Abstract
The peripheral nervous system (PNS) has remarkable regenerative abilities after injury. Successful PNS regeneration relies on both injured axons and non-neuronal cells, including Schwann cells and immune cells. Macrophages are the most notable immune cells that play key roles in PNS injury and repair. Upon peripheral nerve injury, a large number of macrophages are accumulated at the injury sites, where they not only contribute to Wallerian degeneration, but also are educated by the local microenvironment and polarized to an anti-inflammatory phenotype (M2), thus contributing to axonal regeneration. Significant progress has been made in understanding how macrophages are educated and polarized in the injured microenvironment as well as how they contribute to axonal regeneration. Following the discussion on the main properties of macrophages and their phenotypes, in this review, we will summarize the current knowledge regarding the mechanisms of macrophage infiltration after PNS injury. Moreover, we will discuss the recent findings elucidating how macrophages are polarized to M2 phenotype in the injured PNS microenvironment, as well as the role and underlying mechanisms of macrophages in peripheral nerve injury, Wallerian degeneration and regeneration. Furthermore, we will highlight the potential application by targeting macrophages in treating peripheral nerve injury and peripheral neuropathies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unlike the central nervous system (CNS), where damaged neurons are usually unable to regenerate, axons in the peripheral nervous system (PNS) are capable of regeneration after injury. However, the PNS regeneration is often incomplete, and in turn this can lead to neuropathic conditions [6]. Therefore, there is a great deal of interest in elucidating different factors involved in PNS regeneration, and how they regulate regeneration in injured nerves.
When axons in the PNS are injured, the distal portion progressively degenerates (Wallerian degeneration), a process that is produced by the breakdown of both axons and myelin [80]. The intrinsic degeneration of injured axons has been identified as the key event of Wallerian degeneration [38]. However, PNS is not completely isolated, and the injured axons trigger a complex multi-cellular response that involves multiple components [34, 38]. In addition to cellular responses elicited by injured axons, Wallerian degeneration is accompanied by the de-differentiation of Schwann cells and activation of immune response [34, 80]. Moreover, successful axon regeneration relies on a robust regenerative response of injured axons and the coordinated contribution of non-neuronal cells, including immune cells [38]. Indeed, a large body of evidence demonstrates that immune cells, including innate immune cells (neutrophils, macrophages and dendritic cells) and adaptive cells (T and B cells), play a critical role in PNS degeneration and regeneration, and they are recruited into injured sites within hours to days after nerve injury [6]. Among the immune cells operating at the injured sites of peripheral nerves, macrophages are the most notable cell type. A number of studies demonstrated that macrophages not only play a key role for removing myelin debris and modulate the activities of Schwann cells, but also are educated by the local injured microenvironment to promote axonal regeneration by releasing a large number of axonal regeneration-related factors, including extracellular matrix (ECM) proteins, growth factors, cytokines and chemokines [38, 71, 88]. In this review, we first summarize the main properties of macrophages, and then discuss the current knowledge on how monocytes/macrophages are recruited into distal injured sciatic nerves or other nerve tissues as indicated, how they are educated by the injured microenvironment and how they contribute to PNS Wallerian degeneration and axonal regeneration (Fig. 1).
Schematic diagram summarizing the working model for macrophages in peripheral nerve degeneration and regeneration. Peripheral nerve injury induces the disruption of axon/Schwann cell nerve unit, and then upregulates a variety of chemokines, cytokines and other factors to recruit monocytes/macrophages into injured nerves. The infiltrated macrophages on the one hand contribute to the Wallerian degeneration by removing the debris; on the other hand, they are educated by the local injured microenvironment and are polarized to an anti-inflammatory phenotype (M2), thus promoting peripheral nerve regeneration
Macrophages and their phenotypes
Macrophages are mononuclear phagocytes that usually originate from progenitor cells residing within the bone marrow [29, 40]. Recently, studies have demonstrated that tissue-resident macrophages, such as microglia, Kupffer cells and alveolar macrophages, originate from yolk-sac-derived myeloid progenitors [39, 91]. The same type of progenitor cells can also differentiate into other cells of the myeloid lineage, such as neutrophils, eosinophils and dendritic cells (DCs), depending on stimuli. The cytokines granulocyte macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF) and macrophage colony-stimulating factor (M-CSF) are the major stimulus factors for monocyte differentiation from the common myeloid progenitor cells [10]. Upon the emigration from the vasculature into tissues, monocytes can differentiate into distinct populations of macrophages depending on their anatomical location, for example alveolar macrophages in lung, osteoclasts in bone, histiocytes in interstitial connective tissues, Kupffer cells in liver and gut, microglia in brain, and splenic macrophages in spleen (Fig. 2) [10, 19, 40]. Each macrophage population displays a distinct functional profile associated with different gene expression patterns in specific tissue microenvironments [40]. Nonetheless, different populations of macrophages may undertake similar functions upon appropriate stimuli [36]. For example, the specialized phenotype and function of macrophages in gut can be induced from other populations of macrophages following stimulation with intestinal stromal cell products [113]. These findings suggest that macrophages exhibit a remarkable plasticity.
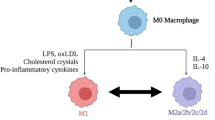
Schematic diagram summarizing the origin of macrophages, their populations and functional phenotypes. Following the stimulation with granulocyte macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF) or macrophage colony-stimulating factor (M-CSF), progenitor cells differentiate into monocytes, which in turn differentiate into macrophages within different tissues and conditions. Macrophages can be divided into distinct populations based on their anatomical locations (left panel) and into different phenotypes based on the distinct functions (right panel). Tissue-resident macrophages include alveolar macrophages (lung), osteoclasts (bone), histiocytes (interstitial connective tissue), Kupffer cells (liver and gut), microglia (brain), splenic macrophages (spleen) and others. The molecules that polarize macrophages toward “classically activated” pro-inflammatory phenotype (M1) or “alternatively activated” anti-inflammatory phenotype (M2, including M2a, M2b, M2c and M2d) as indicated (right panel) for each phenotype. Accordingly, each phenotype of macrophages has its own functions as indicated (right panel). A2AR, adenosine A2A receptor; IC, immune complexes; IFN-γ, interferon gamma; IL, interleukin; IL-1R, IL-1 receptor; LPS, lipopolysaccharides; TLR, toll-like receptor; TNF-α, tumor necrosis factor alpha
In addition to the plasticity, macrophages are also highly heterogeneous (Fig. 2). It has been shown that macrophages can be divided into “classically activated” pro-inflammatory phenotype (M1) and “alternatively activated” anti-inflammatory phenotype (M2) according to the Th1/Th2 dichotomy (Fig. 2) [10, 19, 40]. In view of recent findings about macrophage phenotypes and functions, a revision of Th1/Th2 dichotomy is needed, because other cytokines and factors, such as interleukin (IL)-10, regulatory T [Treg] products and glucocorticoids, that do not fit clearly with the Th1/Th2 response, but are able to polarize macrophages to specific phenotypes [69]. M1 macrophages are the effector and inducer cells in pro-inflammatory responses, and they are activated by lipopolysaccharide (LPS), interferon gamma (IFN-γ) and/or tumor necrosis factor alpha (TNF-α) [10, 19]. In contrast, M2 macrophages are involved in anti-inflammatory responses and activated by exposure to specific cytokines and factors, including IL-4, IL-13, IL-10, immune complexes, hormones or adenosine A2A receptors (A2AR) agonist [10, 19, 32, 33, 35]. These two phenotypes of macrophages have distinct gene and protein expression patterns [19, 35], which have been regarded as the phenotypic markers. These markers include cytokines, chemokines, membrane receptors and enzymes that are involved in distinct functions and processes, thus contributing to the pro- and anti-inflammatory effects of M1 and M2 macrophages, respectively [35]. In general, M1 macrophages are able to kill tumor cells and microorganisms by activating immune responses, whereas M2 macrophages are immunosuppressive cells that promote tissue remodeling and repair, as well as tumor angiogenesis, growth and progression [19, 68]. Further evidence shows that M2 macrophages can be divided into four distinct subtypes, including M2a, M2b, M2c and M2d (Fig. 2) [32, 35, 67, 70]. Each subtype of M2 macrophages has its own distinct phenotypic inducers and markers, as previously well summarized [35, 67]. M2a, M2b and M2c macrophages are considered as the anti-inflammatory cells, whereas M2d macrophages are derived from M1 pro-inflammatory cells following the activation of A2AR [32, 33, 35] and play an important role in wound healing and angiogenesis (Fig. 2) [32].
As discussed above, different phenotypes of macrophages have distinct functions, and macrophages exhibit an extremely high plasticity. Therefore, it is possible to “educate” macrophages to exhibit a beneficial function by polarizing them toward a specific phenotype. For example, tumor-associated macrophages (TAMs) are usually polarized to M2 phenotype [19, 93], where they promote tumor angiogenesis, growth and metastasis, as well as induce drug resistance [93, 99]. Polarization of TAMs toward M1 phenotype exhibits a promising effect in inhibiting tumor growth and progression, as well as in reversing drug resistance [92, 93, 96, 99]. A growing body of evidence demonstrates that macrophage polarization is regulated by a wide range of signaling pathways, which have been reviewed in depth elsewhere [10, 19, 35, 36, 40, 67, 68, 70]. In this review, we focus on discussing the role and underlying mechanisms of macrophage polarization in peripheral nerve regeneration in the context of nerve injury.
Recruitment of monocytes/macrophages into injury sites
It has been shown that a large number of immune cells, including neutrophils, macrophages and T cells, are recruited into injured sites following nerve injury, where they contribute to the pathogenic processes [63, 72]. The recruitment of neutrophils appears early after nerve injury, whereas the infiltration of macrophages into injured nerves is overtly seen starting from 2 to 3 days and peaking at 7 days post-injury (Fig. 3) [5, 74, 80, 88, 118]. Macrophages are the most abundant immune cells infiltrating into degenerating nerves, and they mainly originate from the circulating hematogenous monocytes [80].
Following nerve injury, the disruption of axon/Schwann cell nerve unit triggers Schwann cell de-differentiation and induces the release of multiple factors, including chemokines, cytokines and other factors, that are responsible for macrophage infiltration [80, 81, 121]. Among them, monocyte chemoattractant protein-1 [MCP-1, also known as chemokine (C–C motif) ligand 2, CCL2], leukemia inhibitory factor (LIF), IL-1α, IL-1β and pancreatitis-associated protein III (PAP-III) have been indentified as the major factors regulating monocytes/macrophage recruitment after nerve injury [80, 86, 109, 121, 123]. Both in vitro and in vivo studies have shown that these factors are rapidly produced by Schwann cells after peripheral nerve injury, and then function as the chemoattractants for macrophage infiltration [79, 80, 86, 109, 121]. Additionally, macrophages infiltrated into injured nerves also express and produce several factors, such as CCL2, TNF-α, IL-1α and IL-1β, thus contributing to further recruitment of monocytes/macrophages [52, 109]. These data imply that blockade of these factors would abolish injury-induced macrophage infiltration. However, in vitro evidence shows that addition of MCP-1 neutralizing antibodies at 50 μg/ml to conditional media of Schwann cell culture and peripheral nerve segments is not able to completely block the migration of macrophages [121]. Similar effects are also exhibited in vivo, where blockade of MCP-1 and IL-1β using antibodies does not completely block the macrophage infiltration after peripheral nerve injury [86]. These data suggests that nerve injury may also produce additional factors that contribute to monocyte/macrophage recruitment. Indeed, recent studies provided further evidence showing that some other factors, such as ECM proteins, galectin-1 and the complement system, are also involved in macrophage infiltration after peripheral nerve injury. Collagen VI is a major ECM protein produced by Schwann cells and macrophages in peripheral nerves [20]. Upon sciatic nerve crush injury, the deposition of collagen VI is significantly enhanced in injury sites, where it not only functions as a chemoattractant to recruit monocytes/macrophages (Fig. 3), but also regulates the expression of other chemoattractants, such as MCP-1 and IL-1β [21]. Galectin-1 is 14.5 kDa protein expressed by macrophages, Schwann cells and axons within peripheral nerves, and its expression is significantly elevated after nerve axotomy. The sciatic nerve injury-induced macrophage accumulation is inhibited in galectin-1-deficient mice [37]. The chemotactic function of galectin-1 on macrophages is also supported by the demonstration that injection of oxidized galectin-1 into intact nerve promotes the accumulation of macrophages [37], and galectin-1 enhances the migration of monocytes via p44/42 MAP kinase pathway [66]. Activation of the complement system is a crucial early event upon peripheral nerve injury [26, 94], and the products of activated complement system exhibit a significant role in macrophage migration [8]. In vivo findings show that depletion of complement using cobra venom factor inhibits macrophage infiltration after sciatic nerve injury [25]. Altogether, these findings provide a strong support for the involvement of distinct factors in macrophage infiltration after nerve injury.
By sensing the stimulation provided by distinct factors, monocytes/macrophages are recruited into injured nerves through different signaling pathways. C–C chemokine receptor type 2 (CCR2) is the main receptor of CCL2 in macrophages, and plays a key role for CCL2-induced macrophage migration [19]. Genetic evidence shows that the accumulation of macrophages in injured nerves is significantly inhibited in CCR2-deficient mice, suggesting that CCL2–CCR2 signaling pathway is essential for macrophage infiltration after nerve injury [34, 111]. Toll-like receptors (TLRs) can be activated in injured nerves by endogenous ligands that initiate innate immune response [9]. Ablation of TLR2, TLR4 and MyD88 in mice impairs the production of CCL2 and IL-1β and the infiltration of macrophages, whereas activation of TLR signaling by injection of TLR2 and TLR4 ligands enhances macrophage accumulation in the distal stump of sciatic nerve after injury [9]. Moreover, the production of IL-1β and TNF-α, as well as the infiltration of macrophages in dorsal root ganglion (DRG), is inhibited in TLR2 knockout mice after L5 spinal nerve transection injury [54]. Calcium-binding S100A8/A9 is an endogenous ligand of TLR4 [31, 102], which is important for regulating macrophage function in different pathological conditions [27, 45, 46]. The expression of S100A8 and S100A9 genes is dramatically upregulated in Schwann cells at day 1 post-injury in a sciatic nerve axotomy model [23, 55], where they have the ability to promote macrophage infiltration [23], suggesting the contribution of TLR4-S100A8/A9 signaling pathway in monocyte/macrophage recruitment after nerve injury. These data suggest that TLR and its related signaling pathways are necessary for macrophage infiltration after peripheral nerve injury. P-selectin is a cell adhesion molecule that expresses in the cell surface of macrophages [119] and has an important role in recruiting inflammatory cells into injury sites by binding and interacting with its ligand, P-selectin glycoprotein ligand-1 (PSGL-1) [106, 108]. Genetic evidence demonstrates that the infiltration of macrophages and production of pro-inflammatory cytokines TNF-α and IL-6 are attenuated in P-selectin-deficient mice after partial sciatic nerve ligation [62], suggesting an important effect of P-selectin mediating macrophage infiltration after nerve injury. Intercellular adhesion molecule-1 (ICAM-1) is an inducible surface glycoprotein that is mainly expressed in Schwann cells and nerve blood vein endothelia cells after sciatic nerve injury [126]. It has been shown that ICAM-1 not only involves in inflammation, but also in cell infiltration during the Wallerian degeneration after peripheral nerve injury [12, 17, 101]. Genetic studies showed that ablation of ICAM-1 inhibits macrophage infiltration after sciatic nerve transection injury [125], suggesting the potential role of ICAM-1 in peripheral nerve injury-induced macrophage infiltration. However, further studies are still needed to confirm this conclusion, because other findings show that blockade of upregulated ICAM-1 does not prevent macrophage infiltration after sciatic nerve injury [2, 11]. Prostacyclin receptor is significant for regulating the effect of prostacyclin in peripheral pain sensation. Peripheral nerve injury upregulates the expression of prostacyclin receptor in IL-1β-containing macrophages, and deficiency of prostacyclin receptor reduces the number of macrophages, whereas local administration of prostacyclin receptor agonist promotes the accumulation of IL-1β- and prostacyclin receptor-expressing cells at injury sites [105]. Serum amyloid A (SAA) is major acute phase reactant that can be released into circulation in response to injury [122]. It modulates immune response by promoting the production of inflammatory chemokines, including CCL2, in monocytes [60, 61]. Sciatic nerve axotomy induces the upregulation of SAA1 and SAA3 from Schwann cells through a mechanism involving modulation of IL-6, which in turn enhances macrophage infiltration by upregulating the production of CCL2 [49], thus suggesting that IL-6-SAA-CCL2 signaling pathway is involved in macrophage accumulation in injured nerves. Activation of AKT and PKA signaling is required for macrophage migration [24, 28], which also mediates collagen VI-regulated macrophage infiltration into injured nerves (Fig. 4) [21]. ERK is a central signaling pathway controlling Schwann cell plasticity, and also exhibits an essential role for macrophage infiltration after sciatic nerve injury [81]. Both genetic and pharmacological findings show that inhibition of Raf/MEK/ERK signaling pathway impairs macrophage infiltration into injured nerves [81]. Erythroid-2-related factor 2 (Nrf2) is a transcription factor that is required for monocyte/macrophage recruitment, as demonstrated by the finding that a lower number of macrophages are accumulated in injured nerves of Nrf2-deficient mice, suggesting a significant contribution of Nrf2 and its related pathways in macrophage infiltration upon nerve injury [128]. Altogether, these findings provide insights into the underlying mechanisms of macrophage infiltration after peripheral nerve injury.
Collagen VI-regulated macrophage migration and polarization contribute to peripheral nerve regeneration. Nerve injury induces a robust upregulation of collagen VI derived from Schwann cells and macrophages, which in turn recruits macrophages into injured sites in a paracrine and autocrine manner via AKT and PKA signaling pathways. These recruited macrophages on the one hand are involved in the Wallerian degeneration by removing the debris; on the other hand, they are polarized to M2 phenotype by collagen VI through AKT and PKA signaling pathways, thus enhancing peripheral nerve regeneration. Col VI collagen VI, PNS peripheral nervous system
Role of macrophages in Wallerian degeneration
Wallerian degeneration is a complicated process that is initiated following metabolic or mechanical damage to peripheral nerves and that induces multiple changes including axonal degeneration, myelin breakdown, glial cell proliferation, blood–nerve barrier (BNB) compromise, as well as the infiltration and activation of macrophages [16, 38, 116]. Schwann cells play an important role in the early stage of Wallerian degeneration. Upon injury, Schwann cells begin to de-differentiate in the distal nerve depending on the ubiquitin–proteasome system [59], a process that alters the gene expression profile of Schwann cells and provides an environment for axonal degeneration [38, 59, 76]. Schwann cells are able to remove myelin debris, a component that functions as a barrier to axon regrowth and contains axonal growth inhibitory signals including myelin-associated glycoprotein [38, 48]. Moreover, Schwann cells secrete a large number of trophic factors and ECM molecules promoting axon regeneration [22].
In the later stages of Wallerian degeneration, macrophages are the major cells contributing to remove myelin and axonal debris [30]. It has been demonstrated that the infiltrated macrophages can be seen laden with myelin-derived fat during the process of Wallerian degeneration [88]. Blocking monocytes with silica in mice induces less macrophage infiltration and slower myelin degradation after sciatic nerve injury [7]. Further studies showed that the Wallerian degeneration is delayed in macrophage-depleted animals using distinct pharmacological and genetic approaches [4, 64, 111]. Live cell imaging study also demonstrated that macrophages arrive at the injury sites long before axon fragmentation, and axonal debris can be engulfed by axon fragmentation-triggered invasion of macrophages in zebrafish model [97]. Taken together, these findings support that macrophages play a key role in Wallerian degeneration.
In addition to the predominant effect of hematogenous macrophages, resident macrophages are also involved in Wallerian degeneration. Resident macrophages account for 2–9 % of total cells in peripheral nerves and are endowed with phagocytic abilities [34, 42]. During the process of Wallerian degeneration, resident macrophages are able to induce inflammation via their expression of TLRs and by producing IL-13 and IL-1β [34, 105, 127], suggesting a potential role of these cells in Wallerian degeneration. The direct experimental evidence from chimeric rats shows that as early as 2 days post-sciatic nerve crush injury and before the influx of hematogenous macrophages, resident macrophages are increased and activated, as well as in a proliferating status [75]. These changes are more pronounced at 3 and 4 days after sciatic nerve injury, and the ED1-positive resident macrophages can be still indentified at 28 days after sciatic nerve injury [75]. More importantly, these resident macrophages are involved in early myelin phagocytosis after sciatic nerve injury [75].
Mechanistic studies demonstrated that the complement system is one of the major factors mediating the effect of macrophages in Wallerian degeneration. Depletion of complement by cobra venom factor suppresses the phagocytic activity of macrophages [25]. In the complement cascade, complement 3 (C3) and its receptor, complement receptor 3 (CR3), are the most prominent players in Wallerian degeneration. Decreased expression of CR3 on macrophages is accompanied by the reduction of macrophage-ingested myelin in vitro [13]. CR3 on the surface of macrophages bounds to the degenerating myelin sheaths to initiate phagocytosis [12], which can be blocked by the presence of CR3 antibodies [15]. Studies on co-cultures of macrophages and nerve segments showed that macrophages are unable to invade degenerating nerves and exhibit phagocytic abilities when the culture is incubated with C3-deficient serum [14]. In vivo evidence suggests that complement is activated by endogenous antibodies after peripheral nerve injury, a process that plays an essential role for macrophage accumulation and is critical for C3b-regulated myelin clearance and macrophage-mediated phagocytosis [124].
In addition to the complement system, many other factors and signaling pathways are also involved in macrophage-mediated Wallerian degeneration, especially for the phagocytosis of debris. It has been shown that IL-1β and TNFα are able to promote the phagocytosis of macrophages in vitro [109]. The phagocytic macrophages in injured nerves are dramatically reduced by the treatment with MCP-1 or IL-1β neutralizing antibodies [86], as well as by ablation of collagen VI [21] or of Nrf2 [128]. Upon peripheral nerve injury, the secreted cytokines and factors released by macrophages affect myelin phagocytosis by stimulating the expression of phospholipase A2 (PLA2) [71]. The binding between lysophosphatidylcholine (LPC, which is generated by PLA2) and C-reactive protein activates the classic complement pathway [43, 71], thus contributing to phagocytosis by macrophages. On the other hand, the expression of LPC on the cell surface can function as an “eat me” signal for macrophages, thus promoting phagocytosis [58, 71]. In addition, BNB plays an important role in Wallerian degeneration after peripheral nerve injury. BNB becomes increasingly leaky during Wallerian degeneration after nerve injury and with a peak at 8 days after crush injury, and then gradually regains its barrier function and becomes intact at 30 days after injury when regeneration is completed [107]. Sciatic nerve transection injury induces a robust upregulation of matrix metalloproteinase 2 (MMP-2) and MMP-9 after axotomy, which correlates with the breakdown of BNB and the accumulation of macrophages [110]. Treatment with nonspecific MMP inhibitor or MMP-9-specific antibody delays Wallerian degeneration of the transected peripheral nerve and attenuates macrophage infiltration [110], indicating a potential role of MMP-mediated macrophages and BNB function in Wallerian degeneration. Further evidence shows that peripheral nerve injury-induced BNB dysfunction is regulated by macrophages, as demonstrated by the evidence that chronic nerve compression injury-induced alterations of BNB are attenuated by macrophages depletion [41]. Taken together, these findings allow shedding light on the underlying mechanisms of macrophages in Wallerian degeneration.
Role of macrophages in peripheral nerve regeneration
Although macrophages were originally highlighted for their potent phagocytic activities, more and more studies using different models and/or approaches have demonstrated that they exhibit a pivotal role in peripheral nerve regeneration. Barrette et al. found that depletion of monocytes and macrophages via continuous local delivery at sciatic nerve injury sites or systemic administration of ganciclovir in CD11b-TKmt-30 mice compromises axonal regeneration and locomotor function recovery [4]. Depletion of macrophages with clodronate inhibits peripheral nerve regeneration of both motor and sensory functions [21], as demonstrated by the finding that clodronate-treated mice exhibit lower sciatic functional index score (Fig. 5a), longer time to initial response to toe pinch (Fig. 5b), and longer time to initial toe extension (Fig. 5c) when compared to the control mice [21]. More specifically in the sensory signaling transduction, macrophages play a crucial role in the development of peripheral nerve injury-induced neuropathic pain and in the repair of sensory function [95]. It has been shown that the infiltration of macrophages into injured nerves is delayed in slow Wallerian degeneration mouse (Wlds), which is accompanied by the impairment of thermal hyperalgesia development [77, 115] and peripheral nerve regeneration [83]. Sciatic nerve injury-induced thermal hyperalgesia is alleviated after the depletion of circulating macrophages by clodronate [64]. Taken together, these findings provide strong evidence for an essential role of macrophages during peripheral nerve regeneration and suggest that targeting macrophages is a promising strategy to promote peripheral nerve injury repair and functional recovery.
Depletion of macrophages by clodronate liposome impairs peripheral nerve regeneration. Quantification of the sensory motor function (a), sensory function (b) and motor function (c) of wild-type mice following treatment with PBS liposomes (Control) or clodronate liposomes. The graphs show the sciatic functional index from footprint track before crush (0) and at 7, 11, 14 and 17 days post-crush injury, the initial response time to the pinch using forceps in the digits 3, 4 and 5 after sciatic nerve crush, and the initial extension time to toe spreading reflex, respectively. (n = 5–7; *P < 0.05, **P < 0.01 and ***P < 0.001)
Several lines of studies using genetic and pharmacological approaches supported the idea that macrophage is a notable target for peripheral nerve regeneration. For example, experimental evidence shows that nicotinamide adenine dinucleotide phosphate oxidase 2 (Nox2)-positive macrophages are infiltrated into DRG after sciatic nerve injury and are involved in neuropathic pain hypersensitivity, which is impaired in Nox2-deficient mice [50], suggesting that Nox2 regulates peripheral nerve sensory function after injury by targeting macrophages. Collagen VI-deficient mice exhibit a delayed peripheral nerve regeneration by impairing macrophage function, which is rescued by transplantation of wild-type bone marrow cells [21]. Although these data strongly suggest that lack of Nox2 or of collagen VI impairs peripheral nerve regeneration via inhibition of macrophage function, these studies were carried out in global knockout mice. Further studies are needed to confirm this conclusion using Nox2 or collagen VI conditional knockout mice or treatment strategies specifically targeting macrophages. In addition, pharmacological treatments also support the concept that macrophage is a promising target for improving PNS regeneration [21, 47, 50]. For example, oxidized galectin-1 (GAL-1/Ox) is produced by Schwann cells and injured axons that specifically binds to and stimulates macrophages to produce axonal growth-promoting factor, thus promoting axonal regeneration, a process that is blocked by galectin-1 neutralizing antibodies [47]. Tacrolimus (FK506) is an immunosuppressant agent exhibiting a potent effect on promoting peripheral nerve regeneration [56] probably by enhancing the accumulation of macrophages in injured nerves [57]. Minocycline is a semisynthetic second-generation tetracycline that exhibits an inhibitory effect on nerve regeneration and decreases macrophage infiltration and activation after sciatic nerve injury [51], suggesting a potential action mechanism of minocycline on peripheral nerve regeneration by targeting macrophages.
Macrophages contribute to peripheral nerve regeneration via distinct mechanisms. After their infiltration, macrophages first contribute to peripheral nerve regeneration by removing the inhibitory regeneration signals from myelin debris and paving the way for axonal regrowth. Moreover, these cells produce a wide range of factors, such as proteases and growth-promoting factors/cytokines, and stimulate ECM remodeling to promote peripheral nerve regeneration [44, 87]. In addition, the infiltrated macrophages also stimulate peripheral nerve regeneration by affecting other cell types or components in injured nerves. It has been well demonstrated that Schwann cells exhibit a crucial role in peripheral nerve regeneration via distinct mechanisms [1, 81, 84, 85]. Interestingly, further studies showed that modulation of macrophage function is able to regulate peripheral nerve regeneration by regulating Schwann cell activities in injured nerves, including mitosis and de-differentiation [87], as well as infiltration and/or migration [47, 73]. Moreover, recent evidence shows that sciatic nerve cut injury induces a hypoxic environment in the bridge, a rejoined structure by two stumps following transection, that is selectively sensed by macrophages. These macrophages secret VEGF-A to polarize vasculatures that help Schwann cells to migrate and cross the wound, thus contributing to nerve regeneration [18]. Besides the infiltration into distal nerve after injury, macrophages also accumulate around axotomized cell bodies [65, 104], where they directly stimulate nerve regeneration [83].
More importantly, recent studies demonstrated that the infiltrated macrophages can be educated by the local microenvironment and are further delineated into distinct activation states. Ydens et al. demonstrated that sciatic nerve transection injury triggers an immunosuppressive response where the negative regulators of pro-inflammatory response and the anti-inflammatory cytokine IL-10 are induced, thus providing a microenvironment favoring macrophage M2 polarization [127]. Indeed, they found that M1 macrophage markers, such as inducible nitric oxide synthase (iNOS), IFN-γ and IL-12 p40, are absent, whereas the M2 macrophage markers, such as arginase-1, IL-13, Ym1 and Trem2, are significantly upregulated upon sciatic nerve transaction injury [127]. Further studies demonstrated that monocyte-derived M1 macrophages are only present at early stages after sciatic nerve ligation injury and they are gone by 3–4 days post-injury, when macrophages at injury sites start to be polarized to M2 phenotype and express high levels of arginase-1 and CD206 [78].
Multiple signaling pathways and molecules have been shown to modulate macrophage polarization after peripheral nerve injury (Table 1). For example, sciatic nerve injury induces a robust upregulation of apolipoprotein E (apoE) [100, 114] and collagen VI (Fig. 4) [21], which have the ability to polarize the infiltrated macrophages toward M2 phenotype by activation of p38 mitogen-activated protein kinase and tyrosine kinase [3], and by activation of AKT and PKA pathways, respectively [21]. In addition, CCL2/CCR2 is an important signal molecule that is enhanced upon peripheral nerve injury [53, 103, 117, 120], and can polarize macrophages toward an M2 phenotype [112]. These findings highlight that the recruited monocytes/macrophages are polarized to M2 phenotype through a mechanism that involves apoE, collagen VI and CCL2/CCR2, and their downstream signaling pathways. These findings are confirmed by genetic studies in knockout mice, which showed that GM-CSF-stimulated macrophages from CCR2-deficient mice display M1 phenotype [112] and that macrophage M2 polarization is dramatically impaired in collagen VI null mice after peripheral nerve injury (Fig. 4) [21]. G-protein-coupled receptor 84 (GPR84) is an orphan receptor that is mainly produced by macrophages during inflammation and upregulated upon sciatic nerve injury [82]. Lack of GPR84 polarizes macrophages toward M2 phenotype and inhibits the development of mechanical or thermal hypersensitivity after sciatic nerve ligation injury [82], suggesting that GPR84 is a negative regulator of macrophage M2 polarization in injured nerves. Taken together, these findings indicate that peripheral nerve injury triggers a microenvironment favoring macrophage M2 polarization via a variety of mechanisms.
As discussed above, M1 macrophages are pro-inflammatory cells that activate immune responses, whereas M2 macrophages are immunosuppressive cells that contribute to tissue remodeling and repair [19, 68]. In the context of PNS, macrophages of different phenotypes also exhibit distinct functions in peripheral nerve regeneration. Indeed, recent studies utilized an approach via local delivery of IFN-γ or IL-4 within polymeric nerve guidance channels to polarize macrophages toward M1 and M2 phenotypes, respectively, and the data demonstrated that polarization of macrophage toward M2 phenotype, but not M1 phenotype, promotes peripheral nerve regeneration [73]. Further evidence shows that sciatic nerve injury enhances the expression of IL-4 receptor α chain (IL-4Rα) in macrophages, which in turn mediates the effect of IL-4 on macrophage M2 polarization and sensory function recovery via STAT6 signaling pathway [53]. These findings highlight that macrophage polarization toward M2 phenotype is a promising strategy for promoting peripheral nerve regeneration after injury. Indeed, recent studies demonstrated that parthenolide, a sesquiterpene lactone occurring in the plant feverfew (Tanacetum parthenium), is able to enhance sensory regeneration in sciatic nerve chronic constriction injury model by promoting macrophage polarization to M2 phenotype [89].
As discussed above, IL-10 is a key cytokine that triggers macrophage M2 polarization, suggesting its potential application for promoting peripheral nerve regeneration. However, the therapeutic application of IL-10 has been limited, due to the fact that the in vivo bioactive half-life of IL-10 or its peptide fragments only remains for minutes to hours [98]. Interestingly, Potas et al. recently found that implantation of IL-10 conjugated electrospun poly(ε-caprolactone) (PCL) nanofibrous scaffolds in sciatic nerve effectively promotes macrophage M2 polarization up to 14 days [90]. These findings shed light for the potential application of IL-10 conjugated PCL nanofibrous scaffolds in promoting peripheral nerve regeneration by stimulating macrophage M2 polarization. However, further studies are needed to confirm this finding in peripheral nerve injury models. Taken together, these data suggest that studies focusing on macrophage M2 polarization will have the opportunity to offer novel and practical therapeutic approaches to improve regenerative outcomes following peripheral nerve injury.
Concluding remarks and future perspectives
Macrophages are one kind of prominent immune cells that play a pivotal role in tissue injury and repair. A number of studies indicated that macrophage infiltration and polarization are extensively regulated upon peripheral nerve injury. Moreover, these findings provide clear evidence supporting that the infiltrated and polarized macrophages exhibit a key role in Wallerian degeneration and PNS regeneration. These collective findings not only point at macrophages as a key type of immune cells involved in peripheral nerve regeneration, but also indicate that modulation of macrophage functions represents a promising strategy for improving PNS regeneration and controlling peripheral neuropathies.
Although our understanding of the role and underlying mechanisms of macrophages in Wallerian degeneration and PNS regeneration has increased recently, multiple questions remain unaddressed or only partially understood regarding how monocytes/macrophages are recruited and modulated after nerve injury, how injury triggers macrophage M2 polarization, how macrophages contribute to Wallerian degeneration and PNS regeneration, as well as how to improve peripheral nerve injury outcomes by targeting macrophages. As we discussed above, nerve injury-induced degeneration is a very complicated process that triggers the recruitment of monocytes/macrophages induced by the release and secretion of many factors from injured axons and Schwann cells, as well as from macrophages themselves. Therefore, it is difficult to prevent monocyte/macrophage recruitment and function after nerve injury by the blockade of a sole factor or multiple factors [86, 121]. Further studies on identifying early drivers and key factors upon peripheral nerve injury will greatly enhance the development of effective strategies to control peripheral nerve regeneration. Although our current knowledge indicates that nerve injury triggers a microenvironment favoring macrophage M2 polarization probably via a mechanism involving upregulation of IL-10 [127] and collagen VI [21], further studies are needed to clarify how these factors are modulated upon nerve injury. Recent studies by Mokarram et al. demonstrated that local delivery of IL-4 within polymeric nerve guidance channels promotes peripheral nerve regeneration by polarizing macrophages toward M2 phenotype [73]. These findings shed light on improving the outcomes after peripheral nerve injury by delivery of factors that are able to promote macrophage M2 polarization, such as IL-10 and collagen VI, within nerve guidance channels or other modified scaffolds. In addition to increasing the understanding of molecular mechanisms and key events of macrophages in peripheral nerve injury and regeneration, prospective findings answering these questions may provide novel therapeutic targets for the treatment of PNS injury and peripheral neuropathies and help to develop new therapeutic strategies by adding macrophage M2 polarization-related factors in scaffolds.
References
Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR (2012) c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 75:633–647
Avellino AM, Dailey AT, Harlan JM, Sharar SR, Winn RK, McNutt LD, Kliot M (2004) Blocking of up-regulated ICAM-1 does not prevent macrophage infiltration during Wallerian degeneration of peripheral nerve. Exp Neurol 187:430–444
Baitsch D, Bock HH, Engel T, Telgmann R, Müller-Tidow C, Varga G, Bot M, Herz J, Robenek H, von Eckardstein A, Nofer JR (2011) Apolipoprotein E induces antiinflammatory phenotype in macrophages. Arterioscler Thromb Vasc Biol 31:1160–1168
Barrette B, Hébert MA, Filali M, Lafortune K, Vallières N, Gowing G, Julien JP, Lacroix S (2008) Requirement of myeloid cells for axon regeneration. J Neurosci 28:9363–9376
Bendszus M, Stoll G (2003) Caught in the act: in vivo mapping of macrophage infiltration in nerve injury by magnetic resonance imaging. J Neurosci 23:10892–10896
Benowitz LI, Popovich PG (2011) Inflammation and axon regeneration. Curr Opin Neurol 24:577–583
Beuche W, Friede RL (1986) Myelin phagocytosis in Wallerian degeneration of peripheral nerves depends on silica-sensitive, bg/bg-negative and Fc-positive monocytes. Brain Res 378:97–106
Bianco C, Gotze O, Cohn ZA (1979) Regulation of macrophage migration by products of the complement system. Proc Natl Acad Sci USA 76:888–891
Boivin A, Pineau I, Barrette B, Filali M, Vallières N, Rivest S, Lacroix S (2007) Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J Neurosci 27:12565–12576
Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF (2012) Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 33:3792–3802
Brown HC, Castaño A, Fearn S, Townsend M, Edwards G, Streuli C, Perry VH (1997) Adhesion molecules involved in macrophage responses to Wallerian degeneration in the murine peripheral nervous system. Eur J Neurosci 9:2057–2063
Bruck W (1997) The role of macrophages in Wallerian degeneration. Brain Pathol 7:741–752
Bruck W, Bruck Y, Friede RL (1992) TNF-alpha suppresses CR3-mediated myelin removal by macrophages. J Neuroimmunol 38:9–17
Bruck W, Friede RL (1991) The role of complement in myelin phagocytosis during PNS wallerian degeneration. J Neurol Sci 103:182–187
Bruck W, Friede RL (1990) Anti-macrophage CR3 antibody blocks myelin phagocytosis by macrophages in vitro. Acta Neuropathol 80:415–418
Camara-Lemarroy CR, Guzman-de la Garza FJ, Fernandez-Garza NE (2010) Molecular inflammatory mediators in peripheral nerve degeneration and regeneration. Neuroimmunomodulation 17:314–324
Castano A, Bell MD, Perry VH (1996) Unusual aspects of inflammation in the nervous system: Wallerian degeneration. Neurobiol Aging 17:745–751
Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJ, Garcia Calavia N, Guo Y, McLaughlin M, Rosenberg LH, Quereda V, Jamecna D, Napoli I, Parrinello S, Enver T, Ruhrberg C, Lloyd AC (2015) Macrophage-induced blood vessels guide schwann cell-mediated regeneration of peripheral nerves. Cell 162:1127–1139
Chen P, Bonaldo P (2013) Role of macrophage polarization in tumor angiogenesis and vessel normalization: implications for new anticancer therapies. Int Rev Cell Mol Biol 301:1–35
Chen P, Cescon M, Megighian A, Bonaldo P (2014) Collagen VI regulates peripheral nerve myelination and function. FASEB J 28:1145–1156
Chen P, Cescon M, Zuccolotto G, Nobbio L, Colombelli C, Filaferro M, Vitale G, Feltri ML, Bonaldo P (2015) Collagen VI regulates peripheral nerve regeneration by modulating macrophage recruitment and polarization. Acta Neuropathol 129:97–113
Chen ZL, Yu WM, Strickland S (2007) Peripheral regeneration. Annu Rev Neurosci 30:209–233
Chernov AV, Dolkas J, Hoang K, Angert M, Srikrishna G, Vogl T, Baranovskaya S, Strongin AY, Shubayev VI (2015) The Calcium-binding proteins S100A8 and S100A9 initiate the early inflammatory program in injured peripheral nerves. J Biol Chem 290:11771–11784
Cote SC, Pasvanis S, Bounou S, Dumais N (2009) CCR7-specific migration to CCL19 and CCL21 is induced by PGE(2) stimulation in human monocytes: involvement of EP(2)/EP(4) receptors activation. Mol Immunol 46:2682–2693
Dailey AT, Avellino AM, Benthem L, Silver J, Kliot M (1998) Complement depletion reduces macrophage infiltration and activation during Wallerian degeneration and axonal regeneration. J Neurosci 18:6713–6722
de Jonge RR, van Schaik IN, Vreijling JP, Troost D, Baas F (2004) Expression of complement components in the peripheral nervous system. Hum Mol Genet 13:295–302
Dessing MC, Tammaro A, Pulskens WP, Teske GJ, Butter LM, Claessen N, van Eijk M, van der Poll T, Vogl T, Roth J, Florquin S, Leemans JC (2015) The calcium-binding protein complex S100A8/A9 has a crucial role in controlling macrophage-mediated renal repair following ischemia/reperfusion. Kidney Int 87:85–94
Diaz-Munoz MD, Osma-Garcia IC, Iniguez MA, Fresno M (2013) Cyclooxygenase-2 deficiency in macrophages leads to defective p110gamma PI3K signaling and impairs cell adhesion and migration. J Immunol 191:395–406
Doulatov S, Notta F, Eppert K, Nguyen LT, Ohashi PS, Dick JE (2010) Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat Immunol 11:585–593
Dubovy P (2011) Wallerian degeneration and peripheral nerve conditions for both axonal regeneration and neuropathic pain induction. Ann Anat 193:267–275
Ehrchen JM, Sunderkotter C, Foell D, Vogl T, Roth J (2009) The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol 86:557–566
Ferrante CJ, Leibovich SJ (2012) Regulation of macrophage polarization and wound healing. Adv Wound Care (New Rochelle) 1:10–16
Ferrante CJ, Pinhal-Enfield G, Elson G, Cronstein BN, Hasko G, Outram S, Leibovich SJ (2013) The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Ralpha) signaling. Inflammation 36:921–931
Francesco-Lisowitz A, Lindborg JA, Niemi JP, Zigmond RE (2015) The neuroimmunology of degeneration and regeneration in the peripheral nervous system. Neuroscience 302:174–203
Franco R, Fernandez-Suarez D (2015) Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol 131:65–86
Galli SJ, Borregaard N, Wynn TA (2011) Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol 12:1035–1044
Gaudet AD, Leung M, Poirier F, Kadoya T, Horie H, Ramer MS (2009) A role for galectin-1 in the immune response to peripheral nerve injury. Exp Neurol 220:320–327
Gaudet AD, Popovich PG, Ramer MS (2011) Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation 8:110
Gomez PE, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR (2015) Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518:547–551
Gordon S, Taylor PR (2005) Monocyte and macrophage heterogeneity. Nat Rev Immunol 5:953–964
Gray M, Palispis W, Popovich PG, van Rooijen N, Gupta R (2007) Macrophage depletion alters the blood–nerve barrier without affecting Schwann cell function after neural injury. J Neurosci Res 85:766–777
Griffin JW, George R, Ho T (1993) Macrophage systems in peripheral nerves. A review. J Neuropathol Exp Neurol 52:553–560
Hack CE, Wolbink GJ, Schalkwijk C, Speijer H, Hermens WT, van den Bosch H (1997) A role for secretory phospholipase A2 and C-reactive protein in the removal of injured cells. Immunol Today 18:111–115
Hikawa N, Takenaka T (1996) Myelin-stimulated macrophages release neurotrophic factors for adult dorsal root ganglion neurons in culture. Cell Mol Neurobiol 16:517–528
Hiratsuka S, Watanabe A, Aburatani H, Maru Y (2006) Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol 8:1369–1375
Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K, Shibuya M, Akira S, Aburatani H, Maru Y (2008) The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol 10:1349–1355
Horie H, Kadoya T, Hikawa N, Sango K, Inoue H, Takeshita K, Asawa R, Hiroi T, Sato M, Yoshioka T, Ishikawa Y (2004) Oxidized galectin-1 stimulates macrophages to promote axonal regeneration in peripheral nerves after axotomy. J Neurosci 24:1873–1880
Huang JK, Phillips GR, Roth AD, Pedraza L, Shan W, Belkaid W, Mi S, Fex-Svenningsen A, Florens L, Yates JR 3rd, Colman DR (2005) Glial membranes at the node of Ranvier prevent neurite outgrowth. Science 310:1813–1817
Jang SY, Shin YK, Lee HY, Park JY, Suh DJ, Kim JK, Bae YS, Park HT (2012) Local production of serum amyloid a is implicated in the induction of macrophage chemoattractants in Schwann cells during wallerian degeneration of peripheral nerves. Glia 60:1619–1628
Kallenborn-Gerhardt W, Hohmann SW, Syhr KM, Schröder K, Sisignano M, Weigert A, Lorenz JE, Lu R, Brüne B, Brandes RP, Geisslinger G, Schmidtko A (2014) Nox2-dependent signaling between macrophages and sensory neurons contributes to neuropathic pain hypersensitivity. Pain 155:2161–2170
Keilhoff G, Langnaese K, Wolf G, Fansa H (2007) Inhibiting effect of minocycline on the regeneration of peripheral nerves. Dev Neurobiol 67:1382–1395
Kiguchi N, Kobayashi Y, Saika F, Kishioka S (2013) Epigenetic upregulation of CCL2 and CCL3 via histone modifications in infiltrating macrophages after peripheral nerve injury. Cytokine 64:666–672
Kiguchi N, Kobayashi Y, Saika F, Sakaguchi H, Maeda T, Kishioka S (2015) Peripheral interleukin-4 ameliorates inflammatory macrophage-dependent neuropathic pain. Pain 156:684–693
Kim D, You B, Lim H, Lee SJ (2011) Toll-like receptor 2 contributes to chemokine gene expression and macrophage infiltration in the dorsal root ganglia after peripheral nerve injury. Mol Pain 7:74
Kim Y, Remacle AG, Chernov AV, Liu H, Shubayev I, Lai C, Dolkas J, Shiryaev SA, Golubkov VS, Mizisin AP, Strongin AY, Shubayev VI (2012) The MMP-9/TIMP-1 axis controls the status of differentiation and function of myelin-forming Schwann cells in nerve regeneration. PLoS One 7:e33664
Konofaos P, Terzis JK (2013) FK506 and nerve regeneration: past, present, and future. J Reconstr Microsurg 29:141–148
Kvist M, Danielsen N, Dahlin LB (2003) Effects of FK506 on regeneration and macrophages in injured rat sciatic nerve. J Peripher Nerv Syst 8:251–259
Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, Xu Y, Autenrieth IB, Schulze-Osthoff K, Belka C, Stuhler G, Wesselborg S (2003) Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 113:717–730
Lee HK, Shin YK, Jung J, Seo SY, Baek SY, Park HT (2009) Proteasome inhibition suppresses Schwann cell dedifferentiation in vitro and in vivo. Glia 57:1825–1834
Lee HY, Kim MK, Park KS, Shin EH, Jo SH, Kim SD, Jo EJ, Lee YN, Lee C, Baek SH, Bae YS (2006) Serum amyloid A induces contrary immune responses via formyl peptide receptor-like 1 in human monocytes. Mol Pharmacol 70:241–248
Lee HY, Kim SD, Shim JW, Lee SY, Lee H, Cho KH, Yun J, Bae YS (2008) Serum amyloid A induces CCL2 production via formyl peptide receptor-like 1-mediated signaling in human monocytes. J Immunol 181:4332–4339
Liou JT, Lee CM, Lin YC, Chen CY, Liao CC, Lee HC, Day YJ (2013) P-selectin is required for neutrophils and macrophage infiltration into injured site and contributes to generation of behavioral hypersensitivity following peripheral nerve injury in mice. Pain 154:2150–2159
Liou JT, Liu FC, Mao CC, Lai YS, Day YJ (2011) Inflammation confers dual effects on nociceptive processing in chronic neuropathic pain model. Anesthesiology 114:660–672
Liu T, van Rooijen N, Tracey DJ (2000) Depletion of macrophages reduces axonal degeneration and hyperalgesia following nerve injury. Pain 86:25–32
Lu X, Richardson PM (1993) Responses of macrophages in rat dorsal root ganglia following peripheral nerve injury. J Neurocytol 22:334–341
Malik RK, Ghurye RR, Lawrence-Watt DJ, Stewart HJ (2009) Galectin-1 stimulates monocyte chemotaxis via the p44/42 MAP kinase pathway and a pertussis toxin-sensitive pathway. Glycobiology 19:1402–1407
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25:677–686
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23:549–555
Martinez FO, Gordon S (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6:13
Martinez FO, Sica A, Mantovani A, Locati M (2008) Macrophage activation and polarization. Front Biosci 13:453–461
Martini R, Fischer S, Lopez-Vales R, David S (2008) Interactions between Schwann cells and macrophages in injury and inherited demyelinating disease. Glia 56:1566–1577
Moalem G, Tracey DJ (2006) Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev 51:240–264
Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV (2012) Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials 33:8793–8801
Mueller M, Leonhard C, Wacker K, Ringelstein EB, Okabe M, Hickey WF, Kiefer R (2003) Macrophage response to peripheral nerve injury: the quantitative contribution of resident and hematogenous macrophages. Lab Invest 83:175–185
Mueller M, Wacker K, Ringelstein EB, Hickey WF, Imai Y, Kiefer R (2001) Rapid response of identified resident endoneurial macrophages to nerve injury. Am J Pathol 159:2187–2197
Murinson BB, Archer DR, Li Y, Griffin JW (2005) Degeneration of myelinated efferent fibers prompts mitosis in Remak Schwann cells of uninjured C-fiber afferents. J Neurosci 25:1179–1187
Myers RR, Heckman HM, Rodriguez M (1996) Reduced hyperalgesia in nerve-injured WLD mice: relationship to nerve fiber phagocytosis, axonal degeneration, and regeneration in normal mice. Exp Neurol 141:94–101
Nadeau S, Filali M, Zhang J, Kerr BJ, Rivest S, Soulet D, Iwakura Y, de Rivero Vaccari JP, Keane RW, Lacroix S (2011) Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1beta and TNF: implications for neuropathic pain. J Neurosci 31:12533–12542
Namikawa K, Fukushima M, Murakami K, Suzuki A, Takasawa S, Okamoto H, Kiyama H (2005) Expression of Reg/PAP family members during motor nerve regeneration in rat. Biochem Biophys Res Commun 332:126–134
Namikawa K, Okamoto T, Suzuki A, Konishi H, Kiyama H (2006) Pancreatitis-associated protein-III is a novel macrophage chemoattractant implicated in nerve regeneration. J Neurosci 26:7460–7467
Napoli I, Noon LA, Ribeiro S, Kerai AP, Parrinello S, Rosenberg LH, Collins MJ, Harrisingh MC, White IJ, Woodhoo A, Lloyd AC (2012) A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron 73:729–742
Nicol LS, Dawes JM, La RF, Didangelos A, Clark AK, Gentry C, Grist J, Davies JB, Malcangio M, McMahon SB (2015) The role of G-protein receptor 84 in experimental neuropathic pain. J Neurosci 35:8959–8969
Niemi JP, Francesco-Lisowitz A, Roldan-Hernandez L, Lindborg JA, Mandell D, Zigmond RE (2013) A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration. J Neurosci 33:16236–16248
Painter MW, Brosius LA, Cheng YC, Latremoliere A, Duong K, Miller CM, Posada S, Cobos EJ, Zhang AX, Wagers AJ, Havton LA, Barres B, Omura T, Woolf CJ (2014) Diminished Schwann cell repair responses underlie age-associated impaired axonal regeneration. Neuron 83:331–343
Parrinello S, Napoli I, Ribeiro S, Wingfield Digby P, Fedorova M, Parkinson DB, Doddrell RD, Nakayama M, Adams RH, Lloyd AC (2010) EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell 143:145–155
Perrin FE, Lacroix S, viles-Trigueros M, David S (2005) Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1alpha and interleukin-1beta in Wallerian degeneration. Brain 128:854–866
Perry VH, Brown MC (1992) Role of macrophages in peripheral nerve degeneration and repair. BioEssays 14:401–406
Perry VH, Brown MC, Gordon S (1987) The macrophage response to central and peripheral nerve injury. A possible role for macrophages in regeneration. J Exp Med 165:1218–1223
Popiolek-Barczyk K, Kolosowska N, Piotrowska A, Makuch W, Rojewska E, Jurga AM, Pilat D, Mika J (2015) Parthenolide relieves pain and promotes M2 microglia/macrophage polarization in rat model of neuropathy. Neural Plast 2015:676473
Potas JR, Haque F, Maclean FL, Nisbet DR (2015) Interleukin-10 conjugated electrospun polycaprolactone (PCL) nanofibre scaffolds for promoting alternatively activated (M2) macrophages around the peripheral nerve in vivo. J Immunol Methods 420:38–49
Prinz M, Priller J (2014) Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 15:300–312
Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, Setty M, Leslie CS, Oei Y, Pedraza A, Zhang J, Brennan CW, Sutton JC, Holland EC, Daniel D, Joyce JA (2013) CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med 19:1264–1272
Qian BZ, Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141:39–51
Ramaglia V, Wolterman R, de Kok M, Vigar MA, Wagenaar-Bos I, King RH, Morgan BP, Baas F (2008) Soluble complement receptor 1 protects the peripheral nerve from early axon loss after injury. Am J Pathol 172:1043–1052
Ristoiu V (2013) Contribution of macrophages to peripheral neuropathic pain pathogenesis. Life Sci 93:870–881
Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, Costa S, Vinckier S, Dresselaer T, Åkerud P, De Mol M, Salomäki H, Phillipson M, Wyns S, Larsson E, Buysschaert I, Botling J, Himmelreich U, Van Ginderachter JA, De Palma M, Dewerchin M, Claesson-Welsh L, Carmeliet P (2011) HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell 19:31–44
Rosenberg AF, Wolman MA, Franzini-Armstrong C, Granato M (2012) In vivo nerve-macrophage interactions following peripheral nerve injury. J Neurosci 32:3898–3909
Rotshenker S (2011) Wallerian degeneration: the innate-immune response to traumatic nerve injury. J Neuroinflammation 8:109
Ruffell B, Coussens LM (2015) Macrophages and therapeutic resistance in cancer. Cancer Cell 27:462–472
Saada A, Dunaevsky-Hutt A, Aamar A, Reichert F, Rotshenker S (1995) Fibroblasts that reside in mouse and frog injured peripheral nerves produce apolipoproteins. J Neurochem 64:1996–2003
Scheidt P, Friede RL (1987) Myelin phagocytosis in Wallerian degeneration. Properties of millipore diffusion chambers and immunohistochemical identification of cell populations. Acta Neuropathol 75:77–84
Schiopu A, Cotoi OS (2013) S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease. Mediators Inflamm 2013:828354
Schreiber RC, Krivacic K, Kirby B, Vaccariello SA, Wei T, Ransohoff RM, Zigmond RE (2001) Monocyte chemoattractant protein (MCP)-1 is rapidly expressed by sympathetic ganglion neurons following axonal injury. NeuroReport 12:601–606
Schreiber RC, Shadiack AM, Bennett TA, Sedwick CE, Zigmond RE (1995) Changes in the macrophage population of the rat superior cervical ganglion after postganglionic nerve injury. J Neurobiol 27:141–153
Schuh CD, Pierre S, Weigert A, Weichand B, Altenrath K, Schreiber Y, Ferreiros N, Zhang DD, Suo J, Treutlein EM, Henke M, Kunkel H, Grez M, Nüsing R, Brüne B, Geisslinger G, Scholich K (2014) Prostacyclin mediates neuropathic pain through interleukin 1beta-expressing resident macrophages. Pain 155:545–555
Schulz C, Schafer A, Stolla M, Kerstan S, Lorenz M, von Brühl ML, Schiemann M, Bauersachs J, Gloe T, Busch DH, Gawaz M, Massberg S (2007) Chemokine fractalkine mediates leukocyte recruitment to inflammatory endothelial cells in flowing whole blood: a critical role for P-selectin expressed on activated platelets. Circulation 116:764–773
Seitz RJ, Reiners K, Himmelmann F, Heininger K, Hartung HP, Toyka KV (1989) The blood-nerve barrier in Wallerian degeneration: a sequential long-term study. Muscle Nerve 12:627–635
Semple JW, Freedman J (2010) Platelets and innate immunity. Cell Mol Life Sci 67:499–511
Shamash S, Reichert F, Rotshenker S (2002) The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J Neurosci 22:3052–3060
Siebert H, Dippel N, Mader M, Weber F, Weber F, Brück W (2001) Matrix metalloproteinase expression and inhibition after sciatic nerve axotomy. J Neuropathol Exp Neurol 60:85–93
Siebert H, Sachse A, Kuziel WA, Maeda N, Bruck W (2000) The chemokine receptor CCR2 is involved in macrophage recruitment to the injured peripheral nervous system. J Neuroimmunol 110:177–185
Sierra-Filardi E, Nieto C, Dominguez-Soto A, Barroso R, Sanchez-Mateos P, Puig-Kroger A, Lopez-Bravo M, Joven J, Ardavin C, Rodríguez-Fernandez JL, Sanchez-Torres C, Mellado M, Corbi AL (2014) CCL2 shapes macrophage polarization by GM-CSF and M-CSF: identification of CCL2/CCR2-dependent gene expression profile. J Immunol 192:3858–3867
Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD (2005) Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest 115:66–75
Snipes GJ, McGuire CB, Norden JJ, Freeman JA (1986) Nerve injury stimulates the secretion of apolipoprotein E by nonneuronal cells. Proc Natl Acad Sci USA 83:1130–1134
Sommer C, Schafers M (1998) Painful mononeuropathy in C57BL/Wld mice with delayed wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res 784:154–162
Stoll G, Muller HW (1999) Nerve injury, axonal degeneration and neural regeneration: basic insights. Brain Pathol 9:313–325
Tanaka T, Minami M, Nakagawa T, Satoh M (2004) Enhanced production of monocyte chemoattractant protein-1 in the dorsal root ganglia in a rat model of neuropathic pain: possible involvement in the development of neuropathic pain. Neurosci Res 48:463–469
Taskinen HS, Roytta M (1997) The dynamics of macrophage recruitment after nerve transection. Acta Neuropathol 93:252–259
Tchernychev B, Furie B, Furie BC (2003) Peritoneal macrophages express both P-selectin and PSGL-1. J Cell Biol 163:1145–1155
Toews AD, Barrett C, Morell P (1998) Monocyte chemoattractant protein 1 is responsible for macrophage recruitment following injury to sciatic nerve. J Neurosci Res 53:260–267
Tofaris GK, Patterson PH, Jessen KR, Mirsky R (2002) Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci 22:6696–6703
Uhlar CM, Whitehead AS (1999) Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem 265:501–523
Van SJ, Auvynet C, Sapienza A, Reaux-Le Goazigo A, Combadiere C, Melik Parsadaniantz S (2015) Stromal cell-derived CCL2 drives neuropathic pain states through myeloid cell infiltration in injured nerve. Brain Behav Immun 45:198–210
Vargas ME, Watanabe J, Singh SJ, Robinson WH, Barres BA (2010) Endogenous antibodies promote rapid myelin clearance and effective axon regeneration after nerve injury. Proc Natl Acad Sci USA 107:11993–11998
Vougioukas VI, Roeske S, Michel U, Bruck W (1998) Wallerian degeneration in ICAM-1-deficient mice. Am J Pathol 152:241–249
Yang J, Gu Y, Huang X, Shen A, Cheng C (2011) Dynamic changes of ICAM-1 expression in peripheral nervous system following sciatic nerve injury. Neurol Res 33:75–83
Ydens E, Cauwels A, Asselbergh B, Goethals S, Peeraer L, Lornet G, Almeida-Souza L, Van Ginderachter JA, Timmerman V, Janssens S (2012) Acute injury in the peripheral nervous system triggers an alternative macrophage response. J Neuroinflammation 9:176
Zhang L, Johnson D, Johnson JA (2013) Deletion of Nrf2 impairs functional recovery, reduces clearance of myelin debris and decreases axonal remyelination after peripheral nerve injury. Neurobiol Dis 54:329–338
Acknowledgments
We apologize to all these authors whose papers we could not cite due to space limitations. This work was supported by the University of Padova and the Italian Ministry of Education. P. Chen was supported by a fellowship from the Cariparo Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Chen, P., Piao, X. & Bonaldo, P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol 130, 605–618 (2015). https://doi.org/10.1007/s00401-015-1482-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-015-1482-4