Abstract
Background
Surgical debridement, negative-pressure wound therapy (NPWT) and antibiotics are used for the treatment of open wounds. However, it remains unclear whether this treatment regimen is successful in the reduction and shift of the bacterial load.
Methods
After debridement in the operating room, NPWT, and antibiotic treatment, primary and secondary consecutive microbiological samples of 115 patients with 120 open wounds with bacterial or yeast growth in ≥1 swab or tissue microbiological sample(s) were compared for bacterial growth, Gram staining and oxygen use at a level one trauma center in 2011.
Results
Secondary samples had significantly less bacterial growth (32 vs. 89%, p < .001, OR 17), Gram-positive bacteria (56 vs. 78%, p = .013), facultative anaerobic bacteria (64 vs. 85%, p = .011) and Staphylococcus aureus (10 vs. 46%, p = .002). They also tended to include relatively more Coagulase-negative Staphylococci (CoNS) (44 vs. 18%) and Pseudomonas species (spp.) (31 vs. 7%). Most (98%) wounds were successfully closed within 11 days, while wound revision was needed in 4%.
Conclusions
The treatment regimen of combined use of repetitive debridement, irrigation and NPWT in an operating room with antibiotics significantly reduced the bacterial load and led to a shift away from Gram-positive bacteria, facultative anaerobic bacteria, and S. aureus, as well as questionably toward CoNS and Pseudomonas spp. in this patient cohort. High rates of wound closure were achieved in a relatively short time with low revision rates. Whether each modality played a role for these findings remains unknown.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgical debridement and negative-pressure wound therapy (NPWT) are common treatment modalities for the acute and chronic open wound management [1–3]. The latter effectively drains wound exudate and hematoma through a sterile wound dressing, which consists of black polyurethane ether (PUE) or white polyvinyl alcohol (PVA) foam, a semi-occlusive foil and negative pressure, usually between 50 and 125 mm Hg [4, 5]. It also provides the benefit of continuous wound contraction, tissue granulation, improved perifocal blood supply, and cost-effectiveness because dressing changes are not needed daily [6–9]. Therefore, together with surgical debridement and antibiotics, it is an effective adjunct treatment modality against contaminated and/or infected wounds [10–12].
The influence of NPWT on the reduction of bacterial loads within an open wound cavity is an important contributor to effective treatment. It has been subject to debate since its suggestion in 1997, and, ultimately, requires more studies to clarify the remaining uncertainties [3, 13–20]. Some studies have reported no reduction of the bacterial load [13, 14, 18], while others found a bacterial shift [14, 15], or a reduction of the bacterial load [16, 17]. However, aside from the limited number of these studies [3, 13–20], they not only show discrepancies regarding the location of NPWT application, antibiotic treatment, but were also based on in vitro experiments [13], in vivo animal models [15], topical application [17], or included a rather small sample size [14, 16].
Early administration of antibiotics for open wounds is one of the most important factors in open wound treatment [21]. A recent meta-analysis of randomized controlled trials about patients with open fractures has reported a 59% lower risk of infection with antibiotics compared to placebo [22]. It is common knowledge that antibiotics, usually a first-generation cephalosporin, should be administered as soon as possible and may be continued for 24 h [23]. Surgical debridement is another very important factor in open wound treatment. It is always the goal to fully debride and irrigate all contaminated and necrotic tissue with saline. The exact time frame remains unknown and the historic 6-h rule does apply anymore, but debridement should be done as soon as tolerable for a patient’s condition [24–28]. Bone viability can be evaluated by capacity to bleed and muscle viability by the 4 Cs, color, contractility, consistency, and capacity to bleed [23, 29, 30].
The goal of the present study was to investigate the changes of the bacterial load and a bacterial community shift in a large clinical cohort of mainly trauma patients, who received a treatment regimen of surgical debridement, NPWT in a sterile operating room, and antibiotics.
Materials and methods
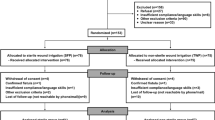
The present study investigated 261 consecutive patients with 280 open wounds treated with surgical debridement and NPWT at a single-level one trauma center (Department of Trauma Surgery, University Hospital of Zürich, Switzerland) in 2011 and is an ancillary to the retrospective cohort study by Osterhoff et al. [4]. Out of the 261 patients reported by Osterhoff et al. [4], 115 patients with 120 wounds were eligible for our study due to a positive microbiological sample. Therefore, none of the presented results has been previously reported. Our study complied with the regulations of the cantonal ethical review board (Zürich, Switzerland).
The inclusion and exclusion criteria are given in Table 1. The flow chart in Fig. 1 depicts the number of patients in this study as well as the measurements and outcome variables. For further patient characteristics, such as comorbidities, one may refer to the study by Osterhoff et al. [4]. Microbiological samples were sterilely acquired in all patients at the first or second surgery and one of the following surgeries before final wound closure. They were obtained after surgical debridement and before NPWT application. Samples were usually incubated for 10 days before final results were documented. Furthermore, the management of infected and traumatic wounds followed a different standardized algorithm at our hospital. Microbiological samples were always acquired immediately in infected wounds, whereas traumatic wounds were first treated with local debridement, irrigation and NPWT for 1–2 days before the wound status in the second look was used for the decision on microbiological sampling. This approach proved beneficial because it avoided over-interpreting contaminated wounds as infected ones. The standardized treatment protocol involved serial surgical debridements in the sterile operating room, usually within 1–5 days. Care was taken to rigorously debride all tissues that could have possibly been infected, but without compromising neurovascular structures. After local debridement and irrigation, provisional wound closure was performed using NPWT with V.A.C® GranuFoam™ (PUE) or V.A.C.® WhiteFoam (PVA) dressing [Kinetic Concepts Inc. (KCI), San Antonio, TX, USA] and their corresponding semi-occlusive drapes and T.R.A.C Pads™ about every 3 days until wound closure. The reasons for different foam use were mostly subjective personal preference of a surgeon. During surgeries, the irrigation regimen consisted of isotonic saline volume for all wounds with added pulse lavage in severely contaminated open traumatic wounds. Postoperatively, negative pressure, usually around 125 mm Hg, was applied. Secondary wound closure was carried out after sufficient granulation tissue was visible without clinical signs of necrosis or infection after at least two rounds of surgical debridements.
Overall, antibiotic use was heterogeneous. An intravenous broad-spectrum antibiotic, most commonly amoxicillin/clavulanic acid, was usually given to high-risk patients, e.g., open fractures, and suspected or confirmed infections, usually for at least 3 days. Wound infection was defined as two or more positive microbiological samples from the wound, abscess formation with one or more positive microbiological samples or wound drainage. In low-risk patients (e.g., fasciotomy due to compartment syndrome), a single shot of intravenous cefazolin was administered 30 min before surgery or oral antibiotics, mostly amoxicillin/clavulanic acid, were given. Afterward, antibiotics were changed according to antibacterial sensitivity tests, usually in an interdisciplinary approach with an infectiologist, particularly in difficult cases. The time period for the antibiotic treatment varied in this group of patients. Generally, trauma patients were mostly treated with amoxicillin/clavulanic acid for 5–7 days, while patients with primary infections received this treatment regimen for 12–14 days or a combination of ciprofloxacin, rifampicin, and clindamycin for 2–4 weeks. This variation is attributable to the individual decision-making process, which combined the results of the secondary microbiological samples, laboratory analysis, and clinical judgement.
The microbiological sample was processed with a standardized semiquantitative technique with laminar flow hoods as described previously [31]. Samples were streaked on one-third of a 5% sheep blood Columbia agar plate (BioMérieux, Geneva, Switzerland). Using a disposable sterile loop, two successive dilutions were obtained by further streaking the initial inoculum on the agar plates over the remaining two-thirds, using a new loop for each streaking maneuver. The samples were then placed into Luria–Bertani broth (Sigma St. Louis, MO, USA) for enrichment for 72 h. The plates were then incubated at 37 °C for 48 h, after which they were assessed for colony growth. After 72 h, the liquid enrichment medium was processed on agar plates identical to the agar plate method described above. Gram staining was performed for morphologic analysis and classification. The bacteria’s oxygen use was evaluated with the catalase reactivity test. Several subcultures were specified by the RapID Staph Plus System (BioMérieux, Geneva, Switzerland).
Data are presented as frequencies and percentages of wounds. Primary and secondary microbiological samples were compared using univariate logistic regression analysis with bacterial growth, Gram staining and oxygen use as independent variables. Clustering within patients was addressed with clustered robust standard errors. Risk stratification and comorbidities were compared between patients with and without bacterial reduction, Gram staining, and oxygen use using Fisher’s exact test. p values less than .05 were considered statistically significant. Stata 13.1 (StataCorp., College Station, TX, USA) was used for statistical analyses.
Results
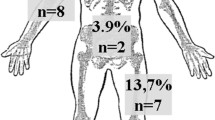
The present study included 115 patients, 31 females and 84 males, with a median age of 47 (IQR 35–64) (range 16–88) years and 120 open wounds. Main causes included a primary infection (e.g., abscess, bursitis, postoperative surgical site infection, spetic arthritis) in 52% (n = 62) and trauma in 46% (n = 55), whereof 18% (n = 21) were open fractures. The wounds were localized at the lower extremity in 51% (n = 61), the trunk, spine and pelvis in 28% (n = 34), the upper extremity in 19% (n = 23), and the abdomen in 2% (n = 2) with varying size. The mean time of NPWT until definite wound closure was 11 days (±10 days). The majority of wounds (98%) were successfully closed. Two (2%) wounds were not closed because one patient died due to an urosepsis in a metastasized breast cancer and one patient had an amputation of a lower leg in an open (Gustilo IIIc) ankle fracture dislocation. The hospital readmission rate was 18% due to various reasons. Furthermore, there were five cases (4%) with delayed (>30 days) wound healing or a reinfection requiring surgical revision.
Bacterial growth was significantly reduced in the secondary samples (32%) compared to the primary samples (89%) (p < .001) and the odds for bacterial growth were reduced by factor 17 [95% confidence interval (CI) 6–46] (Table 2). Of the 11% without bacterial growth in the primary wounds, 82% were acute trauma patients, while 18% had an atraumatic infection. Regarding the positive secondary samples (n = 39), pre-existing bacteria were cultured again in 69% (n = 27) and newly acquired bacteria were detected in 31% (n = 12). Yeast growth was observed in two secondary samples, whereof one patient, who had bacterial growth in the primary sample, with Candida glabrata was treated with anti-mycotics, while the other patient, who did not have bacterial growth in either sample, was interpreted as a contamination.
Gram-positive bacteria was significantly reduced in the secondary samples (56%) compared to the primary samples (78%) and the odds for bacterial growth were reduced by factor 3 (95% CI 1–6) (p < .013) (Table 3). Gram-negative and -positive bacteria did not show significant reductions. Furthermore, if wounds with bacteria were not sterile, no change in Gram staining was slightly more common than a change in Gram staining (Table 4).
Facultative anaerobic bacteria were much more common than bacteria with a different use of oxygen in primary and secondary samples (Table 5). Their growth was significantly reduced from the primary (85%) to the secondary samples (64%) and the odds for bacterial growth were reduced by factor 3 (95% CI 1–8) (p = .011).
The primary samples and pre-existing secondary bacteria mainly showed, among other more rare bacteria, Staphylococcus (S.) aureus, Coagulase-negative Staphylococci (CoNS), and Pseudomonas species (spp.) (mainly Pseudomonas aeruginosa), while newly acquired bacteria in secondary samples mainly revealed CoNS (Table 6). S. aureus was significantly reduced in the secondary samples (10%) compared to the primary samples (44%) and the odds for bacterial growth were reduced by factor 5 (95% CI 2–12) (p = .002). In the primary samples, two methicillin-resistant S. aureus (MRSA) were found. Although not significant, there was a tendency toward an increased percentage of Coagulase-negative Staphylococci (CoNS) and Pseudomonas species (spp.) in the positive secondary samples (n = 39) than in the primary samples (n = 107) (44 and 18 vs. 31 and 7%).
Fifty-seven patients (48%) received an intravenous antibiotic from the beginning of treatment and were, therefore, perceived as high-risk patients. An influence of the perceived stratification into low- and high-risk patients on the results in the secondary wound cultures was not found since no significant differences in bacterial reduction were observed between perceived low- and high-risk patients.
With respect to the reported comorbidities by Osterhoff et al. [4], patients with bacterial reduction had significantly less peripheral vascular disease and immunosuppressive medication (p = 0.023 and p = 0.030, respectively). There were no significant differences for smoking, alcohol and drug abuse, diabetes, and immunodeficiency diseases. Furthermore, PUE foam was used in 58 patients, PVA foam in three patients, and both foams in five patients. Since it was not documented which foam was used in 54 patients, no analysis of different foams was performed.
Discussion
The present follow-up study on a cohort study [4] adds information to the controversial literature [3, 13–20] about bacterial colonization of open wounds treated with our treatment regimen of surgical debridement and irrigation in a sterile operating room, NPWT and antibiotics by investigating a large clinical cohort of mainly trauma patients admitted to our level one trauma center. The applied treatment regimen effectively reduced the bacterial load while keeping newly acquired bacteria to a minimum and was associated with a bacterial community shift away from Gram-positive bacteria, facultative anaerobic bacteria, and S. aureus. It proved to be effective after a relatively short time (mean of 11 days) with a high rate of wound closures (98%) and a low revision rate (4%).
The use of NPWT for open wound management has been controversially discussed, but several studies have shown a clear benefit for patients [11, 12]. A multicenter, randomized controlled trial after diabetic foot amputation showed that NPWT could lead to better and faster wound healing [11]. In a prospective case–control study [12] about the management of diabetic foot ulcers, it was reported that NPWT was more effective, safe, and satisfactory for patients than conventional dressings.
Previous studies [3, 32], who introduced the NPWT in a pig model in 1997, support a reduction of the bacterial load with NPWT. Similarly, in a prospective randomized study [20] of a limited number patients (n = 23) with open fractures, it was reported that only wounds treated with NPWT were much less frequently colonized (8%) than control wounds with gauze dressings. Another study [16] also showed a reduction in the bacterial load in all 21 patients, who were treated with NPWT. In contrast, another study [14] did not find a quantitative reduction of the bacterial load despite favorable wound healing in a well-designed prospective randomized trial of 54 patients, which is in line with other studies [18, 19]. A theoretically possible explanation for these discrepancies may be found in the location of NPWT application, which, in the present study, was exclusively performed in a sterile operating room, including surgical debridements with irrigation at every NPWT procedure, and concomitant antibiotic treatment. This raises the question whether it may be more beneficial to perform NPWT changes in an operating room instead of at a patient’s bedside. This has been appealing because it safes time and does not occupy the operating room. However, this cannot be proven by this study due to the retrospective design using a multimodal therapeutic approach.
The presented treatment regimen was most effective against Gram-positive bacteria because they reduced significantly more often than the remaining bacteria. Additionally, the relative proportion of Gram-negative bacteria in secondary samples was higher than in primary samples, which constitutes a common clinical problem. This shift may have been attributed to an increased resistance of plasmid-containing Gram-negative bacteria with efflux pumps against antibiotics [33]. In a recent study [34], NPWT with instillation was only associated with a bacterial reduction at a short dwell time and when excluding Gram-negative bacteria. This is an upcoming topic, where bacterial reduction may much more depend on the type of solution used.
The facultative anaerobic bacteria, which, among others, included S. aureus and CoNS were the most commonly found subgroup and showed a significant reduction of their bacterial load from primary to secondary samples. Furthermore, anaerobic bacteria, such as Propionibacterium spp. and Bacteroides spp., exclusively became sterile or changed into a different oxygen-using subgroup. Therefore, the presented treatment regimen seems to provide enough oxygen to be effective against anaerobic bacteria. Similarly, another study [14] did not find a difference in the number of anaerobes in wounds treated with or without NPWT.
In a thorough review article [10], diverse effects on certain bacteria have been reported, ultimately suggesting that more studies are needed. In the present study, a tendency toward a relative bacterial shift from S. aureus toward CoNS and Pseudomonas spp. was observed. Similarly, another study [3] reported a decrease in S. aureus. Contrarily, a different study [14] showed an increase in S. aureus and, together with another report [15], observed a decrease in Pseudomonas spp. in their in vivo goat study.
A recent prospective study reported that bacterial loads remained high in foams despite routine changes [18]. Of 68 foams, 97% had ≥1 bacterial type and of 17 patients, 27% had an additional bacterial type. Although this is worrisome, it does not necessarily indicate that these bacteria were in the wounds, i.e., surrounding tissue, as well. Differences to the results of our study may also be explained by the fact that surgical debridement, irrigation, and NPWT changes were performed in a rather sterile operating room, potentially higher negative pressure in certain cases, more frequent foam changes every 1–2 days.
Due to the retrospective design and multimodal treatment regimen with concomitant use of surgical debridement and NPWT application in an operating room as well as antibiotic treatment, this study has several limitations. First, it is not possible to state which particular factor of the treatment regimen was associated with an outcome variable. However, the goal of the present study is to provide information about the bacterial reduction and shift in a large patient cohort that was treated with a commonly used treatment regimen. Although amoxicillin/clavulanic acid was the most commonly used antibiotic, heterogeneous differences in antibiotic treatment represent an important possible confounder. In the clinical setting, this aspect constitutes an issue that is very hard to overcome due to the different preferences of physicians and the need to adapt the antibiotic therapy to the specific bacteria of each wound, but should be considered when planning future trials or treatment algorithms in hospitals. Second, preoperative antibiotic administration likely affects bacterial detection in microbiological samples. It is usually assumed that preoperative antibiotic administration may entail false-negative intraoperative microbiological samples [35]. However, other recent studies have pointed into a different direction in revision arthroplasty, where prophylaxis of infection is very important. In these studies, diagnostic sensitivity of microbiological samples did not differ in case of administration before or after sampling [36, 37]. In heavily contaminated wounds, it seems adequate to administer antibiotics early, but one needs to remember that false-negative microbiological samples may arise. In cases with ambiguous clinical courses, it may be helpful to add broad-range polymerase chain reaction to the bacteriological analysis [38]. Third, our sample size included different wound types with different aetiologies, varying sizes and various locations. Although chronic infections may have been underrepresented and several surgeons were involved, most patients were treated due to trauma or acute infections and the standard operating procedures were applicable to all surgeons because the study was carried out at a level one trauma center. Fourth, the present study used PUE and PVA foams, which could not be presented in a subgroup analysis due to frequent missing data, whereas a previous study [14] exclusively used PUE foams. Fifth, although it is our impression that ≥3 tissue samples should be the gold standard for regular wounds and additional three samples in case of suspecting low-grade infection, the type and number of acquired samples were very heterogeneous, limiting generalizability. It should also be kept in mind that Gram staining in tissue samples often has low sensitivity and certain bacterias (e.g., Propionibacterium) need long incubation periods (10–14 days). One may also consider that one positive histological tissue is proof for infection, which was only done in some patients in this study. Despite these numerous limitations, the current study adds interesting findings to the literature that can be taken into account during NPWT application and for the design of randomized controlled trials, which are already underway [39]. Further studies could compare NPWT changes under sterile conditions in an operating room to bedside changes of NPWT.
Conclusion
The treatment regimen of combined use of repetitive surgical debridement, irrigation and NPWT in a sterile operating room in combination with antibiotic treatment significantly reduces the bacterial load and leads to a bacterial shift away from Gram-positive bacteria, facultative anaerobic bacteria, and S. aureus, as well as a questionable shift toward CoNS and Pseudomonas spp. High rates of wound closure were achieved in a relatively short time with low revision rates. Whether each modality played a role for these findings remains unknown.
References
Peinemann F, Sauerland S (2011) Negative-pressure wound therapy: systematic review of randomized controlled trials. Dtsch Arztebl Int 108:381–389
Evans D, Land L (2001) Topical negative pressure for treating chronic wounds: a systematic review. Br J Plast Surg 54:238–242
Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W (1997) Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 38:553–562
Osterhoff G, Zwolak P, Krüger C et al (2014) Risk factors for prolonged treatment and hospital readmission in 280 cases of negative-pressure wound therapy. J Plast Reconstr Aesthet Surg 67:629–633
Streubel PN, Stinner DJ, Obremskey WT (2012) Use of negative-pressure wound therapy in orthopaedic trauma. J Am Acad Orthop Surg 20:564–574
Timmers MS, Graafland N, Bernards AT et al (2009) Negative pressure wound treatment with polyvinyl alcohol foam and polyhexanide antiseptic solution instillation in posttraumatic osteomyelitis. Wound Repair Regen 17:278–286
Orgill DP, Manders EK, Sumpio BE et al (2009) The mechanisms of action of vacuum assisted closure: more to learn. Surgery 146:40–51
Timmers MS, Le Cessie S, Banwell P, Jukema GN (2005) The effects of varying degrees of pressure delivered by negative-pressure wound therapy on skin perfusion. Ann Plast Surg 55:665–671
Saxena V, Hwang CW, Huang S et al (2004) Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 114:1086–1096 (discussion 97–98)
Birke-Sorensen H, Malmsjo M, Rome P et al (2011) Evidence-based recommendations for negative pressure wound therapy: treatment variables (pressure levels, wound filler and contact layer)—steps towards an international consensus. J Plast Reconstr Aesthet Surg 64(Suppl):S1–S16
Armstrong DG, Lavery LA, Consortium DFS (2005) Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 366:1704–1710
Lone AM, Zaroo MI, Laway BA et al (2014) Vacuum-assisted closure versus conventional dressings in the management of diabetic foot ulcers: a prospective case–control study. Diabet Foot Ankle 5:1–5
Assadian O, Assadian A, Stadler M, Diab-Elschahawi M, Kramer A (2010) Bacterial growth kinetic without the influence of the immune system using vacuum-assisted closure dressing with and without negative pressure in an in vitro wound model. Int Wound J 7:283–289
Mouës CM, Vos MC, van den Bemd GJ, Stijnen T, Hovius SE (2004) Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen 12:11–17
Lalliss SJ, Stinner DJ, Waterman SM et al (2010) Negative pressure wound therapy reduces pseudomonas wound contamination more than Staphylococcus aureus. J Orthop Trauma 24:598–602
Pinocy J, Albes JM, Wicke C, Ruck P, Ziemer G (2003) Treatment of periprosthetic soft tissue infection of the groin following vascular surgical procedures by means of a polyvinyl alcohol-vacuum sponge system. Wound Repair Regen 11:104–109
Deva AK, Buckland GH, Fisher E et al (2000) Topical negative pressure in wound management. Med J Aust 173:128–131
Yusuf E, Jordan X, Clauss M et al (2013) High bacterial load in negative pressure wound therapy (NPWT) foams used in the treatment of chronic wounds. Wound Repair Regen 21:677–681
Weed T, Ratliff C, Drake DB (2004) Quantifying bacterial bioburden during negative pressure wound therapy: does the wound VAC enhance bacterial clearance? Ann Plast Surg 52:276–279 (discussion 79–80)
Stannard JP, Volgas DA, Stewart R, McGwin G Jr, Alonso JE (2009) Negative pressure wound therapy after severe open fractures: a prospective randomized study. J Orthop Trauma 23:552–557
Patzakis MJ, Wilkins J (1989) Factors influencing infection rate in open fracture wounds. Clin Orthop Relat Res 243:36–40
Gosselin RA, Roberts I, Gillespie WJ (2004) Antibiotics for preventing infection in open limb fractures. Cochrane Database Syst Rev 1:CD003764
Halawi MJ, Morwood MP (2015) Acute management of open fractures: an evidence-based review. Orthopedics 38:e1025–e1033
Werner CM, Pierpont Y, Pollak AN (2008) The urgency of surgical débridement in the management of open fractures. J Am Acad Orthop Surg 16:369–375
Skaggs DL, Friend L, Alman B et al (2005) The effect of surgical delay on acute infection following 554 open fractures in children. J Bone Jt Surg Am 87:8–12
Schenker ML, Yannascoli S, Baldwin KD, Ahn J, Mehta S (2012) Does timing to operative debridement affect infectious complications in open long-bone fractures? A systematic review. J Bone Jt Surg Am 94:1057–1064
Fernandez R, Griffiths R (2012) Water for wound cleansing. Cochrane Database Syst Rev 2:CD003861
Crowley DJ, Kanakaris NK, Giannoudis PV (2007) Irrigation of the wounds in open fractures. J Bone Jt Surg Br 89:580–585
Edwards CC, Simmons SC, Browner BD, Weigel MC (1988) Severe open tibial fractures. Results treating 202 injuries with external fixation. Clin Orthop Relat Res 230:98–115
Artz CP, Sako Y, Scully RE (1956) An evaluation of the surgeon’s criteria for determining the viability of muscle during débridement. AMA Arch Surg 73:1031–1035
Osterhoff G, Spirig J, Klasen J et al (2014) Perforation and bacterial contamination of microscope covers in lumbar spinal decompressive surgery. Med Princ Pract 23:302–306
Grauhan O, Navasardyan A, Hofmann M et al (2013) Prevention of poststernotomy wound infections in obese patients by negative pressure wound therapy. J Thorac Cardiovasc Surg 145:1387–1392
Cattoir V, Nordmann P (2009) Plasmid-mediated quinolone resistance in gram-negative bacterial species: an update. Curr Med Chem 16:1028–1046
Kim PJ, Attinger CE, Steinberg JS et al (2014) The impact of negative-pressure wound therapy with instillation compared with standard negative-pressure wound therapy: a retrospective, historical, cohort, controlled study. Plast Reconstr Surg 133:709–716
Al-Mayahi M, Cian A, Lipsky BA et al (2015) Administration of antibiotic agents before intraoperative sampling in orthopedic infections alters culture results. J Infect 71:518–525
Bedenčič K, Kavčič M, Faganeli N et al (2016) Does preoperative antimicrobial prophylaxis influence the diagnostic potential of periprosthetic tissues in hip or knee infections? Clin Orthop Relat Res 474:258–264
Tetreault MW, Wetters NG, Aggarwal V et al (2014) The Chitranjan Ranawat Award: should prophylactic antibiotics be withheld before revision surgery to obtain appropriate cultures? Clin Orthop Relat Res 472:52–56
Levy PY, Fournier PE, Fenollar F, Raoult D (2013) Systematic PCR detection in culture-negative osteoarticular infections. Am J Med 126:1143.e25-33
Seidel D, Lefering R, Neugebauer EA (2013) Treatment of subcutaneous abdominal wound healing impairment after surgery without fascial dehiscence by vacuum assisted closure™ (SAWHI-V.A.C.®-study) versus standard conventional wound therapy: study protocol for a randomized controlled trial. Trials 14:394
Acknowledgements
A similar version of this abstract (“Bacterial Reduction and Community Shift with Negative-Pressure Wound Therapy: The Relevance of Surgical Debridements in the Operating Room”) was presented as an oral presentation at the 16th European Congress of Trauma and Emergency Surgery (ECTES) of the European Society for Trauma and Emergency Surgery (ESTES) in May 2015 in Amsterdam, The Netherlands. We would like to thank Dr. med. Florian P. Maurer for his advice as well as Dr. med. Matthias A. König, Ms. Verena Wilzeck, and Ms. Carmen Krüger for their help with data acquisition.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Jentzsch, T., Osterhoff, G., Zwolak, P. et al. Bacterial reduction and shift with NPWT after surgical debridements: a retrospective cohort study. Arch Orthop Trauma Surg 137, 55–62 (2017). https://doi.org/10.1007/s00402-016-2600-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-016-2600-z