Abstract
Purpose
This prospective randomized study compared acute and chronic anterior cruciate ligament (ACL) reconstruction using ligament advanced reinforcement system (LARS) artificial ligament in young active adults with a 5-year follow-up.
Methods
Fifty-five patients were enrolled in this study and divided into two groups based on the elapsed time between the injury and reconstruction: the acute group (3–7 weeks) and the chronic group (6–11 months). The clinical outcomes were evaluated using the Lysholm knee scoring scale, the Tegner activity rating, a KT-1000 Arthrometer, and the International Knee Documentation Committee (IKDC) scoring system. Isokinetic strength of the quadriceps and hamstring was assessed using the Biodex System 3 isokinetic dynamometer.
Results
Anterior laxity was decreased and quadriceps/hamstring muscle strength was increased in the acute group compared to the chronic group (p > 0.05). There were no statistically significant differences in Lysholm scores, Tegner activity scores, and the IKDC evaluation form between the two groups.
Conclusions
These results suggest that earlier ACL reconstruction using a LARS artificial ligament may provide an advantage in the treatment and rehabilitation of ACL rupture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The anterior cruciate ligament (ACL) is the primary structure that provides knee joint stability. The function of the ACL is to limit excessive anterior displacement during movement. Thus, an ACL rupture can lead to instability and increased abrasion of the knee joint, a higher probability of meniscus injury, and future arthritis.
An ACL rupture is a common sports injury, especially in an otherwise healthy population of active young people. However, the benefits of early or delayed ACL reconstruction, and the optimal time interval between injury and repair remain controversial [1–7]. Noyes et al. [4] concluded that patients with acute injury experience less pain and fewer limitations than chronic cases, and they emphasized the need for earlier reconstruction and joint stabilization in active persons. Cipolla et al. [3] suggested that the “ideal” time for an ACL reconstruction is during the 3–6 weeks after the initial injury, and that patients should follow a well-planned program of exercises to strengthen the quadriceps and hamstrings before surgery. In contrast, Wasilewski et al. [1] reported that knee recovery after acute ACL reconstruction (performed within 1 month after injury) is significantly slower than after chronic reconstruction. Other studies [2, 6] indicate that there are minimal differences in outcome when comparing early and delayed ACL reconstruction. One [2] recommendation is that arthroscopic ACL reconstruction should be performed within 6 weeks of the primary knee injury, as delayed treatment may predispose patients to cartilage lesions and meniscal tears.
Reconstruction of an ACL rupture using the ligament advanced reinforcement system (LARS) graft has become an increasingly popular treatment option[8–14]. As a third-generation artificial ligament, the LARS graft can provide sufficient strength to imitate normal ACL function and adapt to daily activities, competitive sports, or strenuous activities, when implanted into the knee joint. Due to its intrinsic properties, the LARS ligament does not undergo “ligamentization” [15, 16], which is a characteristic of allografts and autografts. This allows the LARS graft to endure stretching and twisting forces during post-operative rehabilitation or mild activities [13], and may provide an advantage in the early return to unrestricted sports and strenuous activities. As such, LARS may be the ideal choice for implantation in young active patients. Indeed, evidence suggests that patients who choose a LARS graft reconstruction can begin their rehabilitation earlier [8, 10]. Several studies have focused on evaluating the outcome of LARS and other grafts such as the bone-patellar tendon-bone (BPTB) and hamstring grafts [17–19]. Data have shown that the therapeutic effects of ACL reconstruction using a LARS ligament can last several years [8, 10, 12–14, 17–19], but there has not been a comparison of the clinical outcome between acute and chronic patients treated with a LARS ligament reconstruction.
The aim of this study was to compare the clinical outcome of ACL reconstruction using a LARS ligament in patients at the acute and chronic stage following knee injury. We hypothesized that patients undergoing earlier ACL reconstruction using a LARS ligament would have better treatment and rehabilitation outcomes than chronic patients.
Materials and methods
Subjects
From March 2004 to April 2007, 62 patients who underwent ACL reconstructions using LARS grafts in our institution were considered eligible to participate in our prospective randomized study.
The inclusion criteria for this study were: (1) unilateral ACL rupture with a normal contralateral knee; (2) primary reconstruction using a LARS graft; (3) a visible ACL remnant by magnetic resonance imaging (MRI) [14]; and (4) patients younger than 50 years of age. The exclusion criteria were: (1) a combined ligament injury or additional existing ligament instability in the affected knee; (2) contralateral knee ligament injury; (3) previous history of knee surgery; (4) infection or septic arthritis in either knee; and (5) patients who declined to participate. In addition, patients who failed to comply with the rehabilitation protocol, were lost to follow-up, or concealed a repeat injury during the study period were excluded.
Informed consent was obtained from all of the patients who participated in this study. The study was authorized by our institutional review board of Taizhou Hospital and was performed in accordance with the ethical standards of the Declaration of Helsinki (revised in 2000) as it was in effect during the study period. All patients were advised about the differences and risks associated with autograft, allograft, and synthetic ligament procedures; the selection of which graft to use was made by the patient. Pursuant to the patients’ decisions, ACL reconstructions with LARS artificial ligaments were performed.
A total 55 patients were finally enrolled in our prospective, randomized study: 27 patients were randomly assigned to the acute group and 28 to the chronic group. We allocated the patients by rolling a dice: those patients with odd digits were assigned to the acute group, and those with even digits to the chronic group. All operations were performed by a treatment team that consisted of two experienced arthroscopic orthopedic sports medicine surgeons (Jia Chen and Aiqun Gu).
Demographic and clinical data before ACL reconstruction using the LARS ligament are shown in Table 1. The acute group underwent surgery 3–7 weeks after the initial ACL injury, and the chronic group underwent surgery 6–11 months after the initial ACL injury. The mean follow-up time was 61.2 months. The acute group included 15 male and 12 female patients with a mean age of 29.4 ± 5.8 years old. The chronic group included 11 male and 17 female patients with a mean age of 31.9 ± 7.0 years old.
Surgical technique
Two senior surgeons experienced in LARS techniques performed all of the reconstructions using arthroscopic techniques. A diagnostic arthroscopy was performed through standard anteromedial and anterolateral portals under adequate anesthesia. The identification of the actual condition of the ACL rupture and intra-articular structure was performed at this time. Any medial or lateral meniscus injuries were treated by partial meniscectomy or meniscus repair at the same time. During arthroscopic diagnosis, the scar tissue that had adhered to the deficient ACL and surrounding tissue was slightly loosened. The ACL remnant was preserved as much as possible, especially on the tibial side. The LARS (Arc-sur-Tille, France) artificial ligament was used for ACL reconstruction in our study.
The surgical procedure and isometric insertion techniques were performed as described by Dericks [13]. Drilling resulted in tibial and femoral tunnels that were 7.5 mm in diameter in all patients. The tibial drill guide was used to locate the intra-articular point of the tibial tunnel in the middle of the tibial ACL footprint. The femoral tunnel was positioned at 10:30 o’clock on the right knee, and at nearly 1:30 o’clock on the left knee. Guided by a cannulated tube, a loop wire was inserted through the tibial tunnel to the center of the ACL stump, and pierced through into the femoral tunnel until it finally reached the lateral thigh. The LARS ligament was introduced by the loop wire, inserted into the intra-articular joint through the ACL stump, and the longitudinal free fibers were retained in the joint. The end of the LARS ligament was fixed in the femoral tunnel with an interference screw. Reliable tension on the LARS ligament was achieved by pulling the tibial end of the LARS and maximally extending and flexing the knee 20 times. The LARS graft in the tibial tunnel was fixed with an interference screw with a knee flexion of 30°. The residual ends of the tibial and femoral LARS ligament were cut off using a customized sharp reamer.
Rehabilitation protocol
A physical therapist (Haitao Jiang) at our institution who was blinded to subject assignment carried out physiotherapeutics and rehabilitation in the two groups. The acute group required four types of treatment interventions during pre-operative rehabilitation, including counseling, symptom relief, physiotherapy, and gradual exercise (Table 2). The chronic group was provided with a comprehensive pre-operative physiotherapy program that did not include interventions for symptom relief.
All of the patients received the same post-operative rehabilitation protocol (Table 3). One day after surgery, several interventions focused on reducing discomfort and complications, such as pain, swelling, and inflammation. Patients also began some initial exercises on the affected extremity. A continuous passive motion (CPM) instrument was set to 0°–30° ROM. After 3 days to 1 week, isometric and isotonic quadriceps exercises were performed to increase thigh muscle strength. At approximately 1 week, patients were allowed to undertake partial weight-bearing activities assisted by crutches. The CPM was set to 0°–40° ROM. In the first month, patients were asked to achieve full weight-bearing activities without crutches, and to begin balance and gait training assisted by a walker. The CPM passive activity was set to 0°–130° ROM. At 2–3 months, patients gradually achieved locomotor activity without any auxiliary tools. After 1 year or more, patients could perform some recreational or non-competitive sports.
Clinical outcome evaluation and statistical analyses
Patients in the two groups were followed for an average of 61.2 months (5.1 years). The KT-1000 Arthrometer (Medmetric, San Diego, CA, USA) was used clinically to evaluate knee stability. The Lysholm score, the Tegner activity score, and the International Knee Documentation Committee (IKDC) scoring systems were used to clinically evaluate knee function outcomes. Muscle strength, including the quadriceps and hamstring, was evaluated relative to the strength of the contralateral limb and is presented as a percentage of the operative knee to the contralateral limb (100 %). Muscle strength was assessed using the Biodex System 3 isokinetic dynamometer (Biodex Medical Inc., New York, USA) at an angular velocity of 60 and 180°/s at 1 year following surgery and at the final follow-up visit. Statistical analysis was performed using SPSS 19.0 software. Data are expressed as mean ± SD. The Levene’s test was used to evaluate whether there was equal variance in the continuous variables. Data that were normally distributed as well as paired data were analyzed using a Student’s t test. Categorical variables were compared using the Chi squared test. The threshold for statistical significance was p < 0.05.
Results
Before ACL reconstruction, there were no significant differences between the groups in terms of the number of patients, gender distribution, age, Lysholm score, Tegner activity score or pre-operative trauma symptoms. Pre-operative recurrent episodes of instability occurred in 33.3 % (9/27) patients in the acute group, while 25 % (7/28) patients in chronic group. And 29.6 % (8/27) and 32 % (9/28) patients suffered from persistent pain pre-operatively in the acute and chronic groups, respectively.
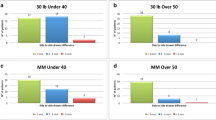
The assessment of knee function is shown in Table 4. After 1 year, the mean Lysholm scores between the acute (93.37 ± 3.89) and chronic groups (91.64 ± 5.12) were not significantly different (p = 0.164). At the final follow-up 5 years after surgery, the Lysholm scores were 95.04 ± 5.10 and 92.64 ± 5.48 for the acute and chronic groups, respectively (p = 0.099). The mean Tegner activity score showed similar trends. One year after reconstruction, there was no significant difference between the acute (6.3 ± 1.1) and chronic (6.1 ± 0.9) groups (p = 0.413). The acute and chronic groups were similar 5 years after the reconstruction, scoring 6.3 ± 1.3 and 6.3 ± 1.2, respectively (p = 0.978). In terms of the overall IKDC rating scale, a “normal” grade was attained by 23/27 (85 %) of the patients in the acute group 1 year after surgery and 20/27 (74 %) patients after 5 years. In the chronic group, 18/28 (64 %) patients attained “normal” function after 1 year and 17/28 (61 %) after 5 years.
Anterior laxity in the knee was assessed using the KT-1000 Arthrometer (30° flexion and 134 N) (Table 5). At the 5-year follow-up, the mean side-to-side difference was 2.35 ± 1.21 and 2.88 ± 1.26 mm in the acute and chronic groups, respectively (p = 0.116). Even though the results were not significantly different, the actual side-to-side difference was slightly higher in the chronic group compared with the acute group at the final follow-up visit.
The Biodex System 3 isokinetic dynamometer was used to record quadriceps and hamstring muscle strength at a velocity of 60 and at 180°/s. The maximal peak torque was evaluated as percentage of the operative knee to the contralateral limb (100 %). The quadriceps strength in the acute group at both the 1 and 5-year follow-ups was greater than in the chronic group (Fig. 1). Similar results were obtained with regard to hamstring strength (Fig. 2). However, there was not a statistically significant difference between the two groups after the LARS reconstruction.
None of the patients had severe symptoms such as synthetic ligament rupture and obvious synovitis of the knee at the final follow-up. There were also no superficial or deep infections in any of the patients, and wound healing post-operatively occurred without complications. One patient in each group was affected by mild arthrofibrosis post-operatively. Only 1 patient in the acute group suffered persistent arthralgia 8 months after the operation due to loosening of the femoral screw (Fig. 3).
Discussion
This midterm follow-up of ACL reconstruction using the LARS ligament showed that patients who underwent earlier ACL reconstruction had improved clinical outcomes, experienced less pain and instability of the injured knee, and achieved earlier rehabilitation compared to patients who delayed surgery. In accordance with our findings, Goradia et al. [5] reported that patients who underwent ACL reconstruction with a triple-strand hamstring tendon graft within 6 weeks of injury achieved higher scores on the Lysholm scale and Cincinnati function scale, and more patients achieved normal grades on the IKDC standard form, when compared to patients who underwent reconstruction at 32.3 months after initial injury. In the current study, post-operative Lysholm scores, Tegner activity scores, and scores on the IKDC evaluation form were slightly better in the acute group, although there were no statistically significant differences between the acute and chronic groups. However, in the chronic group, 25 % of patients experienced recurrent episodes of instability pre-operatively, and 32 % of patients suffered from persistent pre-operative pain. These symptoms were present for 6 months until the patients received their initial surgery. Acute intervention would provide early relief from this discomfort.
Compared with autograft reconstructive surgeries, a LARS graft is able to endure stretching and twisting forces immediately after surgery. However, ligament laxity is an inevitable challenge associated with ligament reconstruction surgery. There is a close relationship between ligament laxity and the ligament stump. Several studies have noted that the LARS graft can be implanted while preserving the remnant of the ACL tissue, especially on the tibial side [13, 14, 20]. The new generation of LARS ligament consists of polyethylene terephthalate (PET). The porosity of this material allows fibroblasts and osteoblast-like cells to grow into the intra-articular multifilament portion [8, 9, 12, 21]. In the present study, the mean anterior laxity was lower in the acute group than in the chronic group. This is consistent with previous work [8] whereby Nau et al. concluded that the mean measured laxity was greater in their chronic LARS group, particularly at 6 months post-operation. It is possible that the ACL remnant forms scar tissue around itself by 6 months after the injury, which prevents new neurovascular tissue from adhering to the fiber of the synthetic ligament. If this is the case, the viable stump would not augment the physical strength of the LARS graft during stretching and twisting [22]. In vivo and in vitro evidence suggests that performing ACL reconstruction earlier after initial rupture can be beneficial for the growth of the preserved ACL remnant [21], and can help avoid a variable pattern of scar formation and associated changes in knee laxity [23]. Furthermore, delayed reconstruction can result in impingement of the ACL remnant [20] and abrasion of the cartilaginous surface.
To date, the optimum timing of reconstruction relative to injury remains controversial. Our study defined an acute timeframe as less than or approximately 6 weeks post injury (patients were operated on 3–7 weeks post injury) and a chronic timeframe as >6 months post injury, which is consistent with previous reports [1, 4, 6, 10, 24, 25]. Noyes et al. [4] defined the mean acute pre-operative interval as 6 weeks and the chronic pre-operative interval as ACL rupture at least 3 months before ACL reconstruction using a patellar tendon autograft. They showed early ACL reconstruction restored stability in more knees and resulted in less pain than chronic ACL reconstruction. However, some studies [1, 7] have suggested that early ACL reconstruction can increase the risk of stiffness, swelling, and other mechanical complications such as a reduced range of motion. In the present study, we defined the “acute” phase not as an immediate operation after ACL rupture, but as the time required to systematically manage rehabilitation and establish a good mental attitude [1, 26].
Implementing a rehabilitation protocol as early as possible has equally obvious benefits for restoring the strength of the quadriceps and hamstring muscles, which play an important role in knee function. The strength of the quadriceps and hamstring muscles determines the level of knee flexion or extension after a reconstruction for ACL deficiency [27]. Schenck et al. [28] found that atrophy in the quadriceps gradually increased after ACL reconstruction. This suggests a need to regain control of the leg and to begin performing exercises to prevent quadriceps/hamstring co-contractions soon after surgery. Patients who undergo reconstruction using hamstring, BPTB, or any other grafts such as an allograft require gradual rehabilitative exercise, which prolongs the time interval between initial injury and reconstruction in comparison to patients treated using a LARS graft. In the present study, patients in the acute group had slightly greater peak quadriceps and hamstring torque than in the chronic group, although there were no statistically significant differences between the two groups 1 and 5 years after surgery. While performing an “earlier” ACL reconstruction and a pre-operative knee rehabilitation protocol may contribute to earlier post-operative rehabilitation of the injured limb and improved patients’ subjective satisfaction.
Importantly, we did not observe any immediate post-operative complications. One patient from each group suffered from mild post-operative arthrofibrosis, which was resolved through gradual rehabilitation, without arthroscopic lysis, to achieve a satisfactory clinical outcome. One patient in the acute group suffered persistent arthralgia 8 months post-operation. This was attributed to femoral screw loosening. The screw tail was rubbing against the hypodermis, causing local swelling and pain, but did not result in anterior knee laxity. The screw was adjusted, and the screw tail drilled into the femoral tunnel. The patient’s symptoms resolved within 2 weeks, consistent with findings from Gao et al. [14].
Our study has several strengths. First, we evaluated clinical outcomes in patients who underwent early and delayed LARS artificial ligament reconstruction for ACL rupture. Second, we implemented a rigid, detailed, and systematic rehabilitation protocol for all patients, which aimed to minimize the complications associated with knee recovery after injury. Finally, we retained the ligament stump in all enrolled patients to facilitate the reconstruction of ligamentous and neurovascular tissues. However, our study is also subject to several limitations. First, we did not investigate the clinical outcome of meniscal injury. Second, the subacute phase defined in some literature was not included. Addressing the role of the subacute phase is a goal for our next research study. Third, the number of cases in our observational cohort was small, and the result could be different in a large sample size. Finally, while the mean follow-up time was 5 years, additional studies are required for longer-term follow-up of patients undergoing ACL reconstructions with LARS graft.
Conclusion
This study suggests that performing ACL reconstruction soon after injury using a LARS graft could provide an advantage for treating and rehabilitating ACL ruptures, as long as the acute reconstruction is based on the foundation of a good rehabilitation protocol that improves the recovery of the patient’s injured limb.
References
Wasilewski SA, Covall DJ, Cohen S (1993) Effect of surgical timing on recovery and associated injuries after anterior cruciate ligament reconstruction. Am J Sports Med 21(3):338–342
Andernord D, Karlsson J, Musahl V et al (2013) Timing of surgery of the anterior cruciate ligament. Arthroscopy 29(11):1863–1871
Cipolla M, Scala A, Gianni E et al (1995) Different patterns of meniscal tears in acute anterior cruciate ligament (ACL) ruptures and in chronic ACL-deficient knees. Knee Surg Sports Traumatol Arthrosc 3(3):130–134
Noyes FR, Barber-Westin SD (1997) A comparison of results in acute and chronic anterior cruciate ligament ruptures of arthroscopically assisted autogenous patellar tendon reconstruction. Am J Sports Med 25(4):460–471
Goradia VK, Grana WA (2001) A comparison of outcomes at 2 to 6 years after acute and chronic anterior cruciate ligament reconstructions using hamstring tendon grafts. Arthroscopy 17(4):383–392
Flint JH, Wade AM, Giuliani J et al (2014) Defining the terms acute and chronic in orthopaedic sports injuries: a systematic review. Am J Sports Med 42(1):235–241
Shelbourne KD, Patel DV (1995) Timing of surgery in anterior cruciate ligament-injured knees. Knee Surg Sports Traumatol Arthrosc 3(3):148–156
Nau T, Lavoie P, Duval N (2002) A new generation of artificial ligaments in reconstruction of the anterior cruciate ligament two-year follow-up of a randomised trial. J Bone Joint Surg Br 84(3):356–360
Viateau V, Manassero M, Anagnostou F et al (2013) Biological and biomechanical evaluation of the ligament advanced reinforcement system (LARS AC) in a sheep model of anterior cruciate ligament replacement: a 3-month and 12-month study. Arthroscopy 29(6):1079–1088
Lavoie P, Fletcher J, Duval N (2000) Patient satisfaction needs as related to knee stability and objective findings after ACL reconstruction using the LARS artificial ligament. Knee 7(3):157–163
Ventura A, Terzaghi C, Legnani C et al (2010) Synthetic grafts for anterior cruciate ligament rupture: 19-year outcome study. Knee 17(2):108–113
Parchi PD, Gianluca C, Dolfi L et al (2013) Anterior cruciate ligament reconstruction with LARS™ artificial ligament results at a mean follow-up of eight years. Int Orthop 37(8):1567–1574
Dericks G Jr (1995) Ligament advanced reinforcement system anterior cruciate ligament reconstruction. Oper Tech Sports Med 3(3):187–205
Gao K, Chen S, Wang L et al (2010) Anterior cruciate ligament reconstruction with LARS artificial ligament: a multicenter study with 3-to 5-year follow-up. Arthroscopy 26(4):515–523
Amiel D, Kleiner JB, Roux RD et al (1986) The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res 4(2):162–172
Falconiero RP, DiStefano VJ, Cook TM (1998) Revascularization and ligamentization of autogenous anterior cruciate ligament grafts in humans. Arthroscopy 14(2):197–205
Liu Z, Zhang X, Jiang Y et al (2010) Four-strand hamstring tendon autograft versus LARS artificial ligament for anterior cruciate ligament reconstruction. Int Orthop 34(1):45–49
Li B, Wen Y, Wu H et al (2009) Arthroscopic single-bundle posterior cruciate ligament reconstruction: retrospective review of hamstring tendon graft versus LARS artificial ligament. Int Orthop 33(4):991–996
Pan X, Wen H, Wang L et al (2013) Bone-patellar tendon-bone autograft versus LARS artificial ligament for anterior cruciate ligament reconstruction. Eur J Orthop Surg Traumatol 23(7):819–823
Ahn JH, Wang JH, Lee YS et al (2011) Anterior cruciate ligament reconstruction using remnant preservation and a femoral tensioning technique: clinical and magnetic resonance imaging results. Arthroscopy 27(8):1079–1089
Trieb K, Blahovec H, Brand G et al (2004) In vivo and in vitro cellular ingrowth into a new generation of artificial ligaments. Eur Surg Res 36(3):148–151
Machotka Z, Scarborough I, Duncan W et al (2010) Anterior cruciate ligament repair with LARS (ligament advanced reinforcement system): a systematic review. BMC Sports Sci Med Rehabil 2(1):29
Crain EH, Fithian DC, Paxton EW et al (2005) Variation in anterior cruciate ligament scar pattern: does the scar pattern affect anterior laxity in anterior cruciate ligament-deficient knees? Arthroscopy 21(1):19–24
Åhlén M, Lidén M (2011) A comparison of the clinical outcome after anterior cruciate ligament reconstruction using a hamstring tendon autograft with special emphasis on the timing of the reconstruction. Knee Surg Sports Traumatol Arthrosc 19(3):488–494
de Roeck NJ, Lang-Stevenson A (2003) Meniscal tears sustained awaiting anterior cruciate ligament reconstruction. Injury 34(5):343–345
Frobell RB, Roos HP, Roos EM et al (2013) Treatment for acute anterior cruciate ligament tear: five year outcome of randomised trial. BMJ 346:f232
Seto JL, Orofino AS, Morrissey MC et al (1988) Assessment of quadriceps/hamstring strength, knee ligament stability, functional and sports activity levels five years after anterior cruciate ligament reconstruction. Am J Sports Med 16(2):170–178
Schenck RC, Blaschak MJ, Lance ED et al (1997) A prospective outcome study of rehabilitation programs and anterior cruciate ligament reconstruction. Arthroscopy 13(3):285–290
Conflict of interest
No funds were received in support of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, J., Gu, A., Jiang, H. et al. A comparison of acute and chronic anterior cruciate ligament reconstruction using LARS artificial ligaments: a randomized prospective study with a 5-year follow-up. Arch Orthop Trauma Surg 135, 95–102 (2015). https://doi.org/10.1007/s00402-014-2108-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-014-2108-3