Abstract
Introduction
Fragility fractures are a growing worldwide health care problem. Hip fractures have been clearly associated with poor outcomes. Fragility fractures of other bones are common reasons for hospital admission and short-term disability, but specific long-term outcome studies of non-hip fragility fractures are rare. The aim of our trial was to evaluate the 1-year outcomes of non-hip fragility fracture patients.
Methods
This study is a retrospective cohort review of 307 consecutive older inpatient non-hip fracture patients. Patient data for analysis included fracture location, comorbidity prevalence, pre-fracture functional status, osteoporosis treatments and sociodemographic characteristics. The main outcomes evaluated were 1-year mortality and post-fracture functional status.
Results
As compared to the expected mortality, the observed 1-year mortality was increased in the study group (17.6 vs. 12.2 %, P = 0.005). After logistic regression, three variables remained as independent risk factors for 1-year mortality among non-hip fracture patients: malnutrition (OR 3.3, CI 1.5–7.1), Charlson comorbidity index (CCI) (OR 1.3, CI 1.1–1.5) and the Parker Mobility Score (PMS) (OR 0.85, CI 0.74–0.98). CCI and PMS were independent risk factors for a high grade of dependency after 1 year. Management of osteoporosis did not significantly improve after hospitalization due to a non-hip fragility fracture.
Conclusion
The outcomes of older non-hip fracture patients are comparable to the poor outcomes of older hip fracture patients, and appear to be primarily related to comorbidities, pre-fracture function and nutritional status. The low rate of patients on osteoporosis medications likely reflects the insufficient recognition of the importance of osteoporosis assessment and treatment in non-hip fracture patients. Increased clinical and academic attention to non-hip fracture patients is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fragility fractures are a major health care problem worldwide. Due to increasing life expectancy and other associated demographic changes, the incidence of fractures and post-fracture disability appear certain to increase [1]. Fragility fractures in older adults are mainly a consequence of osteoporosis. Most clinical outcome studies on fragility fractures are focused on older adults with hip fractures, and describe poor outcomes including up to 30 % 1-year mortality after fracture [2]. Despite advances in surgical and medical care, the excess mortality of hip fracture patient remains high and had not improved over the last decade [3]. Even in patients who survive greater than 1-year post-fracture, long-term functional disabilities are highly prevalent. Within 5-year post-fracture, one quarter of survivors were found to be bedridden and 45 % were not able to walk outside [4]. Due to these historical outcomes, most recent clinical trials and clinical quality improvement efforts for fragility fracture patients have focused mainly on patients with hip fracture. For example, orthogeriatric co-management models have shown to improve some of the outcomes of hip fracture patients [5]. In contrast, other fragility fractures such as proximal humerus, wrist, pelvis or vertebral fractures are under-represented in the current literature. Traditionally, fractures of the thoracolumbar vertebrae, distal radius, proximal femur and proximal humerus have been considered osteoporotic; more recently it has been suggested that pelvic fractures and fractures around the knee should also be considered to be osteoporotic [6, 7]. Kanis et al. [8] have suggested that other femoral fractures as well as fractures of the ribs, clavicle, scapula, sternum and diaphyseal fractures of the tibia and fibula should also be considered to be osteoporotic. The spectrum of adult fractures is increasingly dominated by fragility fractures. About 30 % of fractures in men, 66 % of fractures in women and 70 % of inpatient fractures are potentially osteoporotic [9]. With the exception of proximal femur fractures, epidemiologic and outcome data of other fragility fractures are not commonly published or reported. While a recent meta-analysis showed a 1-year mortality of 16.3 % of older patients with pelvic fracture [10], mortality and functional outcome studies of non-hip fragility fractures remain sparse.
The aim of our trial was to evaluate the mortality and functional outcomes of non-hip fragility fracture patients managed in a level-1 trauma center using a orthogeriatric co-management model [11].
Patients and methods
Study design
The present study is a retrospective cohort study. Data were collected prospectively by clinical routine and analyzed retrospectively. All patients and data were collected at a level-I trauma center in Austria running a Geriatric Fracture Center focused on hip fracture patients. The Geriatric Fracture Center is characterized by an orthogeriatric co-management model [11].
No institutional review and approval were necessary in light of the clinical origin of the data, its retrospective analysis, and use of de-identified patient data.
Study population
We included all in-hospital non-hip fracture patients aged over 70 from September 2009 to October 2010. A total of 307 patients were available for analysis, with a mean age of 83.2 ± 6.7 years; 79.2 % of the cohort was female. We split the study group into seven subgroups based on the anatomical location of the fractures (humerus, wrist, thoracic/rib clavicle/sternum, vertebra, pelvis/sacrum, and lower extremity, including distal femur, and periprosthetic fractures around the knee and tibia) and one group of unspecified fractures (uF), including hand, head, foot and ankle. All patient characteristics are presented in Table 1.
Data collection
Data were collected prospectively by clinical routine. Data extraction was performed by a study nurse and three of the authors (MG, TD and YH) by chart review. Cohort analysis was done by a study nurse and one of the authors (TD). Follow-up ended in October 2011.
Sociodemographic data
For the basic pre-fracture data set, we collected age, gender, pre-fracture residence and place of fall as well as the presence of previous fractures for each patient. At 1-year follow-up, we documented mortality status and place of residence.
Comorbidities
To measure comorbidity prevalence, we applied the Charlson Comorbidities Index (CCI) [12]. The CCI is valuable tool to predict the 1-year mortality for patients with a range of co-morbid conditions (a total of 22). Each condition is assigned with a point value of 1, 2, 3 or 6 depending on the mortality risk associated with this condition, and the total summary score can be used to assess comorbidity burden and predict mortality. CCI was routinely assessed at admission by a geriatrician.
Functional status
To assess the pre-fracture functional status, we used a systematized geriatric screening (GSL) described by Lachs [13]. This short, simple approach can be used by clinicians to routinely screen the functional status of older people. The screening is based on carefully selected evaluations of vision, hearing, arm and leg function, urinary continence, mental status, nutritional status, instrumental and basic activities of daily living, environmental hazards, and social support systems. It contains 15 items and the total score can be used for analysis. This tool is incorporated into the routine clinical practice of our Geriatric Fracture Center at the time of admission.
Mobility was assessed using the Parker Mobility Score (PMS) [14]. This score evaluates the patient’s ability to walk in three settings: inside, outside and going shopping or visiting family. For each of the settings, there are four ordinal responses (0–3) which are summed-up for a total score from 0 to 9, with nine describing maximum independent mobility. We assessed the pre-fracture PMS and follow-up. A significant decrease in post-fracture mobility was defined as a decline in PMS ≥1 point at 1 year.
At follow-up, we evaluated all patients using the Barthel Index (BI) [15]. BI is used to measure performance in basic activities of daily living by documenting the presence or absence of fecal or urinary incontinence, help needed with grooming, toilet use, feeding, transfers (e.g., from bed to chair), walking, dressing, climbing stairs and bathing. The maximum score of 100 points indicates a fully independent patient. This score has been validated for post-fracture assessment of hip fracture patients [16] with a score >80 indicating that a patient who is able to live more or less independently in the community [17].
Mortality
To assess 1-year mortality, the database was crosschecked with the registry of death from the governmental institute of epidemiology. One-year mortality is shown as percentage for the whole study group as well as for different fracture groups. Expected mortality, adjusted for age and gender, was calculated from the registry of the governmental institute of epidemiology.
Complications
Complications were identified through chart review. We looked specifically for thromboembolic events, gastrointestinal diseases (e.g., gastric bleeding), renal failure, cardiovascular events (e.g., myocardial infarction, atrial fibrillation, decompensated heart failure, stroke), pulmonary events (e.g., pneumonia), urinary tract infections and delirium.
Osteoporosis treatment
Treatment of osteoporosis was evaluated at admission and at discharge of the patients. We recorded basic treatment with vitamin D, calcium and specific drug treatment such as bisphosphonate therapy.
Statistical analysis
Statistical analysis was conducted using SPSS version 20.0 (2011). Metric scaled data are reported as arithmetic mean + standard deviation and categorical data as absolute frequency and percentage distribution. Non-parametric statistics (Mann–Whitney U test) were used since normality assumptions were not met for most of the outcome variables. Group effect and main condition effects were tested for significance by the Mann–Whitney U test. The Chi-square test for independence was used to determine a possible relationship between two categorical variables. The significance level was defined by P < 0.05. Multivariate logistic regression analysis was performed to identify factors associated with 1-year mortality, BI (ADL score ≤80) and mobility. Bivariate analyses were based on logistic regression to generate odds ratios (OR) and 95 % confidence intervals (CI). The dependent variable for these analyses was 1-year mortality, BI (ADL score ≤80) and a loss of at least one point in the PMS. Independent variables were gender, age, CCI, pre-fracture PMS, nutrition status and the fracture group.
Results
Fracture location and baseline characteristics (Table 1)
Table 1 shows the clinical characteristics for the overall cohort and by of the fracture location subgroups. The mean age of the patients was 83.2 ± 6.7 years. As compared to the overall cohort, patients who sustained a thoracic or pelvic fracture were older; patients with unspecified fracture locations were younger, with less pre-fracture comorbidity, dependency and osteoporotic treatment. Significant gender differences were seen in two subgroups: patients with humerus fracture were overwhelmingly female (90.5 %), whereas in group of uF males were overrepresented (36.7 %). Pre-fracture living situation differed only in the uF group. Patients with vertebral and pelvic fractures fell more frequently at home, and the prevalence of malnutrition was higher in patients with vertebral fractures (23.6 vs. 14.7 %) compared to the overall cohort. Regarding comorbidity differences, wrist fracture patients had significantly lower CCI scores, whereas patients with pelvic fracture had significantly higher CCI scores. The pre-fracture functional status and the mobility were better in patients in the wrist or uF subgroups. Patients with thoracic fractures had a higher level of baseline functional impairments; patients with pelvic fractures had lower pre-fracture mobility than those in other location subgroups.
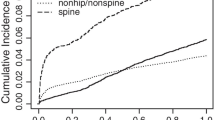
One-year excess mortality (Fig. 1)
The 1-year excess mortality was significantly increased in the study group (P = 0.005). Compared to the calculated expected mortality of 12.2, 17.6 % (n = 54) passed away within the first year. The excess mortality rate differed by fracture location and gender (Table 2). The highest mortality rates were observed in the patients with pelvic, thoracic, vertebral or unclassified fractures.
Predictors of 1-year mortality (Table 3)
Age (P = 0.02), CCI (P < 0.001), GSL (P < 0.001), Parker Mobility Score (P < 0.001) and place of fall (P < 0.009) were significantly correlated with 1-year mortality. Among the 15 items of GSL only nutrition (P < 0.001) and hearing impairment (P = 0.014) showed a significant correlation with the 1-year mortality. After logistic regression, three pre-fracture variables remained as independent risk factors for 1-year mortality: nutrition (OR 3.27, P = 0.001, CI 1.52–7.09), CCI (OR 1.27, P = 0.001, CI 1.11–1.46) and the PMS (OR 0.85, P = 0.024, CI 0.74–0.98).
Functional status (Table 3)
The mean BI scores of total study group as well as for the different fracture locations are shown in Table 3. The 1-year follow-up BI score was available from 210 patients (83 % of survivors). Patients who sustained thoracic, pelvic or vertebral fractures had significant lower BI scores 1 year after the fracture. However, after adjustment for age, sex, CCI, nutrition and the PMS, the fracture location was no longer associated with the BI score. Figure 2 shows the different items of the BI for the study group. In 44.3 % (n = 93) of the patients, we found a BI score ≤80 points. Based on our cutoff these patients were classified as severely impaired in their daily activities and are not able to live independently in the community. After adjustment for age, gender, fracture location and nutrition only CCI (P = 0.002, OR 1.51, CI 1.66–1.97) and PMS (P < 0.001, OR 0.52, CI 0.42–0.64) had significant associations with post-fracture dependence.
Mobility (Fig. 2)
On average, 1-year follow-up PMS was one point less than the pre-fracture score (0.96 ± 2.12, P < 0.001). A significant decrease in the PMS was observed for patients who sustained fractures in the following location subgroups: lower extremity (P = 0.017), pelvis (P < 0.001), thorax (P = 0.005) and uF (P = 0.009). A poor mobility outcome was defined by a loss of at least one point in the PMS after 1 year. After adjustment for age, gender, fracture location and nutrition a high CCI (OR 1.47, P = 0.023, CI 1.06–2.04) and high pre-fracture PMS (OR 1.64, P < 0.001, CI 1.33–2.03) remained independent risk factors for a loss of mobility (Table 4).
Further results
During hospital stay, 73 (23.8 %) patients sustained one or more inpatient complications, 11 (3.6 %) sustained two and 2 (0.7 %) patients three. The most frequent complications were 29 urinary tract infections (9.4 %), 15 cardiovascular events (4.9 %) and 21 patients with delirium (6.8 %).
Only 46.3 % (n = 142) were able to return home after hospital stay; 19.5 % (n = 60) went to a nursing home, 23.5 % (n = 72) were transferred to a geriatric rehabilitation unit, and 9.8 % (n = 30) to another department. One year after the fracture, 38.6 % (81) of the survivors were able to live at home independently. Patients with wrist fractures and uF’s had significantly higher rates of independent living. The vast majority of patients with pelvic fractures (80 %) were not living independently at 1-year post-fracture.
At admission, 42.9 % (n = 129) of patients carried a diagnosis of osteoporosis, 29.6 % (n = 91) were receiving calcium and vitamin D3 and 17.6 % (n = 54) had a specific osteoporosis treatment, mainly bisphosphonates. Among the fracture groups, there was no significant difference proportion of patients diagnosed with osteoporosis or treated with calcium, vitamin D or bisphosphonates. At discharge, the percentage of patients with an osteoporosis diagnosis increased to 48.5 % (n = 146), treatment rates with calcium and vitamin D3 increased to 52.9 % (n = 162) and treatment with specific osteoporosis drugs (e.g., bisphosphonates) to 18.9 % (n = 58).
Discussion
Our trial is the first that describes the pre-fracture characteristics and post-fracture outcomes of older non-hip fracture patients in the setting of a geriatric fracture center. Our study population is a representative cohort of fragility fracture patients treated within a geriatric fracture center [5]. Similar to other studies of fragility fractures and osteoporosis, the majority of our 307 patients were elderly (mean age 83.2 years) and female (80 %). [18]. Many of the demographic, comorbidity and functional characteristics of this cohort are similar to hip fracture patients studied at our hospital 4 years previously [4]. Mean CCI, number of previous fractures, complication rates and length of stay were similar between these hip fracture and non-hip fracture cohorts. Differences between these two cohorts included lower rates of nursing home residence and higher rates of osteoporosis treatment in this current non-hip fracture cohort. More non-hip fracture patients (17.6 %) had osteoporosis treatment at admission than the earlier hip fracture cohort at discharge (11 %) [4]. The higher rates of osteoporosis treatment in the non-hip fracture cohort are possibly due to an increased emphasis on the diagnosis and treatment of osteoporosis reflected in the more recent study. Compared to international rates of osteoporosis treatment, the proportion of pre-treated patients was high [18]. Patients, who sustained a wrist fracture or uF seemed to be younger and healthier than the other patients. In contrary, patients with thoracic, vertebral and pelvic fractures were older, had a higher number of comorbidities and lower functional status on admission.
One-year mortality for our non-hip fracture cohort was 17.6 % and similar to reported mortality rates in hip fracture patients. A recent study from Norway showed an overall mortality rate after 1 year of 21.3 % for hip fracture patients [19]. Abrahamsen et al. describe in their review from 2009 a 1-year hip fracture mortality rate from 8.4 to 36 %. They found also an increased mortality risk following hip fracture that was twice the age-matched control population although less pronounced with advancing age [20]. We found that non-hip fracture patients have also a significantly increased 1-year mortality (17.6 %) compared to an age and gender-matched controls (12.2 %). The relatively less-pronounced excess mortality in our trial may be explained by our older study population. The highest mortality rates were seen in patients suffering from pelvic, thoracic and vertebral fractures; these patients suffered overall mortality rates equal to or in excess of rates found in hip fracture patients. However, the fracture location itself did not have a significant impact on mortality after adjustment for different confounders. Patient’s comorbidities and the pre-fracture functional status had a greater association with outcomes than the fracture location [4]. The differences in the mortality rates among the different fracture groups probably reflect more the general health conditions than the specific fracture or the complications of fracture treatment. Comorbidities, pre-fracture functional and nutrition status were independent risk factors for 1-year mortality.
Similar to hip fracture patients, this cohort of non-hip fracture patients suffered severe outcomes. In addition to the 1-year mortality rate of 17.6 %, only 38.6 % of the surviving patients were able to remain at home. Many of them had 1-year post-fracture BI scores below the threshold for independence. In summary, after 1 year only one-third of non-hip fracture patients were able to live independently at their home.
The most important patient factor that predicted mortality and functional outcomes was the CCI. The CCI is well known as a predictor for the outcomes of older trauma patients as described by Kammerlander et al. [4] in hip fracture patients and Fleischmann et al. [21] in injured older adults.
This study also suggests that pre-fracture functional status in non-hip fracture patients has the same impact as in hip fracture patients. Pre-fracture functionality and mobility as well as the PMS are known to be independent predictors of outcomes of hip fracture patients [4, 14, 22]. One-year post-fracture patients in this cohort suffered an average 1 point decrease in their Parker Mobility Scores, irrespective of fracture location. As expected, we found reduced PMS in patients who sustained fractures of the lower extremity or the pelvis; interestingly, PMS was reduced for thoracic fracture patients as well. The poor mobility outcome of patients who sustained a thoracic fracture is consistent with other poor outcomes and high mortality rates. We observed a high grade of dependency among patients who sustained thoracic, vertebral, pelvic or a lower extremity fracture, but no statistically significant association between post-fracture dependency and fracture location, once these rates were adjusted for other cofactors.
Among hip fracture patients, malnutrition is a known independent risk factor for poor outcomes [26]. Poor nutritional status is associated with falls, leads to a higher rates of complications, longer lengths of stay and higher mortality rates [23]. In our study, the prevalence of malnutrition was low compared to other trials with reported malnutrition prevalence between 6 and 78 % in post-fracture patients [24]. In our trial, we did not use a validated nutritional assessment tool and likely underestimated the true prevalence of malnutrition in our population, especially compared to rates reported by Kaiser et al. (38.7 %) [25]. Despite this, nutritional status had a significant association with mortality within our cohort. In our trial, we did not find an association between malnutrition and functional status or mobility.
Age was not an independent risk factor for mortality. The data about the impact of age on the outcomes of older fragility fracture patients are controversial. We believe that age itself has no impact on mortality. Studies on this topic differ a lot, but many trials that have found age to be a predictor of mortality have not had detailed information regarding comorbidity and functional status, and may have not been able to adequately control for these confounding factors.
Osteoporosis treatment is a cornerstone in the management of fragility fracture patients. Although the pre-treatment rate was not very high and almost half of the patients had already suffered fractures at the time of this study, treatment rates were still higher than in other trials [18]. Unfortunately, osteoporosis treatment rates did not substantially improve between admission and discharge. This may be due to our small personal resources and the historical focus of the Geriatric Fracture Center on hip fracture patients.
Limitations
This study has several important limitations. The present single-center study has a retrospective, uncontrolled design. Our study population is a selected group of non-hip fracture patients of a geriatric fracture center, and may not be easily generalizable to other health care settings or communities. While the total number of patients is sufficient, the proportion of male patients, and some fracture types and fracture locations are under-represented. Male patients were relatively under-represented in this cohort; this likely impaired the ability to identify any impact of gender on outcomes. As with most retrospective cohort studies, data on complications and comorbidity are likely incomplete, as these data depend on variable clinical assessments performed by different health care providers. To minimize that risk, we used validated and standardized scores whenever possible. The accuracy of 1-year functional status assessment was limited by the use of self-reports of the patients or their care givers.
Conclusion
Our results clearly demonstrate poor 1-year outcomes of older non-hip fragility fracture patients. One-year mortality is 17.6 % and similar to that described in hip fracture patients. Mortality risk is independent of fracture location and patient age. Comorbidities, nutrition and pre-fracture functional status have a significant impact on 1-year mortality of non-hip fracture patients. In addition to high mortality rates, non-hip fragility fracture patients also suffer from high rates of disability and loss of function, even for fractures not involving the extremities. Low rates of osteoporosis diagnosis and treatment likely reflect insufficient awareness of the osteoporotic nature of these fractures, and represent an opportunity for improvement in outcomes. These findings strongly suggest that geriatric fracture centers should broaden their focus to include non-hip fragility fracture evaluation and treatment.
References
Leung F, Blauth M, Bavonratanavech S (2010) Surgery for fragility hip fracture—streamlining the process. Osteoporos Int 21(Suppl 4):519–521
Elliott J, Beringer T, Kee F, Marsh D, Willis C, Stevenson M (2003) Predicting survival after treatment for fracture of the proximal femur and the effect of delays to surgery. J Clin Epidemio 56(8):788–795
Finnes TE, Meyer HE, Falch JA, Medhus AW, Wentzel-Larsen T, Lofthus CM (2013) Secular reduction of excess mortality in hip fracture patients >85 years. BMC Geriatr 13:25. doi:10.1186/1471-2318-13-25
Kammerlander C, Gosch M, Kammerlander-Knauer U, Luger TJ, Blauth M, Roth T (2011) Long-term functional outcome in geriatric hip fracture patients. Arch Orthop Trauma Surg 131(10):1435–1444
Kammerlander C, Roth T, Friedman SM, Suhm N, Luger TJ, Kammerlander-Knauer U et al (2010) Ortho-geriatric service—a literature review comparing different models. Osteoporos Int 21(Suppl 4):637–646
Parkkari J, Kannus P, Niemi S, Pasanen M, Järvinen M, Lüthje P et al (1996) Secular trends in osteoporotic pelvic fractures in Finland: number and incidence of fractures in 1970–1991 and prediction for the future. Calcif Tissue Int 59(2):79–83
Kannus P, Niemi S, Palvanen M, Parkkari J, Pasanen M, Järvinen M et al (2001) Continuously rising problem of osteoporotic knee fractures in elderly women: nationwide statistics in Finland in 1970–1999 and predictions until the year 2030. Bone 29(5):419–423
Kanis JA, Oden A, Johnell O, Jonsson B, de Laet C, Dawson A (2001) The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 12(5):417–427
Court-Brown CM, Caesar B (2006) Epidemiology of adult fractures: a review. Injury 37(8):691–697
Krappinger D, Kammerlander C, Hak DJ, Blauth M (2010) Low-energy osteoporotic pelvic fractures. Arch Orthop Trauma Surg 130(9):1167–1175
Kammerlander C, Gosch M, Blauth M, Lechleitner M, Luger TJ, Roth T (2011) The Tyrolean Geriatric Fracture Center: an orthogeriatric co-management model. Z Gerontol Geriatr 44(6):363–367
Charlson M, Szatrowski TP, Peterson J, Gold J (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47(11):1245–1251
Lachs MS, Feinstein AR, Cooney LM Jr, Drickamer MA, Marottoli RA, Pannill FC et al (1990) A simple procedure for general screening for functional disability in elderly patients. Ann Intern Med 112(9):699–706
Parker MJ, Palmer CR (1993) A new mobility score for predicting mortality after hip fracture. J Bone Joint Surg Br 75(5):797–798
Mahoney FI, Barthel DW (1965) Functional evaluation: the Barthel Index. Md State Med J 14:61–65
Bryant DM, Sanders DW, Coles CP, Petrisor BA, Jeray KJ, Laflamme GY (2009) Selection of outcome measures for patients with hip fracture. J Orthop Trauma 23(6):434–441
Kasner SE (2006) Clinical interpretation and use of stroke scales. Lancet Neurol 5(7):603–612
Liu SK, Munson JC, Bell JE, Zaha RL, Mecchella JN, Tosteson AN et al (2013) Quality of osteoporosis care of older medicare recipients with fragility fractures: 2006 to 2010. J Am Geriatr Soc 61(11):1855–1862
Diamantopoulos AP, Hoff M, Hochberg M, Haugeberg G (2013) Predictors of short- and long-term mortality in males and females with hip fracture—a prospective observational cohort study. PLoS One 8(10):e78169. doi:10.1371/journal.pone.0078169
Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C (2009) Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 20(10):1633–1650
Fleischman RJ, Adams AL, Hedges JR, Ma OJ, Mullins RJ, Newgard CD (2010) The optimum follow-up period for assessing mortality outcomes in injured older adults. J Am Geriatr Soc 58(10):1843–1849
Hagino T, Ochiai S, Sato E, Watanabe Y, Senga S, Haro H (2011) Prognostic prediction in patients with hip fracture: risk factors predicting difficulties with discharge to own home. J Orthop Traumatol 12(2):77–80
O’Daly BJ, Walsh JC, Quinlan JF, Falk GA, Stapleton R, Quinlan WR et al (2010) Serum albumin and total lymphocyte count as predictors of outcome in hip fractures. Clin Nutr 29(1):89–93
Bell JJ, Bauer JD, Capra S (2013) The malnutrition screening tool versus objective measures to detect malnutrition in hip fracture. J Hum Nutr Diet 26(6):519–526
Kaiser MJ, Bauer JM, Rämsch C, Uter W, Guigoz Y, Cederholm T, Mini Nutritional Assessment International Group et al (2010) Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc 58(9):1734–1738
Hershkovitz A, Kalandariov Z, Hermush V, Weiss R, Brill S (2007) Factors affecting short-term rehabilitation outcomes of disabled elderly patients with proximal hip fracture. Arch Phys Med Rehabil 88(7):916–921
Conflict of interest
None of the authors has conflict of interest regarding the topics discussed in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gosch, M., Druml, T., Nicholas, J.A. et al. Fragility non-hip fracture patients are at risk. Arch Orthop Trauma Surg 135, 69–77 (2015). https://doi.org/10.1007/s00402-014-2115-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-014-2115-4