Abstract
Chronic traumatic encephalopathy (CTE) is a tauopathy associated with repetitive head impacts (RHI) that has been neuropathologically diagnosed in American football players and other contact sport athletes. In 2013, McKee and colleagues proposed a staging scheme for characterizing the severity of the hyperphosphorylated tau (p-tau) pathology, the McKee CTE staging scheme. The staging scheme defined four pathological stages of CTE, stages I(mild)–IV(severe), based on the density and regional deposition of p-tau. The objective of this study was to test the utility of the McKee CTE staging scheme, and provide a detailed examination of the regional distribution of p-tau in CTE. We examined the relationship between the McKee CTE staging scheme and semi-quantitative and quantitative assessments of regional p-tau pathology, age at death, dementia, and years of American football play among 366 male brain donors neuropathologically diagnosed with CTE (mean age 61.86, SD 18.90). Spearman’s rho correlations showed that higher CTE stage was associated with higher scores on all semi-quantitative and quantitative assessments of p-tau severity and density (p’s < 0.001). The severity and distribution of CTE p-tau followed an age-dependent progression: older age was associated with increased odds for having a higher CTE stage (p < 0.001). CTE stage was independently associated with increased odds for dementia (p < 0.001). K-medoids cluster analysis of the semi-quantitative scales of p-tau across 14 regions identified 5 clusters of p-tau that conformed to increasing CTE stage (stage IV had 2 slightly different clusters), age at death, dementia, and years of American football play. There was a predilection for p-tau pathology in five regions: dorsolateral frontal cortex (DLF), superior temporal cortex, entorhinal cortex, amygdala, and locus coeruleus (LC), with CTE in the youngest brain donors and lowest CTE stage restricted to DLF and LC. These findings support the usefulness of the McKee CTE staging scheme and demonstrate the regional distribution of p-tau in CTE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic traumatic encephalopathy (CTE) is a tauopathy associated with exposure to repetitive head impacts (RHI) [2, 36] that has been reported in a variety of contact sport athletes [14, 27, 29, 34], including American football players [5, 34, 37]. In 2013, in a case series of 68 male brain donors with CTE, McKee and colleagues proposed criteria for the neuropathological diagnosis of CTE. The diagnostic criteria were adopted and refined at the first National Institute of Neurological Disorders and Stroke (NINDS) and National Institute of Biomedical Imaging and Bioengineering (NIBIB) consensus meeting to define the neuropathological criteria for CTE [32]. The 2015 panel also defined the pathognomonic lesion of CTE as “an accumulation of abnormal hyperphosphorylated tau (p-tau) in neurons and astroglia distributed around small blood vessels at the depths of cortical sulci and in an irregular pattern”. Since publication, the NINDS–NIBIB criteria for the neuropathological diagnosis of CTE have been used in multiple studies and across different cohorts [2, 3, 5, 13, 20, 21, 27, 29, 32, 36, 37]. In a few, isolated reports [23, 28], perivascular astrocytic lesions of age-related tau astrogliopathy (ARTAG) have been confused with pathognomonic lesions of CTE, leading to the suggestion that CTE pathology is found in people “not known to have experienced multiple blows to the head” [23]. At the present time, however, there are insufficient data to consider purely astrocytic lesions as diagnostic for CTE. Moreover, there is mounting evidence that the presence of p-tau immunoreactive astrocytes in the pathognomonic lesions of CTE, in addition to neuronal NFT and disordered neurites, is a function of advancing age [12].
In the case series published in 2013, McKee and colleagues proposed a staging system for characterizing the severity of p-tau pathology, the McKee CTE staging scheme. The McKee CTE staging scheme was based on hemispheric 50 µm-thick free-floating sections immunostained for p-tau. Braak method of staging the p-tau pathology of Alzheimer’s disease (AD) [7, 8] was used as a model for staging CTE. Four pathological stages of CTE were defined, ranging from stage I (mild) to stage IV (severe) (Table 1). In stage I CTE, 1 or 2 isolated foci of p-tau NFTs and dot-like neurites are found arranged around small blood vessels at the depths of the sulci, most frequently in the frontal cortex. In stage II CTE, 3 or more CTE lesions are found in multiple cortical regions, the lesions are larger, superficial NFTs are found in adjacent cortices, and there is neurofibrillary pathology in the locus coeruleus and nucleus basalis of Meynert. In stage III CTE, larger, confluent perivascular patches of p-tau NFTs, astrocytes, and dot-like and threadlike neurites are found at the sulcal depths. NFTs are also seen in the superficial cortical laminae. Diffusely distributed NFTs are also found in medial temporal lobe (MTL) structures, including the hippocampus, entorhinal and perirhinal cortices, and amygdala. There is also more widespread brainstem p-tau pathology. By stage III, macroscopic features such as cerebral atrophy, ventricular enlargement, and abnormalities of the septum pellucidum are often found. Neurofibrillary degeneration in the hippocampus in stage III CTE involves CA4, CA2, and CA1. In stage IV CTE, cerebral, MTL, and anterior diencephalic atrophy are found grossly. There is neuronal loss with the corresponding depigmentation of the substantia nigra and locus coeruleus. Perivascular p-tau lesions and NFTs are distributed throughout the cerebral cortex, with pronounced neurofibrillary degeneration of the MTL. Perivascular p-tau lesions and NFTs extend to the diencephalon and brainstem and NFTs are found in the cerebellar dentate nucleus, basis pontis, and spinal cord. Neuronal loss and gliosis are often prominent in the frontal and temporal cortices and there is abundant astrocytic p-tau pathology. Other pathologic lesions, including myelin and axonal loss as well as TAR DNA-binding protein 43 (TDP-43) pathology, are found in the most severe CTE cases.
The McKee CTE staging scheme has been shown to have significant associations with the level of American football play (i.e., high school, college, semi-professional, and professional) and duration of football playing career (a proxy for cumulative exposure to RHI) [1, 2, 34, 36]. The staging scheme has also been linked with age at death [2, 34] and neuroinflammation [13]. By contrast, racial identity, years of education, age of first exposure to RHI, number of concussions, lifetime steroid use, substance abuse, or position played have not been found to have an association with CTE stage [1, 34, 36], although many of these variables were never primary targets of the investigation.
The usefulness of the McKee CTE staging scheme remains unclear, and semi-quantitative and quantitative assessments of regional p-tau pathology are needed to provide a more refined characterization of p-tau distribution in CTE. The objective(s) of the current study were twofold: (1) to test the utility of the McKee CTE staging scheme, and (2) to provide a detailed examination of the regional distribution of p-tau pathology in CTE. We examined the relationship between the McKee CTE staging scheme and semi-quantitative and quantitative assessments of regional p-tau pathology. In addition, years of American football play, age at death, and dementia status were evaluated in relation to CTE stage. We also conducted a k-medoids cluster analysis of semi-quantitative p-tau ratings to identify the patterns of regional p-tau deposition and to determine if these patterns progressed by CTE stage, age at death, dementia status, and years of American football play.
Materials and methods
Brain donors and study design
The sample included 366 deceased individuals with a history of exposure to RHI who donated their brain to the Veterans Affairs (VA)—Boston University School of Medicine (BU)—Concussion Legacy Foundation (CLF)/Understanding Neurological Injury and Traumatic Encephalopathy (UNITE) brain bank between 2008 and 2019 as part of the UNITE research study [1, 2, 36, 38, 44]. The sample included only individuals who were neuropathologically diagnosed with CTE [32]. Most brain donations to the UNITE study originated with the next-of-kin contacting the brain bank near the time of death. The remaining brain donors were referred by medical examiners, recruited by a representative of the CLF, or participated in the Brain Donation Registry during life. The only inclusion criterion for donation was having a history of exposure to RHI (i.e., from contact and collision sport play, military service, and/or domestic violence), regardless of whether symptoms were present during life. Donors were excluded if post-mortem interval exceeded 72 h. Institutional review board approval (IRB) for brain donation, post-mortem clinical record review, interviews with informants, and neuropathological evaluation were obtained through the Boston University Medical Campus (BUMC) IRB.
Neuropathological evaluation
Neuropathological evaluation occurred blinded to clinical data and was reviewed by three neuropathologists (BH, TS, and AM); any discrepancies in the neuropathological diagnosis were resolved by discussion and consensus of the group. Pathological processing and evaluation were conducted using previously published methodology [47, 48]. Brain weight and macroscopic features were recorded during initial processing. Twenty-two sections of paraffin-embedded tissue were stained for Luxol fast blue, hematoxylin and eosin (LHE), Bielschowsky’s silver, p-tau (AT8), alpha-synuclein, beta-amyloid (Aß), and phosphorylated TDP-43 (pTDP-43) using methods described previously [33].
Well-established criteria were used for the neuropathological diagnosis of neurodegenerative diseases, including AD [39, 41], LBD defined by LBD-Brainstem (LBD-B), LBD-transitional and neocortical (LBD-T, LBD-NC) [35], frontotemporal lobar degeneration (FTLD) [6, 11, 16, 30, 31], and motor neuron disease (MND) [10]. The National Institute on Aging (NIA)-Reagan Institute criteria were used for the neuropathological diagnosis of AD in this study and not the NIA-Alzheimer’s Association (AA) neuropathological diagnostic criteria for AD. One study suggested that the addition of Thal staging for Aß as required by the NIA-AA criteria for AD might have limited value in the prediction of antemortem clinical function beyond Braak NFT stage and CERAD neuritic plaque scores [42], although, the analyses for that study may have been underpowered. Lewy body pathology in the brainstem, limbic (transitional), and neocortical (diffuse) regions were used to define LBD because of their contributions to clinical syndromes (i.e., Parkinson’s disease dementia, Lewy body dementia) (for a review, see Jellinger et al. [25].
Neuropathological diagnosis of CTE was made using criteria defined by the NINDS-NIBIB Consensus Conference [32] with modifications. The 2015 panel defined the pathognomonic lesion of CTE as “an accumulation of abnormal hyperphosphorylated tau (p-tau) in neurons and astroglia distributed around small blood vessels at the depths of cortical sulci and in an irregular pattern”. For this study, the neuropathological diagnosis of CTE required the presence of at least one pathognomonic p-tau lesion in the cortex, and purely astrocytic perivascular p-tau lesions were considered non-diagnostic. Supportive features for the diagnosis of CTE included neurofibrillary tangles (NFTs) in superficial cortical layers (layers II/III) of the cerebral cortex and pretangles, NFTs, or dendritic dystrophy in CA2 and CA4 of the hippocampus [32]. In each case, the CTE p-tau pathology was classified into four stages using the McKee staging criteria, as shown in Table 1 [34].
Semiquantitative assessment of pathology
Independent semi-quantitative assessments of the density of p-tau pathology were performed by the same three aforementioned neuropathologists (BH, TS, and AM) at the time of initial diagnosis (blinded to clinical data) using semi-quantitative rating scales (0–3 scale; 0 = none, 1 = mild, 2 = moderate, and 3 = severe) in 14 regions. AT8-immunostained, 10 µm-thick paraffin-embedded sections of the following regions were evaluated: dorsolateral frontal cortex (DLF), rolandic cortex (RC), inferior frontal cortex (IF), inferior parietal cortex (IP), superior temporal cortex (ST), CA1-hippocampus, CA2-hippocampus, CA4-hippocampus, entorhinal cortex (EC), amygdala, thalamus, substantia nigra (SN), locus coeruleus (LC), and the dentate nucleus of the cerebellum (DN). These regions were a priori selected because of their involvement in CTE [32, 36, 37]. Other regions were not included due to excessive missing data and/or lack of involvement in CTE. Semi-quantitative measurements were also recorded for diffuse and neuritic Aß plaques, vascular Aß, pTDP-43 immunoreactive inclusions and neurites, and alpha-synuclein immunoreactive Lewy bodies and Lewy neurites.
Quantitative assessment of AT8 pathology
Using digitally scanned slides at 20 × magnification, the density of total AT8 staining was quantitatively measured in 7 regions, including the DLF, hippocampus subfields CA1, CA2/CA3 (CA2 and CA3 combined), CA4, subiculum, and the LC on a Leica Aperio ImageScope (Leica Biosystems). Slide scanning methods have been described elsewhere [13]. The gray matter was highlighted from the pia to the boundary between the white matter and gray matter. Leica’s image analysis and automated counting software (Aperio positive pixel algorithm, Version 9, Leica Biosystems) were calibrated for positive staining to detect AT8-immunoreactivity within the region of interest. Counts were normalized to the area measured and presented as the density of positively stained pixels within the analyzed region (positive pixels/mm2). For the DLF, p-tau density was measured at the gyral crest (defined as the top third of two connecting gyri) and at the depth of the cortical sulcus (defined as the bottom third of two connecting gyri). These regions had the most complete quantitated data at the time of the data freeze (July 2019) and were prioritized for slide scanning due to their involvement in the clinical and neuropathological pathogenesis of CTE and/or other neurodegenerative diseases.
Clinical evaluation
Retrospective clinical evaluations were performed using online surveys and structured and semi-structured telephone interviews between researchers and informants of brain donors [1, 37, 38, 44]. Researchers conducting these evaluations were blind to the neuropathological analysis and informants were interviewed before receiving the results of the neuropathological examination. For all participants, a clinician (JM, MLA, DHD, RAS, or BD) obtained a detailed history, including a timeline of cognitive, behavioral, mood, and motor symptomology. Clinician’s also assessed for symptoms associated with mental illness, such as depression, anxiety, and posttraumatic stress disorder. Clinicians qualitatively summarized the clinical presentation (e.g., presence and course of symptoms, and functional independence) into a narrative and a dementia diagnosis (at time of death) was adjudicated using modified Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria. To assist with the determination of dementia, informants were administered the Functional Activities Questionnaire (FAQ) to determine the presence and severity of functional impairment. The FAQ is a 10-item scale of instrumental activities of daily living and scores range from 0 to 30, with higher scores reflecting greater severity of functional impairment. Demographic, athletic, military, and other exposure to RHI history characteristics were self-reported by informants using an online survey (and confirmed by clinicians during telephone interviews).
Sample sizes
There were no missing data for CTE stage or age at death (N = 366). For analyses that examined dementia as an outcome, the sample of 366 was reduced to 359 following exclusion of cases with missing data for antemortem dementia. There were missing data across the brain regions for the semi-quantitative and quantitative measurements of p-tau. Reasons for missingness included poor tissue quality, exhausted tissue sources, or incomplete brain specimens that did not include the area of analysis. In addition, for the quantitative measurements, only a subset of cases had been scanned by the time of the data freeze (July 2019), as mentioned above. Analyses were restricted to brain donors who had complete data across all brain regions, resulting in a sample size of 287 for the semi-quantitative scales of p-tau and 176 for the quantitative measurements of p-tau density. For the semi-quantitative scales, there was no statistically significant difference in CTE stage between the analytic sample and those excluded (mean difference 0.11, t = 0.85, p = 0.40); however, brain donors excluded for missingness were younger (mean difference 5.65, t = 2.37, p = 0.02) and less likely to have dementia (48.1% vs 63.6%, Pearson Chi-square 6.15, p = 0.01). Brain donors excluded for missingness on the quantitative measurements of p-tau density were also younger (mean difference 4.76, t = 2.43, p = 0.02), less likely to have dementia (51.9% versus 68.8%, Pearson Chi-square 10.61, p = 0.001), and more likely to have a lower CTE stage (mean difference 0.27, t = 2.53, p = 0.01) compared to those included.
Statistical methods
Three neuropathologists rated CTE stage and the density of p-tau using 4-point semi-quantitative scales (0–3). We conducted an interrater reliability study to examine rater concordance for CTE stage and select regions, which included DLF, CA1-hippocampus, EC, and LC. The three neuropathologists (AM, TS, and BH) used the semi-quantitative scales to rate these regions for randomly selected sixteen cases across the CTE stage severity spectrum. These 16 cases included 6 with stage I CTE, 4 for stage II, 3 for stage III, and 3 for stage IV. We intentionally overrepresented CTE stages I and II, given our hypothesis that agreement for mild CTE would be lowest. Kendall’s coefficient of concordance (Kendall’s W) was used to evaluate rater agreement. Kendall’s W statistic is used for evaluating agreement among raters on ranked ordinal variables.
Outcomes for the statistical analyses included CTE stage (ordinal scale), semi-quantitative regional ratings of p-tau severity (ordinal scale), quantitative regional measurements of p-tau density (continuous scale), and yes/no dementia (binary scale). The quantitative regional measurements of p-tau density were log-transformed due to a non-normal distribution. Spearman’s rho correlations were first conducted to test the association of CTE stage with each of the semi-quantitative scales of p-tau severity, as well as with the regional quantitative measurements of p-tau density. Ordinal (for ordinal outcomes) and linear (for continuous outcomes) regressions tested the association between age at death and the following: CTE stage, regional semi-quantitative scales of p-tau severity, and quantitative measurements of p-tau density. For analyses that examined the semi-quantitative and quantitative variables as outcomes, separate models were performed for each brain region and p values were false discovery rate (FDR) adjusted using Benjamini–Hochberg methods. An FDR-adjusted p value less than 0.05 defined statistical significance.
Additional analyses were conducted to examine the utility of the McKee CTE staging scheme. Binary logistic regression was used to examine the relationship between CTE stage and dementia, controlling for age at death. This model was repeated with education level (no high school/some high school, high school degree, some college, college degree, and more than a college degree/graduate degree) and racial identity (White vs other) added as covariates to determine their effects on the CTE stage estimate for dementia. To support the association between CTE stage and dementia, post hoc linear regressions were conducted to evaluate the relationship between CTE stage and FAQ scores (n = 307 due to missing FAQ data). Our previous findings in deceased former American football players from the UNITE study showed that years of football play are associated with p-tau pathological severity, including CTE stage [1, 13, 36, 43]. We sought to test this association in this specific UNITE sample. Ordinal logistic regression was used to test the association between years of football play and CTE stage in 304 individuals whose primary sport was American football (based on the sport played the longest), controlling for age at death. For the above models, identical post hoc analyses were repeated controlling for the absence/presence of co-morbid neurodegenerative disease diagnoses (i.e., AD, LBD, FTLD, and MND), and severity of arteriolosclerosis and white matter rarefaction. Sample size for these post hoc analyses was reduced due to missing data on LBD (n = 1), FTLD (n = 4), arteriolosclerosis (n = 4), and white matter rarefaction (n = 7). There were only two brain donors who had prion disease and this variable was not entered into the model due to the small cell size that would preclude reliable estimates. Instead, the post hoc analyses were done with these two brain donors excluded.
A k-medoids cluster analysis was performed on the 14 semi-quantitative rating scales of p-tau severity to identify regional patterns of p-tau deposition and to determine if these patterns differed by CTE stage. The Gap Statistic method was used to identify the optimal number of clusters, based on examination of up to ten cluster sizes [45]. Using the identified number of clusters, we compared each brain donor’s cluster assignment to their CTE stage to determine the regions affected by p-tau at each stage of the disease. We also examined the relationship between cluster membership and age at death, dementia status, and years of football play.
Results
Of the 366 brain donors who met the neuropathological diagnostic criteria for CTE, 58 (15.8%) had stage I, 79 (21.6%) had stage II, 127 (34.7%) had stage III, and 102 (27.9%) had stage IV CTE. A majority of the sample included individuals whose primary sport (based on the sport played the longest) was American football (N = 304 of 356; primary sport was not able to be determined for ten brain donors due to missing data). Demographic, clinical, athletic, and neuropathologic characteristics are shown in Tables 2, 3, and 4. Supplementary Table 1 also provides descriptive data on the presence of cognitive, behavioral/mood, headache, and motor symptoms by CTE stage.
Interrater reliability
The three neuropathologists demonstrated very good agreement for CTE stage (Kendall’s W = 0.92, p < 0.0001) and for the semi-quantitative ratings of the DLF (Kendall’s W = 0.93, p < 0.0001), CA1-hippocampus (Kendall’s W = 0.92, p < 0.0001), EC (Kendall’s W = 0.83, p < 0.0001), and LC (Kendall’s W = 0.96, p < 0.0001).
CTE stage: association with semi-quantitative p-tau severity (N = 287) and quantitative p-tau density (N = 176)
Spearman’s rho correlations showed that higher CTE stage was associated with higher scores on all 14 semi-quantitative scales of p-tau severity (ρ = 0.42–0.73, all p’s < 0.001). Likewise, for the quantitative measures of p-tau density, higher CTE stage corresponded to greater log-transformed p-tau density for each region assessed (ρ = 0.55–0.77, all p’s < 0.001) (Fig. 1, Table 4).
Box plots of quantitative measurements of regional p-tau accumulation by CTE stage. Spearman’s rho correlations showed that greater p-tau density was associated with a higher CTE stage for all regions assessed (p’s < 0.001 for all). The sample size was reduced to 176 as this represents the sample with available quantitative data across all 7 brain regions at the time of this data freeze (July 2019). The mid-point line in the box represents the median, the interquartile range box represents the middle 50%, and the whiskers represent the bottom and top 25% of data value. Y-axis values are positive pixels (mm2) and are on a logarithmic scale
CTE stage: association with age (N = 366)
The age range of the sample was 17–100 years old (mean 61.86, SD 18.90; median 66). The nature, severity, and distribution of CTE-related p-tau pathology followed an age-dependent progression (Fig. 2). Older age was associated with increased odds for having a higher CTE stage (OR 1.08, 95% CI 1.06–1.09, p < 0.001). This effect remained when co-morbid neurodegenerative disease and severity of arteriolosclerosis and white matter rarefaction were included in the model (OR 1.07, 95% CI 1.05–1.09, p < 0.001). Older age corresponded to increased semi-quantitative ratings of p-tau for all 14 regions (Fig. 3): DLF (OR 1.03, 95% CI 1.02–1.04, p < 0.001), RC (OR 1.04, 95% CI 1.03–1.05, p < 0.001), IF (OR 1.05, 95% CI 1.04–1.06, p < 0.001), IP (OR 1.03, 95% CI = 1.02–1.04, p < 0.001), ST (OR = 1.04, 95% CI = 1.02–1.05, p < 0.001), CA1 (OR 1.05, 95% CI 1.05–1.08, p < 0.001), CA2 (OR 1.06, 95% CI 1.05–1.08, p < 0.001), CA4 (OR 1.07, 95% CI 1.05–1.08, p < 0.001), EC (OR 1.06, 95% CI 1.05–1.07, p < 0.001), amygdala (OR 1.07, 95% CI 1.05–1.08, p < 0.001), thalamus (OR 1.06, 95% CI 1.06–1.07, p < 0.001), SN (OR 1.06, 95% CI 1.05–1.08, p < 0.001), LC (OR 1.04, 95% CI 1.02–1.05, p < 0.001), and DN (OR 1.06, 95% CI 1.04–1.08, p < 0.001).
Association between age at death and regional p-tau progression. Heat map of semi-quantitative p-tau pathology (0–3, 3 most severe) for 14 brain regions (N = 287); in addition to exclusion of those for missing data across all regions, the three individuals in the 90–100 decades of age at death were not included in the heat map due to insufficient sample size. In each region, 0 = no NFTs, 1 = 1 NFT per 20X field, 2 = 2–3 NFTs per 20X field, 3 = ≥ 4 NFTs per 20 × field. Darker color is indicative of greater p-tau pathology. The color scale for the heat map is based on the distribution of all values. The values are averages of semi-quantitative p-tau pathology among the individuals for each age group. DLF dorsolateral frontal cortex, RC rolandic cortex, IF inferior frontal cortex, IP inferior parietal cortex, EC entorhinal cortex, SN substantia nigra, LC locus coeruleus
Older age was associated with greater quantitative p-tau density (log-transformed) for each region assessed: DLF gyral crest (standardized beta 0.52, p < 0.001), DLF depths of sulcus (standardized beta 0.50, p < 0.001), CA1 (standardized beta 0.75, p < 0.001), CA2/3 (standardized beta 0.72, p < 0.001), CA4 (standardized beta 0.75, p < 0.001), subiculum (standardized beta 0.76, p < 0.001), and LC (standardized beta = 0.59, p < 0.001) (Fig. 4).
Scatter plots of the association between age at death and quantitative measurements of p-tau density. The sample size was reduced to 176 as this represents the sample with available quantitative data across all three brain regions at the time of this data freeze (July 2019). Older age was associated with greater quantitative p-tau density for each region assessed (p’s < 0.05). For the dorsolateral frontal cortex, p-tau density was measured at the depth of the cortical sulcus (defined as the bottom third of two connecting gyri) and at the gryal crest (defined as the top third of two connecting gyri). For quantitative hippocampal subfield analysis, CA2 and CA3 were combined into one field denoted as CA2/3. Y-axis values are positive pixels (mm2) and are on a logarithmic scale
CTE stage: association with dementia (N = 359)
Of the 359 brain donors with CTE who did not have missing data for antemortem dementia, 216 (60.2%) were determined to have had dementia. Like CTE stage, the presence of dementia followed an age-dependent progression and older age at death was associated with increased odds for having dementia (OR 1.10, 95% CI 1.08–1.12, p < 0.001). Binary logistic regression controlling for age at death showed that for every one level increase in the stage of CTE, there was a 1.64X increased odds for antemortem dementia at time of death (95% CI 1.19–2.27, p = 0.003) (Table 5, Fig. 5); statistical significance remained when controlling for education level and racial identity (OR 1.63, 95% CI 1.18–2.26, p = 0.003). Post hoc linear regressions showed that increases in CTE stage also corresponded to higher scores on the FAQ controlling for age at death (standardized beta 0.21, p < 0.001), as well as when education level and racial identity were added to the model (standardized beta 0.22, p < 0.001). Note that the sample size was reduced by one for that above models that included racial identity as a covariate due to missing data on this variable.
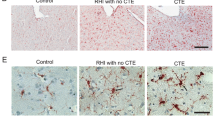
Dementia status by CTE stage and p-tau accumulation in the dorsolateral frontal cortex and hippocampus. Left: pie chart of the number (%) of brain donors with dementia by CTE stage (N = 359). For percents, denominator is total brain donors with dementia (n = 216). Right: In CTE, p-tau deposition often begins in the dorsolateral frontal cortex (DLF), with the hippocampus becoming involved in later disease stages (i.e., stage III). The density of p-tau in the DLF and hippocampus and the size of the pathognomonic CTE lesions increase with age. These regions are, therefore, sensitive markers of the progression and severity of the disease. The images on the right are exemplary of the severity of pathology in these regions by age and dementia status. a Mild perivascular accumulation of p-tau at the depths of the cortical sulcus in the DLF in a non-demented 30-year-old with stage II CTE; b severe perivascular accumulation of p-tau at the depths of the sulcus in the DLF in a demented 80 year old with stage IV CTE; c absence of hippocampal p-tau accumulation in a non-demented 44-year-old with stage II CTE; d hippocampal p-tau accumulation in a demented 69-year-old with stage III CTE. Positive staining for p-tau is depicted in red, while hematoxylin counterstain is in blue. Scale bar represents 200um (a, b) and 2 mm (c, d)
The statistically significant association between CTE stage and dementia remained after controlling for co-morbid neurodegenerative disease and severity of arteriolosclerosis and white matter rarefaction, along with age at death (OR 1.51, 95% CI 1.03–2.21, p = 0.036). Supplementary Table 2 shows the contributions of the co-morbid pathologies. Education level and racial identity were not included here because of their lack of association with dementia in this sample, as shown in Table 5.
CTE Stage: association with years of American football play (N = 304)
Among the 304 brain donors whose primary sport was American football, 41 (13.5%) had stage I CTE, 60 (19.7%) had stage II CTE, 114 (37.5%) had stage III CTE, and 89 (29.3%) had stage IV CTE. More years of American football play were associated with increased odds for having a higher stage of CTE (OR 1.10, 95% CI 1.06–1.15, p < 0.001), controlling for age at death. The association between years of American football play and CTE stage also remained when co-morbid neurodegenerative disease and arteriolosclerosis and white matter rarefaction were included in the model (OR 1.14, 95% CI 1.09–1.19, p < 0.001). The effects of age at death on CTE stage and quantitative measurements of p-tau density remained when years of American football play were included as a covariate (data not shown).
Cluster analysis of semi-quantitative scale of p-tau severity (N = 287)
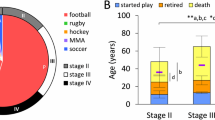
Based on the k-medoid cluster analysis of the 14 semi-quantitative rating scales of p-tau severity, the optimal number of clusters was 5 (Fig. 6a). The five clusters ranged from minimal, focal p-tau pathology to severe, widespread p-tau pathology and corresponded with the McKee CTE staging scheme (Fig. 6b, c). As the severity of p-tau increased across the clusters, CTE stage age at death, years of American football, frequency of dementia, and the presence of co-morbid neurodegenerative disease increased.
K-medoids cluster analysis of 14 semi-quantitative rating scales of regional p-tau pathology and their association with CTE stage. A k-medoids cluster analysis with the 14 semi-quantitative rating scales was conducted to determine the different patterns of regional p-tau deposition and to ascertain how these patterns differed by CTE stage. a Using the Gap Statistic method, five clusters best fitted the data. (Ten cluster sizes were examined. After five clusters, there was minimal difference between the clusters.) b Heat map of the medoids of each cluster. The 14 brain regions on the x-axis are those that were rated for p-tau severity using a 0–3 scale, with 0 being none and 3 being severe p-tau involvement. The five clusters are represented on the y-axis. The median score of p-tau severity for each brain region is graphed using the shown color scale; darker colors reflecting more severe p-tau. c Association of cluster assignment and CTE stage. DLF dorsolateral frontal cortex, RC rolandic cortex, IF inferior orbitofrontal cortex, IP inferior parietal cortex, ST superior temporal cortex, EC entorhinal cortex, SN substantia nigra, LC locus coeruleus
Cluster 1, N = 31 (CTE stage I)
Donors in cluster 1 most frequently had CTE stage I (n = 29/31). It was characterized by mild p-tau severity of DLF and LC and the absence of p-tau across all other regions. This cluster included brain donors who were young (mean 38.74, SD 15.41) and had the fewest years of American football play (mean 10.02, SD 4.98). Dementia was less frequent (n = 7/30, 23%) and only five (15.6%) had a co-morbid neurodegenerative disease diagnosis.
Cluster 2, N = 66 (CTE stage II)
Donors in cluster 2 most frequently had CTE stage II (n = 41/66) and, to a lesser extent, CTE stage I (n = 15/66). Cluster 2 showed increased pathology in the LC; mild pathology in DLF and other neocortical regions (with the exception of the RC); mild pathology in MTL structures (EC, amygdala, hippocampal subfields CA1 and CA2) and in the substantia nigra. The mean age at death was higher than cluster 1 (mean 55.47, SD 19.57), as were the years of American football play (mean 13.32, SD 5.21). Dementia frequency increased (30/64, 46.9%), but the rate of co-morbid neurodegenerative disease diagnosis was similar to cluster 1 (n = 10, 15.2%).
Cluster 3, N = 84 (CTE stage III)
Donors in cluster 3 most frequently had CTE stage III (n = 57/84). Compared to cluster 2, cluster 3 showed increased p-tau pathology in DLF, RC, ST, and all MTL regions, including CA4. There was also mild p-tau pathology of the thalamus. Cluster 3 had a mean age at death of 66.14 (SD 15.00), mean years of American football play were 13.83 (SD 5.32), and 53/82 (64.6%) had dementia. Thirty-one (36.9%) had a co-morbid neurodegenerative disease diagnosis.
Cluster 4, N = 39 (CTE stage IV)
Donors in cluster 4 most frequently had CTE stage IV (n = 26/39). Cluster 4 had severe p-tau pathology in all neocortical regions: DLF, RC, IF, IP, ST, as well as EC, with less severe pathology in the amygdala, hippocampus, thalamus, and brainstem. Mild involvement of the DN was present. The mean age at death of cluster 4 was 73.00 (SD 13.89) and brain donors of this cluster played 13.94 (SD 5.29) years of football on average. Cluster 4 had the greatest proportion of brain donors with dementia (36/39, 92.3%) and co-morbid neurodegenerative disease (n = 29/39, 74.4%).
Cluster 5, N = 67 (CTE stage IV)
Cluster 5 was a second larger cluster associated with stage IV CTE (n = 45/67), although many had stage III CTE (n = 20). Brain donors in cluster 5 had severe p-tau pathology in DLF, IF, IP, and ST, and moderate pathology in the RC. There was severe p-tau pathology in all MTL and brainstem regions. Mild and moderate p-tau pathology was present in the DN and thalamus, respectively. The mean age at death of this cluster was 72.21 (SD 10.36). Brain donors of this cluster had the highest mean years of American football play (mean 16.70, SD = 6.12). The frequency of dementia was high (n = 52/65, 80.0%) and a majority, 38 (56.7%), had a co-morbid neurodegenerative disease diagnosis; there were no statistically significant differences in these variables between clusters 4 and 5.
Discussion
In this sample of 366 brain donors who met neuropathological diagnostic criteria for CTE and were staged for CTE severity using the McKee CTE staging scheme, higher CTE stage was associated with higher scores on semi-quantitative scales of p-tau severity in 14 brain regions and higher quantitative p-tau density in 7 regions. In addition, higher CTE stage was significantly associated with older age at death, increased odds for having antemortem dementia, and greater years of American football play. Cluster analysis of the semi-quantitative p-tau scores in the 14 regions demonstrated 5 clusters of pathology ranging from mild, focal p-tau deposition to severe, widespread p-tau pathology that corresponded to the McKee stages of CTE. Clusters 1, 2, and 3 were associated with CTE stages I, II, and III, respectively; clusters 4 and 5 were associated with stage IV. As p-tau severity increased across the 5 clusters, age at death, years of American football, frequency of dementia, and presence of co-morbid neurodegenerative disease also increased.
Across all four stages of CTE and all ages at death, the semi-quantitative and quantitative ratings of p-tau density, as well as the cluster analysis demonstrated a predilection for p-tau pathology in five key brain regions (DLF, ST, EC, amygdala, and LC), with CTE in the youngest brain donors and lowest CTE stage primarily restricted to DLF and LC. P-tau pathology in the LC is common in other tauopathies, such as AD, and may contribute to the neuropsychiatric symptoms characteristic of early (“preclinical”) disease [9, 17, 19, 49]. The extent to which minor amounts of p-tau pathology in the amygdala, EC, and LC (or other regions) found in young brain donors with low CTE stage (e.g., stage I) contribute to the heterogenous cognitive, mood, and behavioral disturbances reported by family members of brain donors with CTE has not been investigated [37]. Furthermore, the isolated p-tau lesions found in low CTE stage might be hallmarks of more profound molecular, cellular, or synaptic alterations than can be captured by light microscopic examination alone.
CTE is distinguished from AD by the lack of Aß plaques and a distinctive pattern, type, and regional distribution of p-tau pathology. Early p-tau pathology in CTE is cortical and consists of patchy, irregular perivascular clusters of NFTs, neuritic processes that are often dot-like, and variably, astrocytes, at the depths of the sulci [32] that enlarge over time and progressively involve the gyral banks, gyral crests, and superficial laminae of the neocortex. P-tau pathology in AD, by contrast, initially appears as NFTs and threadlike neurites in the MTL and later progresses to the neocortex in a diffusely laminar pattern affecting layers 3 and 5. In the hippocampus, p-tau pathology in CTE shows the involvement of CA2 and CA4, as well as CA1, whereas hippocampal regions CA2 and CA4 are relatively spared in AD. NFTs are also found in the mammillary bodies, base of the pons, and cerebellar dentate nucleus of the cerebellum in advanced CTE, and are uncommon in AD.
The latent development of p-tau pathology in MTL structures in CTE might be precipitated by a previous sub-threshold traumatic brain injury that increases neuronal vulnerability to p-tau neurodegeneration with increasing age. This p-tau neurodegeneration may be enhanced by other age-related changes, such as lowered cerebral blood flow [51], loss of blood–brain barrier integrity [18, 40], increased neuroinflammation [13, 46], altered metabolism, and decreased neurogenesis and neuroplasticity [15]. Other mechanisms of pathological protein propagation might also contribute to the spread of p-tau in CTE, including prion templating [50] and age-related decline in glymphatic clearance [26].
The McKee CTE staging scheme was associated with age at death, dementia status, and years of American football play. Autopsy studies are inherently cross-sectional and there are difficulties in drawing inferences regarding disease progression. With that caveat in mind, the severity and distribution of CTE-related p-tau pathology were strongly associated with age. The semi-quantitative and quantitative data and cluster analyses demonstrated marked increases in p-tau pathology in widespread neocortical regions, hippocampus, EC, and amygdala in CTE stages III and IV, as well as in age groups 40 years and older. Although most individuals with low CTE (stage I or II) were under the age of 40 at death, the presence of low stage CTE in advanced middle-age and older American football players, whose football careers ended decades earlier, suggests that the onset of p-tau and/or pathological progression in CTE is heterogeneous. Many factors may increase susceptibility to p-tau pathology, including genetic factors, differences in RHI exposure (e.g., duration, intensity, and age of first exposure), and other environmental variables, which are directions for future study.
In addition to CTE stage and age at death associations, in stages III and IV, quantitative analysis revealed increased p-tau pathology in the gyral crests of the neocortex suggesting intracortical spread of the p-tau pathology. Furthermore, severity of p-tau pathology corresponded to increased odds for having antemortem dementia: for every one level increase in the stage of CTE, there was a 1.64X increased odds for dementia, controlling for age at death (the OR remained similar after controlling for racial identity and education level). This association remained statistically significant after accounting for neurodegenerative disease co-morbidity, arteriolosclerosis, and white matter rarefaction. The binary logistic regression that evaluated this association assumes that the increase in odds for dementia is equal across the CTE stages. The odds ratio might have been suppressed by the lower levels of disease stages (e.g., stage I). By comparison, clinical–pathological correlation studies investigating AD neuropathological changes and AD dementia have classified brain donors with low likelihood of AD as being part of the disease absent group [24]. Measurement error in dementia (which was based on retrospective reports from informants) may have also affected the accuracy of the estimated effect between CTE stage and dementia. We only examined dementia as the primary clinical outcome and, as previously described, family members of brain donors with CTE have reported a combination of cognitive, behavioral, and mood symptoms, as well as parkinsonism (Supplementary Table 2). Clinical–pathological correlation studies by our team and others are investigating the specific clinical phenotypes associated with CTE, as well as the contributions of mixed neuropathologies. Notably, the ORs for co-morbid pathologies and dementia in the present study were large with wide-ranging 95% CI (SupplementaryTable 1), particularly for AD. This is because the effects of these co-morbid pathologies on dementia are based on brain donors who have more severe disease and at least two diseases (the entire sample has CTE). Consequently, dementia is much more common and in fact, all but one of the brain donors with co-morbid AD had dementia. In contrast, the effect of CTE stage on dementia (which is per unit increase in CTE stage) is based in part on the 40% of brain donors who did not have a co-morbid neurodegenerative disease.
The present study showed that greater years of American football (a proxy for cumulative exposure to RHI) was associated with higher CTE stage. Duration of football playing career has consistently emerged as a strong correlate of CTE pathology, along with age at death, in the previous reports from our brain bank [2, 13, 34, 36], and other autopsy samples [4, 5, 29]. A recent autopsy case study (n = 8) reported CTE in brain donors who did not have a history of exposure to RHI [23]. In that report, based on the images and data presented, the lesions were not diagnostic of CTE and more consistent with aging-related tau astrogliopathy (ARTAG). This confusion underscores the need to refine the neuropathological diagnostic criteria of CTE to allow a better distinction between CTE and other neurodegenerative diseases, including ARTAG. Nevertheless, the presence of CTE lesions in individuals who were not known to be exposed to RHI does not negate exposure to RHI as a risk factor for CTE pathology.
The current study is not without limitations. This autopsy sample was male, demographically homogenous, and predominantly American football players. Future research is needed to confirm the universal usefulness of the McKee CTE staging scheme via examination in demographically and athletically diverse autopsy samples. Interrater reliability of the McKee CTE staging scheme is an important component to support its utility. Here, three neuropathologists demonstrated very good agreement in their ratings of CTE stage and semi-quantitative p-tau pathology. To establish the interrater reliability of the McKee CTE staging scheme, similar ratings will need to be performed using independent neuropathologists across different institutions. This study also used NIA-Reagan and not the NIA-AA criteria for the neuropathological diagnosis of AD for reasons described in the Methods. Thereby, a binary ascertainment of AD (present/not present) was made and such an approach might not have accounted for low levels of AD pathology. That being said, the NIA-Reagan and NIA-AA diagnoses of AD in this data set were correlated and the effects of age at death and years of American football play on CTE stage, as well as the effect of CTE stage on dementia remained statistically significant when the NIA-AA diagnosis of AD was used instead of the NIA-Reagan (data not shown).
The generalizability of autopsy studies is limited, because selection into brain banks is dependent on dementia status, depression, marital status, age, sex, race, education, and other factors [22]. The accuracy of estimated effects is affected only if both the predictor and the outcome are differentially association with selection. The primary purpose of this study was to examine the usefulness of the McKee CTE staging scheme by examining its correspondence with measures of p-tau severity and density (e.g., semi-quantitative and quantitative scales). Because are all measures of p-tau, the influence of selection bias is likely to be constant and have a non-differential effect. Our prior studies among brain donors from the VA-BU-CLF/UNITE brain bank have also shown that the associations between exposure to RHI and CTE pathology remain after accounting for factors that influence selection [1, 36]. In the current study, analyses were restricted to brain donors who had complete data across all brain regions, resulting in reduced sample sizes for the semi-quantitative and quantitative scales of p-tau. Those excluded from analyses were younger, less likely to have dementia, and more likely to have lower CTE stage (for the quantitative measures). The exclusion of these brain donors may have led to an under- or overestimation of estimated effects related to age, dementia, and CTE stage. The semi-quantitative ratings are subjective, however, the addition of quantitative assessments is a strength and attenuates concerns regarding rater bias. At the time of the data freeze, quantitated assessments of AT8 pathology were available for seven brain regions that play a key role in the clinical and neuropathological pathogenesis of CTE and/or other neurodegenerative diseases. There are ongoing efforts to provide a more widespread quantitative assessment of AT8 pathology throughout the brain in the UNITE sample.
Conclusions
Findings from the present study support the usefulness of the McKee CTE staging scheme in assessing CTE pathological severity and support their continued use in the study of CTE. The findings additionally demonstrate the distinctive regional distribution of p-tau pathology in CTE, as well as link p-tau severity in CTE with older age, dementia, and greater years of American football play. Future research is needed to clarify the clinical correlates of CTE across the different stages of the disease, and identify RHI- and non-RHI-related risk factors that enhance susceptibility and course progression.
References
Alosco ML, Mez J, Tripodis Y, Kiernan PT, Abdolmohammadi B, Murphy L et al (2018) Age of first exposure to tackle football and chronic traumatic encephalopathy. Ann Neurol 83:886–901. https://doi.org/10.1002/ana.25245
Alosco ML, Stein TD, Tripodis Y, Chua AS, Kowall NW, Huber BR et al (2019) Association of white matter rarefaction, arteriolosclerosis, and tau with dementia in chronic traumatic encephalopathy. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2019.2244
Armstrong RA, McKee AC, Stein TD, Alvarez VE, Cairns NJ (2019) Cortical degeneration in chronic traumatic encephalopathy and Alzheimer's disease neuropathologic change. Neurol Sci 40:529–533. https://doi.org/10.1007/s10072-018-3686-6
Bieniek KF, Blessing MM, Heckman MG, Diehl NN, Serie AM, Paolini MA 2nd et al (2020) Association between contact sports participation and chronic traumatic encephalopathy: a retrospective cohort study. Brain Pathol 30:63–74. https://doi.org/10.1111/bpa.12757
Bieniek KF, Ross OA, Cormier KA, Walton RL, Soto-Ortolaza A, Johnston AE et al (2015) Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol 130:877–889. https://doi.org/10.1007/s00401-015-1502-4
Bigio EH (2008) Update on recent molecular and genetic advances in frontotemporal lobar degeneration. J Neuropathol Exp Neurol 67:635–648. https://doi.org/10.1097/NEN.0b013e31817d751c
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259. https://doi.org/10.1007/BF00308809
Braak H, Braak E (1995) Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging 16:271–278. https://doi.org/10.1016/0197-4580(95)00021-6
Braak H, Del Tredici K (2011) The pathological process underlying Alzheimer's disease in individuals under thirty. Acta Neuropathol 121:171–181. https://doi.org/10.1007/s00401-010-0789-4
Brownell B, Oppenheimer DR, Hughes JT (1970) The central nervous system in motor neurone disease. J Neurol Neurosurg Psychiatry 33:338–357. https://doi.org/10.1136/jnnp.33.3.338
Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ et al (2007) TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol 171:227–240. https://doi.org/10.2353/ajpath.2007.070182
Cherry JD, Kim SH, Stein TD, Pothast MJ, Nicks R, Meng G et al (2020) Evolution of neuronal and glial tau isoforms in chronic traumatic encephalopathy. Brain Pathol. https://doi.org/10.1111/bpa.12867
Cherry JD, Tripodis Y, Alvarez VE, Huber B, Kiernan PT, Daneshvar DH et al (2016) Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol Commun 4:112. https://doi.org/10.1186/s40478-016-0382-8
Corsellis JA, Bruton CJ, Freeman-Browne D (1973) The aftermath of boxing. Psychol Med 3:270–303. https://doi.org/10.1017/s0033291700049588
de Miranda AS, Zhang CJ, Katsumoto A, Teixeira AL (2017) Hippocampal adult neurogenesis: does the immune system matter? J Neurol Sci 372:482–495. https://doi.org/10.1016/j.jns.2016.10.052
Dickson DW (2009) Neuropathology of non-Alzheimer degenerative disorders. Int J Clin Exp Pathol 3:1–23
Ehrenberg AJ, Suemoto CK, Franca Resende EP, Petersen C, Leite REP, Rodriguez RD et al (2018) Neuropathologic correlates of psychiatric symptoms in Alzheimer's disease. J Alzheimers Dis 66:115–126. https://doi.org/10.3233/JAD-180688
Erdo F, Denes L, de Lange E (2017) Age-associated physiological and pathological changes at the blood-brain barrier: a review. J Cereb Blood Flow Metab 37:4–24. https://doi.org/10.1177/0271678X16679420
Eser RA, Ehrenberg AJ, Petersen C, Dunlop S, Mejia MB, Suemoto CK et al (2018) Selective vulnerability of brainstem nuclei in distinct tauopathies: a postmortem study. J Neuropathol Exp Neurol 77:149–161. https://doi.org/10.1093/jnen/nlx113
Falcon B, Zivanov J, Zhang W, Murzin AG, Garringer HJ, Vidal R et al (2019) Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature 568:420–423. https://doi.org/10.1038/s41586-019-1026-5
Forrest SL, Kril JJ, Wagner S, Honigschnabl S, Reiner A, Fischer P et al (2019) Chronic traumatic encephalopathy (CTE) is absent from a european community-based aging cohort while cortical aging-related tau astrogliopathy (ARTAG) is highly prevalent. J Neuropathol Exp Neurol 78:398–405. https://doi.org/10.1093/jnen/nlz017
Haneuse S, Schildcrout J, Crane P, Sonnen J, Breitner J, Larson E (2009) Adjustment for selection bias in observational studies with application to the analysis of autopsy data. Neuroepidemiology 32:229–239. https://doi.org/10.1159/000197389
Iverson GL, Luoto TM, Karhunen PJ, Castellani RJ (2019) Mild chronic traumatic encephalopathy neuropathology in people with no known participation in contact sports or history of repetitive neurotrauma. J Neuropathol Exp Neurol 78:615–625. https://doi.org/10.1093/jnen/nlz045
James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA (2016) TDP-43 stage, mixed pathologies, and clinical Alzheimer's-type dementia. Brain 139:2983–2993. https://doi.org/10.1093/brain/aww224
Jellinger KA, Korczyn AD (2018) Are dementia with Lewy bodies and Parkinson's disease dementia the same disease? BMC Med 16:34. https://doi.org/10.1186/s12916-018-1016-8
Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D et al (2014) Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76:845–861. https://doi.org/10.1002/ana.24271
Lee EB, Kinch K, Johnson VE, Trojanowski JQ, Smith DH, Stewart W (2019) Chronic traumatic encephalopathy is a common co-morbidity, but less frequent primary dementia in former soccer and rugby players. Acta Neuropathol 138:389–399. https://doi.org/10.1007/s00401-019-02030-y
Ling H, Holton JL, Shaw K, Davey K, Lashley T, Revesz T (2015) Histological evidence of chronic traumatic encephalopathy in a large series of neurodegenerative diseases. Acta Neuropathol 130:891–893. https://doi.org/10.1007/s00401-015-1496-y
Ling H, Morris HR, Neal JW, Lees AJ, Hardy J, Holton JL et al (2017) Mixed pathologies including chronic traumatic encephalopathy account for dementia in retired association football (soccer) players. Acta Neuropathol 133:337–352. https://doi.org/10.1007/s00401-017-1680-3
Litvan I, Hauw JJ, Bartko JJ, Lantos PL, Daniel SE, Horoupian DS et al (1996) Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol 55:97–105. https://doi.org/10.1097/00005072-199601000-00010
Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J et al (2010) Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 119:1–4. https://doi.org/10.1007/s00401-009-0612-2
McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Keene CD, Litvan I et al (2016) The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 131:75–86. https://doi.org/10.1007/s00401-015-1515-z
McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE et al (2009) Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 68:709–735. https://doi.org/10.1097/NEN.0b013e3181a9d503
McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH et al (2013) The spectrum of disease in chronic traumatic encephalopathy. Brain 136:43–64. https://doi.org/10.1093/brain/aws307
McKeith IG (2006) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis 9:417–423. https://doi.org/10.3233/jad-2006-9s347
Mez J, Daneshvar DH, Abdolmohammadi B, Chua AS, Alosco ML, Kiernan PT et al (2020) Duration of American football play and chronic traumatic encephalopathy. Ann Neurol 87:116–131. https://doi.org/10.1002/ana.25611
Mez J, Daneshvar DH, Kiernan PT, Abdolmohammadi B, Alvarez VE, Huber BR et al (2017) Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA 318:360–370. https://doi.org/10.1001/jama.2017.8334
Mez J, Solomon TM, Daneshvar DH, Murphy L, Kiernan PT, Montenigro PH et al (2015) Assessing clinicopathological correlation in chronic traumatic encephalopathy: rationale and methods for the UNITE study. Alzheimers Res Ther 7:62. https://doi.org/10.1186/s13195-015-0148-8
Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW et al (2012) National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 123:1–11. https://doi.org/10.1007/s00401-011-0910-3
Mooradian AD (1988) Effect of aging on the blood-brain barrier. Neurobiol Aging 9:31–39. https://doi.org/10.1016/s0197-4580(88)80013-7
Newell KL, Hyman BT, Growdon JH, Hedley-Whyte ET (1999) Application of the National Institute on Aging (NIA)-Reagan Institute criteria for the neuropathological diagnosis of Alzheimer disease. J Neuropathol Exp Neurol 58:1147–1155. https://doi.org/10.1097/00005072-199911000-00004
Serrano-Pozo A, Qian J, Muzikansky A, Monsell SE, Montine TJ, Frosch MP et al (2016) Thal amyloid stages do not significantly impact the correlation between neuropathological change and cognition in the Alzheimer disease continuum. J Neuropathol Exp Neurol 75:516–526. https://doi.org/10.1093/jnen/nlw026
Stein TD, Montenigro PH, Alvarez VE, Xia W, Crary JF, Tripodis Y et al (2015) Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol 130:21–34. https://doi.org/10.1007/s00401-015-1435-y
Stern RA, Daneshvar DH, Baugh CM, Seichepine DR, Montenigro PH, Riley DO et al (2013) Clinical presentation of chronic traumatic encephalopathy. Neurology 81:1122–1129. https://doi.org/10.1212/WNL.0b013e3182a55f7f
Tibshirani R, Walther G, Hastie T (2001) Estimating the number of clusters in a data set via the gap statistic. J R Stat Soc Ser B (Stat Methodol) 63:411–423
Udeochu JC, Shea JM, Villeda SA (2016) Microglia communication: Parallels between aging and Alzheimer's disease. Clin Exp Neuroimmunol 7:114–125. https://doi.org/10.1111/cen3.12307
Vonsattel JP, Aizawa H, Ge P, DiFiglia M, McKee AC, MacDonald M et al (1995) An improved approach to prepare human brains for research. J Neuropathol Exp Neurol 54:42–56. https://doi.org/10.1097/00005072-199501000-00006
Vonsattel JP, Del Amaya MP, Keller CE (2008) Twenty-first century brain banking. Processing brains for research: the Columbia University methods. Acta Neuropathol 115:509–532. https://doi.org/10.1007/s00401-007-0311-9
Wilson RS, Nag S, Boyle PA, Hizel LP, Yu L, Buchman AS et al (2013) Brainstem aminergic nuclei and late-life depressive symptoms. JAMA Psychiatry 70:1320–1328. https://doi.org/10.1001/jamapsychiatry.2013.2224
Woerman AL, Aoyagi A, Patel S, Kazmi SA, Lobach I, Grinberg LT et al (2016) Tau prions from Alzheimer's disease and chronic traumatic encephalopathy patients propagate in cultured cells. Proc Natl Acad Sci USA 113:E8187–E8196. https://doi.org/10.1073/pnas.1616344113
Xing CY, Tarumi T, Liu J, Zhang Y, Turner M, Riley J et al (2017) Distribution of cardiac output to the brain across the adult lifespan. J Cereb Blood Flow Metab 37:2848–2856. https://doi.org/10.1177/0271678X16676826
Acknowledgements
We would like to acknowledge the many families who contributed to this work, Christopher Nowinski, Ph.D. and Lisa McHale from the Concussion Legacy Foundation, and the clinical and neuropathology research staff of the BU CTE Center, VA Boston Healthcare System and Edith Nourse Rogers VA Medical Center This work was supported by grant funding from: NIA (AG057902, AG06234, RF1AG054156, K23AG046377), NINDS (U54NS115266, U01NS086659, and K23NS102399), National Institute of Aging Boston University AD Center (P30AG13846; supplement 0572063345-5); Department of Veterans Affairs Merit Award (I01-CX001038), the Nick and Lynn Buoniconti Foundation, and BU-CTSI Grant Number 1UL1TR001430. JC is funded by the Alzheimer’s Association (AARF-17-529888). The views, opinions, and/or findings contained in this article are those of the authors and should not be construed as an official Veterans Affairs or Department of Defense position, policy, or decision, unless so designated by other official documentation. Funders did not have a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alosco, M.L., Cherry, J.D., Huber, B.R. et al. Characterizing tau deposition in chronic traumatic encephalopathy (CTE): utility of the McKee CTE staging scheme. Acta Neuropathol 140, 495–512 (2020). https://doi.org/10.1007/s00401-020-02197-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-020-02197-9