Abstract
Introduction
Femoral head (FH) osteonecrosis (ON) and subsequent segmental collapse is a major concern following displaced femoral neck fractures (FNF). We aimed to quantify residual perfusion to the FH following FNF and evaluate the viability of the FH overtime after surgical fixation.
Materials and methods
Twenty-three patients with FNF underwent dynamic contrast-enhanced (DCE)-MRI to estimate bone perfusion in the FH, using the contralateral side as control. Following open anatomic reduction and a length/angle-stable fixation, a special MRI sequence evaluated the FH for ON changes over time at 3 and 12 months after surgery.
Results
We found significant compromise of both arterial inflow [83.1%—initial area under the curve (IAUC) and 73.8%—peak) and venous outflow (243.2%—elimination rate (K el)] in the FH of the fractured side. The supero-medial quadrant suffered the greatest decrease in arterial inflow with a significant decrease of 71.6% (IAUC) and 68.5% (peak). Post-operative MRI revealed a high rate (87%—20/23) of small ON segments within the FH, and all developed in the anterior aspect of the supero-medial quadrants. Fracture characteristics, including subcapital FNF, varus deformity, posterior roll-off ≥20° and Pauwel’s angle of 30°–50° demonstrated a greater decrease in perfusion compared to contralateral controls.
Conclusion
FNF significantly impaired the vascular supply to the FH, resulting in high incidence of small ON segments in the supero-medial quadrant of the FH. However, maintained perfusion, probably through the inferior retinacular system, coupled with urgent open anatomic reduction and stable fixation resulted in excellent clinical and radiographic outcomes despite a high rate of small ON segments noted on MRI.
Level of evidence
Level I: Prognostic Investigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteonecrosis (ON) of the femoral head (FH) is a multifactorial disease process culminating in cellular demise within the FH secondary to compromised blood flow [1,2,3]. The initial stages are often asymptomatic, but the condition can continue to deteriorate and become debilitating [1, 3]. Development of ON and subsequent segmental collapse of the FH is major concern following displaced femoral neck fractures (FNF), and its occurrence seems to dictate functional prognosis [4]. Surgical decision-making for treatment of this fracture usually depends upon the overall risk of developing this devastating complication and potential for re-operations. Historically, the reported incidence of ON (25–45%) [5], late segmental collapse (25%) [6] and re-operations (20-64%) [7,8,9,10,11,12] is considerable, and often leads to arthroplasty [2, 20]. The presumed etiologic factor of this complication is decreased FH arterial inflow and venous outflow caused by either direct disruption of the terminal vessels and/or indirect tamponade secondary to development of an intra-articular fracture hematoma [13,14,15,16,17,18,19,20].
Functional vascular assessment of the FH using dynamic contrast-enhanced (DCE) MRI has previously demonstrated decreased blood flow in both the arterial and venous systems following FNF, suggestive of both an inflow and outflow problem [13]. We aimed to quantify residual perfusion to the FH following FNF, using DCE-MRI, and evaluate the viability of the FH overtime after surgical fixation, using a special MRI sequence. Our hypothesis was twofold: (1) the area of the FH that exhibits the most prominent decrease in perfusion is more likely to develop ON, and (2) subcapital FNF and angular deformity, including varus malposition and posterior roll-off, can further compromise FH perfusion.
Arterial supply to the femoral head
Three arterial systems provide the FH blood supply including the (1) retinacular arteries, (2) foveolar artery, and (3) intraosseous nutrient artery [21,22,23,24,25,26,27]. The retinacular systems (superior, inferior and anterior) are the primary arterial supply of the FH [21, 24, 26,27,28,29,30,31], with the superior retinacular arteries providing the greatest contribution [21, 23, 26,27,28, 30,31,32,33,34,35,36]. Both the superior and inferior retinacular systems are the intra-articular terminal branches of the medial femoral circumflex artery [22, 24, 27], and they course within the retinacula of Weitbrecht (fibrous extensions of the capsule wall) [21, 23, 27, 34, 37,38,39]. The superior retinacular system courses within the superior retinacula of Weitbrecht adjacent to the femoral neck, penetrating the posterior superior aspect of the femoral head–neck junction and arborizes to supply the superior/weight bearing portion of the FH [27, 28, 32]. The foveolar system flows through the ligamentum teres and branches to supply the peri-foveolar area [28, 32]. The inferior retinacular system runs through the inferior retinacula of Weitbrecht (elevated off the femoral neck), piercing the posterior-inferior aspect of the femoral head–neck junction and arborizes to supply the inferior FH [27, 28, 32]. There is a vast anastomosis between the retinacular and foveolar arterial systems [28, 32, 40].
Materials and methods
Our Institutional Review Board approved this prospective study. The senior author treated all patients enrolled in the study using the same surgical technique [41, 42]. Inclusion criteria consisted of: (1) an isolated injury; (2) age ≥18 years; (3) complete set of advanced imaging (including pre- and post-operative MRI) and (4) radiographic follow-up duration of >12 months.
Pre-operative dynamic contrast-enhanced (DCE) MRI
Preoperatively, we obtained fat-suppressed DCE-MRIs, a technique that provides an estimate of bone perfusion in vivo over time for both the injured and uninjured proximal femur simultaneously [13]. Using a power injector, we injected 0.1 mM/kg of gadolinium-diethylenetriamine penta-acetic acid (Gd-DTPA) (Magnevist®; Bayer HealthCare Pharmaceuticals Inc., Wayne, NJ) at a rate of 2 cc/s. The DCE-MRI sequence used a coronal fat-suppressed 3D spoiled gradient echo pulse sequence (liver acquisition with volume acquisition, LAVA) with a temporal resolution of 7 s/image over 45 time points for a scan time of 6 min. We used the Brix 2-compartment model to analyze the DCE-MRI uptake curves in the normal and injured FH [13]. We created time intensity curves of the entire FH and defined quadrants with the average of all subjects’ model parameters. Reflecting the arterial inflow are the following parameters: A (signal amplitude), k ep (exchange rate between plasma and extravascular extracellular space), IAUC (initial area under the curve) and peak [13, 43]. We used the Brix model equation to calculate both the IAUC and peak value 90 s after injection. K el (elimination rate) reflects venous outflow.
Surgical technique and post-operative care
The surgical technique consists of open anatomic reduction, intraoperative compression and a length- and angle-stable construct [41, 42] (Fig. 1). A strategically placed endosteal strut (fibular allograft), serving as a biologic dowel, was used to reconstruct the comminuted femoral neck and to augment the screw construct (consisting of two fully threaded cannulated screws). Transfixing through the fibular allograft creates a non-sliding fixed angle construct between the host bone and the allograft. This construct provides increased fixation stability enhancing osseous union and FH revascularization [41]. Several authors have recognized that anatomical reduction and stable fixation gives the best chances for success following open reduction and internal fixation (ORIF) of FNF [6, 41, 44,45,46,47,48,49].
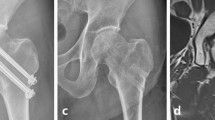
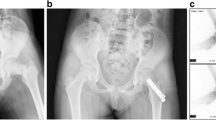
a Anteroposterior (AP) and lateral pre-operative radiographs of a 54-year-old male with a displaced femoral neck fracture including displacement in both the coronal and sagittal plane (with varus displacement and posterior roll-off). b The 12-month AP and lateral radiographs illustrate the fixation construct, and the maintenance of anatomic reduction, femoral neck length and hip joint space with neither radiographic signs of femoral head osteonecrosis nor segmental collapse
Weight bearing status was limited to 20% of patient body weight for the first 3 months. Passive range of motion began immediately after surgery, but strengthening exercises were delayed for 6 weeks. At 3 months, patients advanced to full-weight bearing. Clinical and radiographic follow-up consisted of a visit at 2 and 6 weeks, then every 3 months up to a year, and finally at 2 years.
Post-operative multi-acquisition variable-resonance image combination (MAVRIC) MRI
Postoperatively, we obtained a MAVRIC-MRI 3 and 12 months after surgery. This sequence improves image quality by reducing metallic artifacts caused by implants [50,51,52]. All MRI examinations were performed with a 1.5-T clinical scanner system (450 DVMR, system General Electric Health Care, Waukesha, WI) using an 8-channel phased-array cardiac coil (GE Healthcare, Waukesha, WI). MAVRIC images were obtained with the parameters: TR, 1000 ms; TE, 10–14 ms; RBW, ±125 kHz; slice thickness, 3.5 mm; slice spacing, 0 mm; acquisition matrix, 512 × 256; NEX, 0.5; ETL, 8; FOV, 38–44 cm. Scans were evaluated by an experienced musculoskeletal MRI radiologist (HGP) for signs of ON, characterized as the serpentine low signal-intensity focus within the high signal intensity of the fatty subchondral bone. We correlated the size and location of the ON segments with the injury DCE-MRI perfusion analysis and fracture characteristics.
Statistical analysis
We used the Shapiro–Wilk test to assess normality for all continuous variables. When the assumption of normality was not violated, independent sample t tests were used for pre-operative dynamic MRI parameters between fracture and non-fracture sides of the femoral head both at each quadrant and between the entire femoral head. Wherever normality was violated, we used Mann–Whitney U tests. We used similar analyses to assess differences between various fracture characteristics. In comparisons of three categories or more, we used one-way ANOVA to assess differences in dynamic MRI measures for normally distributed data, whereas Kruskal–Wallis tests were used for any non-parametric assessments. Two-factor ANOVA models were generated comparing mean differences in pre-operative dynamic MRI parameters between two independent factors in fracture characteristics and fracture group, as well as between post-operative MAVRIC-MRI characteristics and fracture group. All analyses were conducted using SPSS version 22.0 (IBM Corp., Armonk, NY).
Results
Thirty-seven patients with FNF presented at our institution during the study period (October 2009 to July 2013). Five patients were indicated for arthroplasty treatment and five (non-displaced fractures) underwent closed reduction and percutaneous pinning. Twenty-seven patients were indicated for open reduction and internal fixation, but one patient decline participation in the study, one patient suffered fracture non-union and underwent total hip arthroplasty and two patients suffered early catastrophic failure (one was non-complaint with weight bearing restrictions and the other suffered a fall). Twenty-three patients (4 males, 19 females) with displaced/unstable fractures met inclusion criteria. At time of injury, average patient age was 60.1 years (range of 30–79). All patients achieved osseous union, and none demonstrated radiographic signs of ON or FH segmental collapse at latest follow-up on standardized anteroposterior (AP) and lateral radiographs. Average radiographic follow-up was 18.7 months (range of 12–45). As previously reported [41], all patients had excellent clinical and radiographic outcomes and maintained an anatomical reduction over time. All patients also recovered a painless normal gait. Table 1 lists distribution of fracture characteristics (fracture location, coronal/sagittal deformity and Pauwel’s angle) at time of injury.
We obtained averaged time intensity curves, based on the DCE-MRI’s fit parameters, of the entire FH and the four quadrants for both the fractured side and contralateral matched control (Fig. 2). Analysis revealed a significant (p < 0.05) compromise of both arterial inflow (83.1 and 73.8% decrease in IAUC and peak, respectively) and venous outflow (243.2% decrease of K el) in the fractured side FH when compared to the contralateral control. A greater decrease was noted in arterial inflow within the injury side superior-medial quadrant with a significant decrease of 71.6% (IAUC) and 68.5% (peak) when compared to the uninjured side. Fracture characteristics, including subcapital FNF, varus deformity, posterior roll-off ≥20° and a 30–50° Pauwel’s angle demonstrated a greater decrease in perfusion (IAUC and peak) when compared to contralateral controls, although these differences were not statistically significant. All cases maintained significant FH perfusion despite suffering a great decrease in blood flow following an FNF. None of the patients demonstrated ON changes in the pre-operative MRI.
Post-operative MAVRIC-MRI revealed a high rate (87%; 20/23) of FH ON segments. All ON segments developed in the anterior aspect of the supero-medial quadrants (area of largest decrease in perfusion on pre-op DCE-MRI). FHs which developed ON showed a significantly higher decrease in perfusion, on pre-op DCE-MRI, to both the entire FH (70.5%; p = 0.001) and to the supero-medial quadrant (71.4%; p = 0.007) when compared to those that did not develop ON (Fig. 3). Comparing fracture side to the contralateral control, those that developed ON segments had greater decreases in perfusion to both the entire FH (79.5% p = 0.002 versus 25.4% p = 0.361) and the supero-medial quadrant (66.6% p = 0.040 versus 47.1% p = 0.184) than those without ON. All fractures demonstrated decreased venous outflow (K el) when compared to controls, but those that developed ON segments did not demonstrate a greater decrease in outflow than those that did not develop ON. This suggests a decrease in venous outflow (K el) does not correlate with the ON development. The ON segments decreased in size over time (6.68 → 5.84 cm3) and only involved a small percentage of the FH (13.9 → 12.8%). Based on MRI data, 20% (4/20) of those patients with ON segments demonstrated minimal subchondral collapse (<2 mm), but none were symptomatic nor showed signs of collapse upon latest radiographic follow-up.
Depicts a post-operative MAVRIC-MRI revealing the osteonecrosis segment in the superior quadrant of the femoral head. The graphs demonstrate the average time–intensity curves, based on the pre-operative dynamic contrast-enhanced-MRI’s fit parameters, of the entire FH and the supero-medial quadrant comparing those patients who developed osteonecrosis segments and those who did not
Discussion
Development of ON in the FH following FNF is a major concern, and usually dictates surgical treatment. Using DCE-MRI, we demonstrated maintenance of substantial perfusion to the entire FH, despite suffering a significantly compromised blood flow following a displaced FNF. A decreased FH perfusion of more than 67% resulted in subsequent ON development based on MAVRIC-MRI. All ON segments developed in the anterior superior-medial FH segment, which correlated with (1) area of greatest decrease in dynamic perfusion on pre-operative DCE-MRI; (2) the area mainly supplied by the superior retinacular artery and (3) the most distant region from the presumed residual sources of perfusion (inferior retinacular and foveolar arterial systems). Our cohort had a high ON incidence (87%) based on MRI, but ON segments were small (13% of the FH) and did not appear to lead to FH collapse on radiographs nor negatively affect the clinical outcomes at latest follow-up. Osteonecrosis segment and subchondral collapse on MRI were not noted on radiographs given the tomographic nature of MR acquisition and superior tissue contrast, yielding superior sensitivity to focal ON and subchondral collapse.
A small study group, hindering the study power for outcome prediction, limits our investigation. However, the primary purpose of the study was still achieved, which was to evaluate residual perfusion preoperatively and correlate it with the post-operative MRI findings of ON in the FH. We understand that a longer clinical and radiographic follow-up is required to assess the FH viability. However, at latest follow-up (18.7 months; range 12–45), patients were asymptomatic without radiographic evidence of segmental collapse in any patient. Additionally, based on MRI findings at 12 months postoperatively, no patient presented any of the prognostic factors for progression of the ON segment to FH collapse [3, 53,54,55]. Additionally, we rely on indirect measures, using advanced imaging, to evaluate biologic changes and FH viability. However, direct measures (histological) are not achievable in clinical practice outside an animal model. Nevertheless, MAVRIC-MRI provides an excellent non-invasive method for assessment of the FH around the metal implants.
Fracture of the femoral neck acts synergistically to affect the extra/intra-osseous arterial inflow, the sinusoidal flow and/or the extraosseous/intraosseous venous outflow of the FH leading to cellular death. Both the superior retinacular system and the intraosseous nutrient artery course in close proximity to or within the femoral neck and are prone to injury in the setting of femoral neck fracture [26, 27]. This leaves the inferior retinacular system (protected by the inferior retinacula of Weitbrecht and elevated off the femoral neck) [27, 56,57,58] and the foveolar systems as the remaining vascular supply to the FH [4]. The protective setting of the inferior retinacula of Weitbrecht was first detailed by Smith in 1953 [59]. More recently, Papadakis el al. [58] reported an intact inferior retinacula of Weitbrecht in 98% (108/110) of displaced FNF.
In our study, the injury DCE-MRIs demonstrated markedly decreased perfusion to the entire FH, but mainly to the superior head. This area is primarily supplied by the superior retinacular system [27, 28, 32], which presumably is disrupted. Notably, significant perfusion to the entire FH was maintained, particularly in the inferior head where the inferior retinacular system predominates [27, 28, 32], suggesting this vessel is preserved. Quadrant analysis revealed a prominent decrease in perfusion to the supero-medial quadrant, as well as significantly delayed washout in the injured side as compared to the uninjured side. This delayed washout may signify an outflow problem caused by either increased intraosseous/intraarticular pressure and/or disruption of the venous system. Accumulation of hemarthrosis increases intracapsular pressure creating a tamponade effect that jeopardizes both arterial inflow and venous outflow of the retinacular system [4, 13, 14, 16, 18,19,20]. Our cohort demonstrated a significant decrease in venous outflow in the fractured hips when compared to the control side, but we did not appreciate a significant difference when comparing between the developments of ON segments. All our patients underwent surgical fixation urgently (<24 h post-injury), limiting ability to evaluate tamponade effect over time. The remaining arterial supply can be compromised by rotatory and coronal malalignment of the FH [56]. Our results revealed varus deformity and posterior roll-off ≥20° led to a greater decrease in FH perfusion, perhaps due to compression of the inferior posterior retinacular arteries. An open anatomic reduction may help restore the FH perfusion by decompressing the tamponade effect created by the hemarthrosis and by unkinking the preserved retinacular vessels.
An interruption of blood flow following an FNF has been established as the primary etiologic factor in posttraumatic ON [2]. Several investigators have used different techniques to study the residual perfusion to the FH following FNF to identify the patients at risk for ON development and segmental FH collapse. Heuck et al. [60] implemented subtraction arteriography to show 97% of cases with established ON demonstrated changes in the arterial system supplying the FH. Intraosseous oxygen pressure measurements showed a correlation between elevated intraosseous pressure and decreased oxygen pressure noted in FH with ON [61, 62]. Sugamoto et al. [63.], using Doppler-laser flowmetry, documented severe vascular damage to the FH in displaced FNFs. Using bone scintigraphy, several authors reported great results with 85–90% accuracy in detecting pathologic scintigraphy changes that then developed ON [64,65,66,67,68,69]. Einert et al. [44] used 3-phase bone scintigraphy to evaluate residual perfusion and FH viability following ORIF of FNF, and reported that ON of the FH developed if there was persistent impairment of FH perfusion at 3 and 6 months postoperatively.
DCE-MRI demonstrated encouraging results for the early evaluation of FH osteonecrosis risk following FNFs using non-invasive means. Kamano et al. [70.] evaluated 29 FNF with DCE-MRI <24 h after injury and demonstrated excellent prognoses for those that had complete FH enhancement. Konishiike et al. [71.] performed a similar evaluation of 22 FNF and reported that in those with no enhancement, the DCE-MRI has high predictive value for subsequent ON risk with 89% accuracy. They detected ON of the FH when there was ≥70% decrease in perfusion. In our study, subjects that developed ON had decreased perfusion ranging between 67 and 78%.
The reported prognostic factors for progression of ON to FH collapse include: (1) extent of the ON segment; (2) location of the lesion and (3) presence of bone marrow edema in the proximal femur [3]. Nam et al. [54] reported an FH collapse rate of 46–83% of medium- to large-size lesions (>30–50%) and 5% for small lesions (<30%). All except one of the ON segments in our cohort were small lesions (<30%; range 3.0–24.6%), with one patient with a medium lesion (31.8%). Nishii et al. [55] reported higher progression of collapse in lesions involving >2/3 of the weight bearing area. Ito et al. [53] reported significant association between presence of bone marrow edema in the proximal femur and subsequent symptomatic collapse of the FH. None of the ON segments identified in our cohort occupied >2/3 of the weight bearing area nor were associated with bone marrow edema. In 1971, Garden [14] suggested hip joint incongruity, as result of severe malreduction following osseous union, leads to a remodeling process/late segmental collapse in the FH. In our cohort, all patients had an anatomic reduction and the fixation led to osseous union in anatomic position with hip joint congruity, potentially avoiding this remodeling process. Small ON segments, based on MRI, were detected in 87% of our cohort. Nevertheless, at latest follow-up no radiographic signs of FH collapse were noted.
Conclusion
An intracapsular femoral neck fracture compromises FH vascularity affecting both the inflow and outflow systems that lead to cellular death and segmental ON of the FH. Impaired vascularity and residual decrease perfusion of more than 67% will lead to ON segments. However, impairment of FH vascularity is not the sole etiologic factor for post-operative complications and FH collapse. In our cohort, an urgent open anatomic reduction coupled with an angle- and length-stable construct resulted in excellent clinical and radiographic outcomes despite high rate of small ON segments noted on MRI. We need a longer follow-up to evaluate the absolute fate of FH with ON changes.
References
Lavernia CJ, Sierra RJ, Grieco FR (1999) Osteonecrosis of the femoral head. J Am Acad Orthop Surg 7(4):250–261
Mont MA, Hungerford DS (1995) Non-traumatic avascular necrosis of the femoral head. J Bone J Surg Am Vol 77(3):459–474
Zalavras CG, Lieberman JR (2014) Osteonecrosis of the femoral head: evaluation and treatment. J Am Acad Orthop Surg 22(7):455–464. doi:10.5435/JAAOS-22-07-455
Ehlinger M, Moser T, Adam P, Bierry G, Gangi A, de Mathelin M et al (2011) Early prediction of femoral head avascular necrosis following neck fracture. Orthop Traumatol Surg Res. 97(1):79–88
Nikolopoulos KE, Papadakis SA, Kateros KT, Themistocleous GS, Vlamis JA, Papagelopoulos PJ et al (2003) Long-term outcome of patients with avascular necrosis, after internal fixation of femoral neck fractures. Injury 34:525–528 (England)
Garden RS (1971) Malreduction and avascular necrosis in subcapital fractures of the femur. J Bone Joint Surg Br Vol 53(2):183–197
Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD (2008) Surgical management of hip fractures: an evidence-based review of the literature. I: Femoral neck fractures. J Am Acad Orthop Surg 16(10):596–607
Masson M, Parker MJ, Fleischer S (2003) Internal fixation versus arthroplasty for intracapsular proximal femoral fractures in adults. Cochrane Database Syst Rev (2):CD001708
Roden M, Schon M, Fredin H (2003) Treatment of displaced femoral neck fractures: a randomized minimum 5-year follow-up study of screws and bipolar hemiprostheses in 100 patients. Acta Orthop Scand 74(1):42–44
Parker MJ, Khan RJ, Crawford J, Pryor GA (2002) Hemiarthroplasty versus internal fixation for displaced intracapsular hip fractures in the elderly. A randomised trial of 455 patients. J Bone Joint Surg Br 84(8):1150–1155
Puolakka TJ, Laine HJ, Tarvainen T, Aho H (2001) Thompson hemiarthroplasty is superior to Ullevaal screws in treating displaced femoral neck fractures in patients over 75 years. A prospective randomized study with two-year follow-up. Ann Chir Gynaecol 90(3):225–228
Lu-Yao GL, Keller RB, Littenberg B, Wennberg JE (1994) Outcomes after displaced fractures of the femoral neck. A meta-analysis of one hundred and six published reports. J Bone Joint Surg Br 76(1):15–25
Dyke JP, Lazaro LE, Hettrich CM, Hentel KD, Helfet DL, Lorich DG (2013) Regional analysis of femoral head perfusion following displaced fractures of the femoral neck. J Magn Reson Imaging 41(2):550–554. doi:10.1002/jmri.24524
Beck M, Siebenrock KA, Affolter B, Notzli H, Parvizi J, Ganz R (2004) Increased intraarticular pressure reduces blood flow to the femoral head. Clin Orthop Relat Res 424:149–152
Kregor PJ (1996) The effect of femoral neck fractures on femoral head blood flow. Orthopedics 19(12):1031–1036 (quiz 7–8)
Bachiller FG, Caballer AP, Portal LF (2002) Avascular necrosis of the femoral head after femoral neck fracture. Clin Orthop Relat Res 399:87–109
Calandruccio RA, Anderson WE 3rd (1980) Post-fracture avascular necrosis of the femoral head: correlation of experimental and clinical studies. Clin Orthop Relat Res 152:49–84
Wingstrand H, Stromqvist B, Egund N, Gustafson T, Nilsson LT, Thorngren KG (1986) Hemarthrosis in undisplaced cervical fractures. Tamponade may cause reversible femoral head ischemia. Acta Orthop Scand 57(4):305–308
Bonnaire F, Schaefer DJ, Kuner EH (1998) Hemarthrosis and hip joint pressure in femoral neck fractures. Clin Orthop Relat Res 353:148–155
Bonnaire FA, Weber AT (2002) The influence of haemarthrosis on the development of femoral head necrosis following intracapsular femoral neck fractures. Injury 33(Suppl 3):C33–C40
Tucker FR (1949) Arterial supply to the femoral head and its clinical importance. J Bone Joint Surg Br Vol 31B(1):82–93
Howe WW Jr, Lacey T, Schwartz RP (1950) A study of the gross anatomy of the arteries supplying the proximal portion of the femur and the acetabulum. J Bone Joint Surg Am 32(A:4):856–866
Harty MY (1953) Blood supply of the femoral head. Br Med J 2(4848):1236–1237
Ogden JA (1974) Changing patterns of proximal femoral vascularity. J Bone Joint Surg Am Vol 56(5):941–950
Judet J, Judet R, Lagrange J, Dunoyer J (1955) A study of the arterial vascularization of the femoral neck in the adult. J Bone Joint Surg Am 37-A(4):663–680
Sevitt S, Thompson RG (1965) The distribution and anastomoses of arteries supplying the head and neck of the femur. J Bone Joint Surg Br Vol 47:560–573
Lazaro LE, Klinger CE, Sculco PK, Helfet DL, Lorich DG (2015) The terminal branches of the medial femoral circumflex artery: the arterial supply of the femoral head. Bone Joint J 97-b(9):1204–1213
Harrison MH, Schajowicz F, Trueta J (1953) Osteoarthritis of the hip: a study of the nature and evolution of the disease. J Bone Joint Surg Br 35-B(4):598–626
Trueta J. The normal vascular anatomy of the human femoral head during growth. J Bone Joint Surg Br 39-B(2):358–394
Gautier E, Ganz K, Krugel N, Gill T, Ganz R (2000) Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg Br Vol 82(5):679–683
Wolcott WE (1943) The evolution of the circulation in the developing femoral head and neck. Surg Gynecol Obstet 77:185
Trueta J (1957) The normal vascular anatomy of the human femoral head during growth. J Bone Joint Surg Br 39-b(2):358–394
Beaule PE, Campbell P, Lu Z, Leunig-Ganz K, Beck M, Leunig M et al (2006) Vascularity of the arthritic femoral head and hip resurfacing. J Bone Joint Surg Am Vol 88(Suppl 4):85–96
Kalhor M, Beck M, Huff TW, Ganz R (2009) Capsular and pericapsular contributions to acetabular and femoral head perfusion. J Bone Joint Surg Am Vol 91(2):409–418
Lavigne M, Kalhor M, Beck M, Ganz R, Leunig M (2005) Distribution of vascular foramina around the femoral head and neck junction: relevance for conservative intracapsular procedures of the hip. Orthop Clin North Am 36(2):171–176 (viii)
Beaule PE, Ganz R, Leunig M (2008) Blood flow to the femoral head and hip resurfacing arthroplasty. Der Orthopade. 37(7):659–666
Walmsley T (1916) A note on the retinacula of Weitbrecht. J Anat 51:61–64
Kalhor M, Horowitz K, Gharehdaghi J, Beck M, Ganz R (2012) Anatomic variations in femoral head circulation. Hip Int 22(3):307–312. doi:10.5301/HIP.2012.9242
Gojda J, Bartonicek J (2012) The retinacula of Weitbrecht in the adult hip. Surg Radiol Anat 34(1):31–38
Boraiah S, Dyke JP, Hettrich C, Parker RJ, Miller A, Helfet D et al (2009) Assessment of vascularity of the femoral head using gadolinium (Gd-DTPA)-enhanced magnetic resonance imaging: a cadaver study. J Bone Joint Surg Br 91:131–137 (England)
Lazaro LE, Birnbaum JF, Farshad-Amacker NA, Helfet DL, Potter HG, Lorich DG (2016) Endosteal biologic augmentation for surgical fixation of displaced femoral neck fractures. J Orthop Trauma 30(2):81–88
Lorich D, Lazaro L, Boraiah S (2013) Master technique in orthopaedic surgery: femoral neck fracture open reduction and internal fixation, 3rd edn. Lippincott Williams and Wilkins, Philadelphia
Tofts PS (1997) Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging JMRI 7(1):91–101
Einert A, Bonnaire F, Simon GH, Kuner E, Moser E (1996) 3-phase bone scintigraphy: perfusion and vitality of the femur head after media femoral neck fracture and osteosynthesis. Aktuelle Radiol 6(5):219–224
Frenyo S, Kazar G, Manninger J (1995) Intracapsular fractures of the hip. J Bone Joint Surg Am 77(7):1130
Szita J, Cserhati P, Bosch U, Manninger J, Bodzay T, Fekete K (2002) Intracapsular femoral neck fractures: the importance of early reduction and stable osteosynthesis. Injury 33(Suppl 3):C41–C46
Boraiah S, Paul O, Gardner MJ, Parker RJ, Barker JU, Helfet D et al (2010) Outcomes of length-stable fixation of femoral neck fractures. Arch Orthop Trauma Surg 130(12):1523–1531
Garden RS (1964) Stability and union in subcapital fractures of the femur. J Bone Joint Surg Br 46:630–647
Garden RS (1974) Reduction and fixation of subcapital fractures of the femur. Orthop Clin North Am 5(4):683–712
Koch KM, Brau AC, Chen W, Gold GE, Hargreaves BA, Koff M et al (2011) Imaging near metal with a MAVRIC-SEMAC hybrid. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med 65(1):71–82
Hayter CL, Koff MF, Shah P, Koch KM, Miller TT, Potter HG (2011) MRI after arthroplasty: comparison of MAVRIC and conventional fast spin-echo techniques. AJR Am J Roentgenol 197(3):W405–W411
Koch KM, Hargreaves BA, Pauly KB, Chen W, Gold GE, King KF (2010) Magnetic resonance imaging near metal implants. J Magn Reson Imaging JMRI 32(4):773–787
Ito H, Matsuno T, Minami A (2006) Relationship between bone marrow edema and development of symptoms in patients with osteonecrosis of the femoral head. AJR Am J Roentgenol. 186:1761–1770 (United States)
Nam KW, Kim YL, Yoo JJ, Koo KH, Yoon KS, Kim HJ (2008) Fate of untreated asymptomatic osteonecrosis of the femoral head. J Bone Joint Surg Am 90:477–484 (United States)
Nishii T, Sugano N, Ohzono K, Sakai T, Haraguchi K, Yoshikawa H (2002) Progression and cessation of collapse in osteonecrosis of the femoral head. Clin Orthop Relat Res 400:149–157
Smith FB (1959) Effects of rotatory and valgus malpositions on blood supply to the femoral head observations at arthroplasty. J Bone Joint Surg 41(5):800–815
Garden RS (1961) Low-angle fixation in fractures of the femoral neck. J Bone Joint Surg Br 43:647–663
Papadakis SA, Segos D, Kouvaras I, Dagas S, Malakasis M, Grivas TB (2009) Integrity of posterior retinaculum after displaced femoral neck fractures. Injury 40(3):277–279
Smith LD (1953) Hip fractures; the role of muscle contraction or intrinsic forces in the causation of fractures of the femoral neck. J Bone Joint Surg Am 35-a(2):367–383
Heuck A, Reiser M, Schmucker F, Lehner K, Gmeinwieser J, Kahn T et al (1987) Selective digital subtraction arteriography in necrosis of the femoral head. Skeletal Radiol 16(4):270–274
Watanabe Y, Terashima Y, Takenaka N, Kobayashi M, Matsushita T (2007) Prediction of avascular necrosis of the femoral head by measuring intramedullary oxygen tension after femoral neck fracture. J Orthop Trauma 21(7):456–461
Kiaer T, Pedersen NW, Kristensen KD, Starklint H (1990) Intra-osseous pressure and oxygen tension in avascular necrosis and osteoarthritis of the hip. J Bone Joint Surg Br Vol 72(6):1023–1030
Sugamoto K, Ochi T, Takahashi Y, Tamura T, Matsuoka T (1998) Hemodynamic measurement in the femoral head using laser Doppler. Clin Orthop Relat Res 353:138–147
Meyers MH, Telfer N, Moore TM (1977) Determination of the vascularity of the femoral head with technetium 99m-sulphur-colloid. J Bone Joint Surg Am Vol 59(5):658–664
D’Ambrosia RD, Shoji H, Riggins RS, Stadalnik RC, DeNardo GL (1978) Scintigraphy in the diagnosis of osteonecrosis. Clin Orthop Relat Res 130:139–143
Phillips TW, Aitken GK, MacKenzie RA (1986) Sulphur colloid bone scan assessment of femoral head vascularity following subcapital fracture of the hip. Clin Orthop Relat Res 208:52–54
Lucie RS, Fuller S, Burdick DC, Johnston RM (1981) Early prediction of avascular necrosis of the femoral head following femoral neck fractures. Clin Orthop Relat Res 161:207–214
Greiff J (1980) Determination of the vitality of the femoral head with 99mTc-Sn-pyrophosphate scintigraphy. Acta Orthop Scand 51(1):109–117
Turner JH (1983) Post-traumatic avascular necrosis of the femoral head predicted by preoperative technetium-99m antimony-colloid scan. An experimental and clinical study. J Bone Joint Surg Am 65(6):786–796
Kamano M, Narita S, Honda Y, Fukushima K, Yamano Y (1998) Contrast enhanced magnetic resonance imaging for femoral neck fracture. Clin Orthop Relat Res 350:179–186
Konishiike T, Makihata E, Tago H, Sato T, Inoue H (1999) Acute fracture of the neck of the femur. An assessment of perfusion of the head by dynamic MRI. J Bone Joint Surg Br 81(4):596–599
Acknowledgements
We gratefully acknowledge the assistance from the project support staff (Nadine C. Pardee, Jacqueline Birnbaum and Amelia Ni) for coordination of scheduling and the logistics for acquisition of the study data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Each author certifies that he has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest with the submitted article.
Funding
The study was supported by a research grant from the AO Research Fund of the AO Foundation (AO Research Grant F-09-6H) and by Depuy Synthes Trauma.
Ethical approval
Institutional ethical board approval has been received for this research study.
Rights and permissions
About this article
Cite this article
Lazaro, L.E., Dyke, J.P., Thacher, R.R. et al. Focal osteonecrosis in the femoral head following stable anatomic fixation of displaced femoral neck fractures. Arch Orthop Trauma Surg 137, 1529–1538 (2017). https://doi.org/10.1007/s00402-017-2778-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-017-2778-8