwards, the heel wedge was removed so that the foot was placed in neutral position for another 2 weeks allowing simultaneous increased weight bearing. After 6 weeks, the functional boot was removed. A 1 cm heel lift for another 12 weeks was used to reduce Achilles tendon tension. As a part of the standardized aftercare all patients received diclofenac as pain medication for at least 7 days.
Measurement of the clinical outcome
The clinical outcome after Achilles tendon repair was assessed with the Thermann score and the Ankle Hindfoot Scale developed by the American Foot and Ankle Society (AOFAS). In addition, we documented postoperative wound complications, neurological complications, re-ruptures, the presence of PTC, and internal disease if present.
The Achilles tendon was examined at the last follow-up by ultrasound including the entire tendon from the calcaneus to the muscular transition of the triceps surae muscle in two planes. Calcifications were identified as hyperechogenic structures with acoustical shadows and their largest diameter was measured. In case of more than one PTC lesion within one Achilles tendon, we used the largest one for the calculation of the median PTC size within the complete group of PTC-positive patients. Additionally, the Achilles tendon diameter was measured in both planes 3 cm above the footprint on each side according to previous publications [15]. Figure 1 shows an example of a calcification of the Achilles tendon in ultrasonography.
Statistical analysis
The study population was divided into two groups. Patients with PTC were enrolled into group I, and patients without PTC were enrolled into group II. Measured data was transferred into the software package JMP (SAS Institute Inc., JMP, Version 12.2.0, Cary, NC, USA) to perform statistical analysis. Data were screened for normality of distribution applying Shapiro–Wilk W test. Descriptive statistics were carried out to calculate the medians and ranges. Wilcoxon test was used to identify significant differences between the group of patients with PTC and the group of patients without PTC according to the Thermann and the AOFAS score. A p < 0.05 was considered statistically significant.
Results
Nine of 81 patients who were available for follow-up examination developed PTC (11.1%). Three patients had one PTC lesion, two patients had two PTC lesions and one patient had three PTC lesions. In three PTC-positive patients, the size measurement was missing. The patients with PTC (n = 9) were enrolled into group I. All patients with PTC were male with a median age of 52 years (range 26–76 years). The size measurements revealed calcifications with a median size of 5.3 mm (range 2.0–14.5 mm). Three patients with PTC suffered from hyperuricemia (33%), three from hypercholesterinemia (33%), two from arterial hypertension (22%), one from type two diabetes mellitus (11%), one from coronary heart disease (11%), one from carotid artery stenosis (11%) and one patient had a stroke in the medical history (11%). The patients were assessed positive for hyperuricemia and hypercholesterinemia when they were under medication for these diseases. Table 2 shows the patients’ characteristics of the overall study population as well as of the subgroups. In group I, the median subjective Thermann score was 41 (range 32–45), the median objective Thermann score was 53 (range 36–55) and the median overall Thermann score was 94 (range 68–100). The median overall AOFAS score was 97 (range 85–100).
Group II included 72 patients without any signs of PTC (56 men, 16 women). The median age of the patients was 46 years (range 24–70 years). The most common internal diseases within this group were hypercholesterinemia (ten patients, 14%), hyperuricemia (seven patients, 10%), arterial hypertension (five patients, 7%) and type two diabetes mellitus (four patients, 6%). Within this group, the median subjective Thermann score was 41 (range 32–45), the median objective Thermann score was 47 (range 28–55) and the median overall Thermann score was 88.5 (range 60–100). The median overall AOFAS score was 97 (range 85–100). Tables 3 and 4 show the clinical results according to the Thermann score and the AOFAS score with absolute frequencies of group I and group II.
Significant differences were not detected between group I (with PTC) and group II (without PTC) according to the median subjective Thermann score (p = 0.91), the median objective Thermann score (p = 0.19) and the median overall Thermann score (p = 0.21), as well as the median overall AOFAS score (p = 0.37) (see Tables 5, 6).
Wound complications after percutaneous Achilles tendon repair such as infection, suture granuloma, hematoma and wound dehiscence occurred in nine patients within the patient population available for follow-up (n = 81, 0 in group I vs. 9 in group II). At the time of follow-up, 13 patients reported to have dysesthesia in the sural nerve area of the operated foot (1 in group I vs. 12 in group II). Re-ruptures occurred in four patients in the population available for follow-up. None of them had PTC. Table 7 shows the frequency of postoperative complications within both groups.
Discussion
The treatment options for Achilles tendon rupture consist of conservative treatment, open repair with or without augmentation, and percutaneous minimal invasive repair. To our best knowledge, this is the first study investigating the incidence and impact of PTC after percutaneous Achilles tendon repair including the analysis of risk factors. Our study revealed a PTC incidence of 11.1% with a standardized postoperative aftercare and medication. All patients with PTC were male with a median age of 52 years. Hyperuricemia and hypercholesterinemia were more common within group I (33 vs. 10%, 33 vs. 14%).
The results suggest a correlation between PTC and male gender as well as with advanced age. In addition, the present study highlights hyperuricemia and hypercholesterinemia as potential risk factors for PTC. Studies correlating PTC with internal diseases after percutaneous repair are still unavailable, so that further investigations are necessary.
The incidence of PTC in the present study was similar to the published results of Bleakney et al. [15]. They found tendon calcification in ten of 70 patients with a rupture of the Achilles tendon. In this study the patients had been treated conservatively, with open or percutaneous repair. Kraus et al. reported a rate of 28% of peri- and intratendinous calcifications after open Achilles tendon repair within a study population of 36 patients. Further, they found that patients with peri- and intratendinous calcifications lager than 10 mm had a poor functional outcome. An analysis of 104 patients who had open Achilles tendon repair by Ateschrang et al. found PTC in 14.4% of the patients. According to the Thermann score, negative effects were not detected on the clinical outcome [17]. In a study with 101 patients who had surgery for Achilles tendon rupture, Leppilahti et al. found intratendinous calcifications in 62% of the cases by ultrasonography [18].
In our study, the occurrence of PTC after percutaneous Achilles tendon repair had no influence on the clinical outcome. Significant differences were not detected between group I (with PTC) and group II (without PTC) according to the Thermann and the AOFAS scores.
We found an average PTC size of 5.3 mm within group I, whereby only one was larger than 10 mm. The relatively small size could be the reason for the nearly equal clinical outcome within group I (with PTC) compared to group II (without PTC). In the study of Kraus et al. only patients with a tendon calcification larger than 10 mm demonstrated relevantly impaired outcomes. To our knowledge, further experiences about tendon calcification after percutaneous Achilles tendon repair are not yet available.
The PTC rate in our study was lower compared to the rates reported by Kraus et al. with 28% and Leppilahti et al. with 62%. It is possible that the regular administration of diclofenac for at least 7 days postoperatively could be a reason for the lower incidence and smaller extent of PTC. Diclofenac is a non-selective anti-inflammatory drugs (NSAID). Non-selective NSAIDs inhibit both isoforms of the cyclooxygenase (COX-1 and COX-2), which is the first enzyme in the prostaglandin synthetic pathway. At the site of injury prostaglandins mediate inflammation and pain. After a tendon injury or surgery, NSAIDs influence the tendon cell proliferation and the fibroblast collagen synthesis and they as well decrease the adhesion formation after the injury [19]. Further, previous studies reported an anti-calcifying effect of NSAIDs in the tendon healing [20].
Beyond that, many details of the influence of NSAIDs on the healing of injured tendons are not fully understood [21]. In a recent review by Chen et al. among others three animal studies dealing with the influence of NSAIDs on the healing of the Achilles tendon were included [22]. The results were inconsistent. Forslund et al. found that indomethacin and celecoxib can improve the healing of injured Achilles tendons in rats [23]. Whereas, Thomas et al. found that indomethacin had no influence on the tendon healing in rabbits [24]. Parecoxib and indomethacin had a negative influence on the tendon healing in rats concerning different parameters such as tensile strength or transverse and sagittal tendon diameter in the study by Dimmen et al. [25]. Chen et al. concluded that there is insufficient evidence of a detrimental effect of NSAIDs in soft tissue healing.
No clinical trial has yet investigated the effect of diclofenac on the human Achilles tendon healing. This aspect should be a subject for future trials. Further, many efforts have been done to improve healing by usage of different growth factors, bone morphogenetic proteins and bone marrow aspirate [10].
Re-ruptures of the Achilles tendon occurred in four patients (4.9%). In none of them, PTC was detected. PTC was no risk factor for a re-rupture of the Achilles tendon. Our overall re-rupture rate was comparable to other studies [3, 6, 26]. Concerning postoperative wound complications and neurological complications our results were comparable to other reports [3, 6, 26]. Further, PTC was no risk factor for wound and neurological complications.
Patients were enrolled into the study over a period of over 7 years. A standardized follow-up-examination was performed in all patients included into the study. The wide range of follow-up time is the result of the long time of enrollment. The follow-up was similar in both groups of patients (PTC-positive patients 70 months (range 15–107 months) vs. PTC-negative patients 64 months (range 15–110 months)).
Conclusion
PTC after percutaneous Achilles tendon repair seems to be gender- and age related. Especially, male individuals seem to be at risk to develop PTC. Furthermore, advanced age seems to be associated with a higher risk for PTC. Hyperuricemia and hypercholesterinemia could be risk factors for PTC. In the present study, PTC had no negative influence on the clinical postoperative outcome. The perioperative NSAID administration could influence the incidence and diameter of PTC and should be investigated via further trials.
References
Wong J, Barrass V, Maffulli N (2002) Quantitative review of operative and nonoperative management of achilles tendon ruptures. Am J Sports Med 30:565–575
Willits K, Amendola A, Bryant D et al (2010) Operative versus nonoperative treatment of acute Achilles tendon ruptures: a multicenter randomized trial using accelerated functional rehabilitation. J Bone Jt Surg Am 92:2767–2775. doi:10.2106/JBJS.I.01401
Jones MP, Khan RJ, Carey Smith RL (2012) Surgical interventions for treating acute Achilles tendon rupture: key findings from a recent Cochrane review. J Bone Jt Surg 94:CD003674. doi:10.2106/JBJS.J.01829
Soroceanu A, Sidhwa F, Aarabi S et al (2012) Surgical versus nonsurgical treatment of acute Achilles tendon rupture. J Bone Jt Surg 94:2136. doi:10.2106/JBJS.K.00917
Wilkins R, Bisson LJ (2012) Operative versus nonoperative management of acute Achilles tendon ruptures: a quantitative systematic review of randomized controlled trials. Am J Sports Med 40:2154–2160. doi:10.1177/0363546512453293
Holm C, Kjaer M, Eliasson P (2014) Achilles tendon rupture—treatment and complications: a systematic review. Scand J Med Sci Sports. doi:10.1111/sms.12209
Cooper MT (2015) Acute Achilles tendon ruptures: does surgery offer superior results (and other confusing issues)? Clin Sports Med 34:595–606. doi:10.1016/j.csm.2015.06.001
Zhang H, Tang H, He Q et al (2015) Surgical versus conservative intervention for acute Achilles tendon rupture: a PRISMA-compliant systematic review of overlapping meta-analyses. Medicine (Baltimore) 94:e1951. doi:10.1097/MD.0000000000001951
Porter MD, Shadbolt B (2015) Randomized controlled trial of accelerated rehabilitation versus standard protocol following surgical repair of ruptured Achilles tendon. ANZ J Surg 85:373–377. doi:10.1111/ans.12910
Shapiro E, Grande D, Drakos M (2015) Biologics in Achilles tendon healing and repair: a review. Curr Rev Musculoskelet Med 8:9–17. doi:10.1007/s12178-015-9257-z
Ma GW, Griffith TG (1977) Percutaneous repair of acute closed ruptured achilles tendon: a new technique. Clin Orthop Relat Res 128:247–255
Haji A, Sahai A, Symes A, Vyas JK (2004) Percutaneous versus open tendo Achilles repair. Foot Ankle Int 25:215–218
Cretnik A, Kosanovic M, Smrkolj V (2005) Percutaneous versus open repair of the ruptured Achilles tendon: a comparative study. Am J Sports Med 33:1369–1379. doi:10.1177/0363546504271501
Del Buono A, Volpin A, Maffulli N (2014) Minimally invasive versus open surgery for acute Achilles tendon rupture: a systematic review. Br Med Bull 109:45–54. doi:10.1093/bmb/ldt029
Bleakney RR, Tallon C, Wong JK et al (2002) Long-term ultrasonographic features of the Achilles tendon after rupture. Clin J Sport Med 12:273–278
Kraus R, Stahl J-P, Meyer C et al (2004) Frequency and effects of intratendinous and peritendinous calcifications after open Achilles tendon repair. Foot Ankle Int 25:827–832
Ateschrang A, Gratzer C, Weise K (2008) Incidence and effect of calcifications after open-augmented Achilles tendon repair. Arch Orthop Trauma Surg 128:1087–1092. doi:10.1007/s00402-007-0441-5
Leppilahti J, Forsman K, Puranen J, Orava S (1998) Outcome and prognostic factors of achilles rupture repair using a new scoring method. Clin Orthop Relat Res 346:152–161
Su B, O’Connor JP (2013) NSAID therapy effects on healing of bone, tendon, and the enthesis. J Appl Physiol 115:892–899. doi:10.1152/japplphysiol.00053.2013
O’Brien EJO, Frank CB, Shrive NG et al (2012) Heterotopic mineralization (ossification or calcification) in tendinopathy or following surgical tendon trauma. Int J Exp Pathol 93:319–331. doi:10.1111/j.1365-2613.2012.00829.x
Dideriksen K (2014) Muscle and tendon connective tissue adaptation to unloading, exercise and NSAID. Connect Tissue Res 55:61–70. doi:10.3109/03008207.2013.862527
Chen MR, Dragoo JL (2013) The effect of nonsteroidal anti-inflammatory drugs on tissue healing. Knee Surg Sports Traumatol Arthrosc 21:540–549. doi:10.1007/s00167-012-2095-2
Forslund C, Bylander B, Aspenberg P (2003) Indomethacin and celecoxib improve tendon healing in rats. Acta Orthop Scand 74:465–469. doi:10.1080/00016470310017802
Thomas J, Taylor D, Crowell R, Assor D (1991) The effect of indomethacin on Achilles tendon healing in rabbits. Clin Orthop Relat Res 272:308–311
Dimmen S, Engebretsen L, Nordsletten L, Madsen JE (2009) Negative effects of parecoxib and indomethacin on tendon healing: an experimental study in rats. Knee Surg Sport Traumatol Arthrosc 17:835–839. doi:10.1007/s00167-009-0763-7
Bhandari M, Guyatt GH, Siddiqui F et al (2002) Treatment of acute Achilles tendon ruptures: a systematic overview and metaanalysis. Clin Orthop Relat Res 400:190–200
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The study received the approval of the institutional ethic committee. Informed consent was obtained from all individual participants included in the study. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ateschrang, A., Körner, D., Joisten, K. et al. Incidence and risk factors for postoperative Achilles tendon calcifications after percutaneous repair. Arch Orthop Trauma Surg 138, 203–210 (2018). https://doi.org/10.1007/s00402-017-2829-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-017-2829-1