Abstract
Neuronal injury from ischemic stroke is aggravated by invading peripheral immune cells. Early infiltrates of neutrophil granulocytes and T-cells influence the outcome of stroke. So far, however, neither the timing nor the cellular dynamics of neutrophil entry, its consequences for the invaded brain area, or the relative importance of T-cells has been extensively studied in an intravital setting. Here, we have used intravital two-photon microscopy to document neutrophils and brain-resident microglia in mice after induction of experimental stroke. We demonstrated that neutrophils immediately rolled, firmly adhered, and transmigrated at sites of endothelial activation in stroke-affected brain areas. The ensuing neutrophil invasion was associated with local blood–brain barrier breakdown and infarct formation. Brain-resident microglia recognized both endothelial damage and neutrophil invasion. In a cooperative manner, they formed cytoplasmic processes to physically shield activated endothelia and trap infiltrating neutrophils. Interestingly, the systemic blockade of very-late-antigen-4 immediately and very effectively inhibited the endothelial interaction and brain entry of neutrophils. This treatment thereby strongly reduced the ischemic tissue injury and effectively protected the mice from stroke-associated behavioral impairment. Behavioral preservation was also equally well achieved with the antibody-mediated depletion of myeloid cells or specifically neutrophils. In contrast, T-cell depletion more effectively reduced the infarct volume without improving the behavioral performance. Thus, neutrophil invasion of the ischemic brain is rapid, massive, and a key mediator of functional impairment, while peripheral T-cells promote brain damage. Acutely depleting T-cells and inhibiting brain infiltration of neutrophils might, therefore, be a powerful early stroke treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is a leading cause of death in industrialized countries and a major cause of morbidity. The most common type is the ischemic stroke, which is caused by occlusion of a brain-supplying artery followed by an energy failure which in turn leads to neuronal death. Despite intensive research, the treatment options for established strokes are limited. The only approved therapy is thrombolysis, which is reserved for patients admitted into the clinic within the first few hours after stroke onset and who do not suffer from intracerebral bleeding.
The development of additional therapies has been unsuccessful so far [50], probably since most treatments have focused on neuroprotective drugs. Another promising target, however, is the postischemic inflammatory cascade following stroke, since its contribution to brain damage is clearly evident [15, 66]. Postischemic inflammation comprises early infiltration of polymorphonuclear neutrophils (PMNs) and monocytes/macrophages into the injured brain parenchyma. This is accompanied by the activation of microglia and expression of proinflammatory cytokines, adhesion molecules, and other inflammatory mediators [16, 46, 47, 67]. Also peripheral T-cells are known to play important roles in post-stroke development, although the exact mechanisms remain unclear [33].
Despite these established inflammatory events after ischemia, the main impact of postischemic inflammation is controversially discussed [11, 67]. While some researchers conclude, that even massive PMN infiltrations are less important than T-cells for the outcome of stroke [39], those favoring a detrimental role of infiltrating PMN argue that these cells contribute to tissue damage by releasing oxygen radicals, proteases, and proinflammatory cytokines [5, 28]. Indeed, experimental strategies interfering with PMN infiltration into the injured parenchyma were neuroprotective [6, 8, 23, 44, 53, 55], and we showed there was a strong exacerbation of neuronal death by PMN in primary neuronal cultures and organotypic hippocampal slices [14, 47]. We also demonstrated that brain-resident microglia interacted with invading PMN thereby protecting neurons from overt pathology [47]. However, it remained open whether similar events would also happen in vivo.
Based on these insights, interfering with cerebral infiltration of PMN after stroke should be beneficial. This hypothesis was tested in a number of clinical trials either by the use of antibodies against the key adhesion molecule LFA-1 or its main ligand ICAM-1, or by blocking LFA-1 via neutrophil inhibitory factor rNIF [50]. However, the trials were terminated in clinical phase II (rNIF), or phase III [Enlinomab (anti-ICAM-1) study], either due to a lack of clinical benefit or from serious adverse events, and even increased mortality in the Enlinomab Study [10].
While all these studies focused on LFA-1-ICAM-1 for brain entry of PMN, probably a more relevant target might be the adhesion pair VLA-4 (very-late-antigen-4) and VCAM-1 (vascular cell adhesion molecule 1). Autoaggressive T-cells use the VLA-4-VCAM-1 axis to enter the CNS, and consequently its blockade is effective against multiple sclerosis [54]. Experiments in mice showed that PMN also expresses VLA-4 and the brain entry of PMN could be inhibited by blocking VLA-4 with antibodies or downregulating VACM-1 [39]. Importantly, both these therapies have been beneficial for the outcome of stroke.
Nevertheless, this study concluded that T-cells rather than PMN were the key cell type determining the overall outcome of the stroke [39]. This conclusion, however, fails to explain why the observed benefit of anti-VLA-4 in behavioral tests occurred between 24 and 72 h after the insult, a time point where T-cell accumulation into the brain was still limited and which is too early to account for a significant adaptive T-cell response [39]. Furthermore, the conclusion that huge numbers of highly activated infiltrating PMN are irrelevant for neuronal death, compared to 10 times fewer T-cells, is in contrast to a number of published studies from different groups including our own [14, 21, 45, 47, 58].
Thus, conclusions on the role of PMN and T-cells for the direct effects on brain parenchyma and outcome of stroke needed a re-evaluation, and the role of microglia in this respect had not been studied at all. We, therefore, observed the response of individual PMN and microglia within the first minutes after stroke-related blood–brain barrier irritation by intracranial two-photon microscopy. Furthermore, we investigated the direct effects of VLA-4 blockade on the brain-invasive behavior of PMN. Finally, we tested the impact of VLA-4-blockade and a direct depletion of myeloid cells, PMN or T-cells on stroke outcome.
Our data demonstrate the rapid response of PMN and microglia to blood vessel activation in association with stroke thereby showing that microglia directly interacted with invading PMN. Interaction of PMN with activated blood vessels was blocked by anti-VLA-4 leading to a strongly reduced neutrophil infiltration and significant amelioration of stroke outcome after 72 h that was also achievable by direct PMN removal. However, while the depletion of T-cells had no significant positive effect on the behavioral impairment after stroke, it strongly reduced the infarct volume.
Materials and methods
Transgenic mice
LysM-eGFP [19] mice were used to visualize PMN. The LysM-promoter is expressed in many myeloid cells, however, in the LysM-eGFP-expressing cells it has been found that neutrophils are especially brighter (~10×) than other myeloid cells such as monocytes/macrophages ([25] and supplemental Figure 4). A cross to CX3CR1+/gfp-mice [29] LysM-eGFP was used to simultaneously image PMN and microglia. In peripheral immune cells, the CX3CR1 promoter is active in dendritic cells and monocytes/macrophages. However, within the brain parenchyma, it is exclusively expressed in microglia which are recognizable by their typical ramified morphology in these mice.
All animal care was in accordance with institutional guidelines. Experiments were performed in accordance with National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals with local government approval.
Surgical preparation
To allow for the in situ addition of substances during imaging sessions, the jugular vein of the mice was catheterized. Catheterization and focal cerebral ischemia were performed in one operation step. The mice were anesthetized by inhalation of 2 % halothane and their rectal temperature was maintained at 37 ± 0.5 °C using a heating pad (Temperature control, TSE Systems, Bad Homburg, Germany).
A longitudinal incision was made over the joining of the anterior jugular, acromeodeltoid, and cephalic veins. Two threads were passed under the jugular vein below and the cephalic thread was tied to prevent bleeding. The catheter (Alzet, Cupertino, CA, USA) was pushed 13 mm into the lumen through an incision below the ligature and fixed with the second thread. A blunt needle (16G) was inserted through the interscapula incision and pushed subcutaneously until the end came out through an incision in the neck. The implanted catheter was flushed with saline containing 200 U Heparin/ml. The incisions in the skin were sutured.
Permanent middle cerebral artery occlusion
Focal cerebral ischemia was induced by permanent occlusion of the middle cerebral artery (pMCAO). After removal of the temporalis muscle, a burr hole was drilled in the temporal bone overlying the MCA above the zygomatic arch. The MCA was electrocoagulated using a cauterizer (Fine Science Tools Inc., Heidelberg, Germany). For analyzing the infarct volumes from animals with a pMCAO, six C57/Bl6 mice were sacrificed 24 h after surgery. The mice were transcardially perfused with 0.9 % NaCl and 4 % PFA/PBS. The brains were removed and cut into 1-mm slices using a matrix. The thick slices were stored in a 30 % sucrose solution until they sunk and were frozen in −40 °C cooled Methyl-2-butane. After this procedure, ten 20-µm coronal sections were cut from each 1-mm slice. The 20-µm sections were stained with toluidine-blue and the infarct size was measured in microscopic images (Zeiss Axiovert200, Zeiss, Jena, Germany) using the AxioVision software (Zeiss).
Two-photon intravital microscopy
Anesthesia was induced as described [34]. Animals were fixed in a self-drafted frame, and their rectal temperature was maintained at 37 ± 0.5 °C using a heating pad (DC Temperature Control, FHC, Bowdoin, Canada). The skull of the animals was thinned over the assumed ischemic penumbra by an electric drill (Dremel, BD Breda, The Netherlands). Two-photon imaging was performed using an upright LSM710 microscope (Zeiss), with a MaiTai Deep-See laser (Spectra Physics, Darmstadt, Germany) and a Zeiss 20× water-immersion objective (1.0 NA). 50 µl of 10 mg/ml Rhodamine/dextran (70 kDa, hydrodynamic size ~10.2 nm, [7]) in sterile 0.9 % saline was applied through the catheter to label blood vessels and to indicate the leakage of the blood–brain barrier vessels. Fluorescence was detected by non-descanned detectors following excitation at 850 nm.
Movie analysis
The microscopic experiments and the analysis of the movies were done by different persons to avoid any knowledge of used treatment (blind analysis). Images were obtained in 5-s intervals for 1-h periods. 30 min were recorded before and 30 min after application of antibody or isotype through the catheter during microscopy. Movies were analyzed for rolling and adhered PMN. Adherence was defined as resting more than 30 s. PMNs moving at the inner vessel wall were defined as rolling cells. Anti-VLA-4 [anti-mouse CD49d antibody (Clone R1-2)] and isotype control (IGg2b) were from eBioscience (San Diego, CA, USA). IMARIS (Bitplane, Zurich, Switzerland) was used for analyzing the movies and Z-stacks for the distances of PMN to their nearest vessel, and for the automated tracking and speed measurements of rolling PMN.
Flow cytometric analyses
Animals were sacrificed by intracranial perfusion with PBS/Heparin (1 %) solution while blood samples were taken. Brain hemispheres were separated and equally sized pieces of the ischemic area and the contralateral side were isolated using a 4-mm punch. Tissue was homogenized in PBS containing 1 % FCS and filtrated (40 µm cell-strainer). The cell suspension was fixed in 4 % PFA/PBS. After erythrocyte lysis, blood was also fixed in 4 % PFA/PBS. Cells were resuspended in a solution containing anti-Gr-1-APC (BD, Heidelberg, Germany) and incubated for 15 min at 4 °C. All probes were measured using a Canto II FACS (BD) or a MACSQuant (Miltenyi, Bergisch Gladbach, Germany). To examine the VLA-4 expression in human blood, probes were taken from 2 healthy test persons. PMNs in these samples were stained with CD66b-FITC (BD) and CD49d-PE (BD). Using anti-VLA-4 antibodies in mouse blood (FITC-labeled, eBioscience, Frankfurt, Germany) and brain samples (PE-labeled, in Lys-EGFP-mice, BD), the VLA-4 expression on mouse PMNs was detected. Statistics were performed using Sigma Stat software. For additional VLA-4 analyses in murine blood and on isolated PMN from bone marrow, we used anti-CD49d (PE, Biolegend, Fell, Germany) and anti-CD29 (PE-Cy7, Biolegend) in non-fixed samples. P values are: *p < 0.05.
Determination of VLA-4 expression by quantitative real-time PCR
PMNs from the bone marrow of mice were negatively isolated with immunomagnetic beads (Miltenyi) according to the manufacturer’s protocol. Isolated neutrophils were incubated for 30 min at 37 °C in 1-ml complete medium (CM) with either phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich, Hamburg, Germany) (1 µM), formyl-met-leu-phe (fMLP, Sigma-Aldrich) (100 µM), lipopolysaccharide (LPS, Sigma-Aldrich) (2.5 ng/ml) or PBS as control. To isolate total mRNA, cells were pelleted (350× g/5 min/4 °C) and lysed with 350 µl of RLT-buffer using the RNeasy kit (Qiagen, Hilden, Germany) following the manufacturer’s guidelines. The total mRNA concentration was determined using a NanoDrop 2000C (Thermo-Fisher Scientific, Reinach, Switzerland) and up to 12 µl of sample was used for reverse transcription using the QuantiTect Reverse Transcription Kit (Qiagen). For quantification of VLA-4, primers for alpha (Itga4, Qiagen) and beta chain (Itgb1, Qiagen) as well as β-actin (Actb, Qiagen) as housekeeping gene were used. The qPCR was run in a total volume of 25 µl containing 12.5 µl of 2× QuantiFast SYBR Green PCR Master Mix (Qiagen), 100 ng DNA and 2.5 µl Primer (1 µM/reaction). The amplification was performed on a RotorGene Q PCR system (Qiagen) with the initial step of 5 min at 95 °C followed by 40 cycles, each of them 10 s at 95 °C and 30 s at 60 °C. The obtained ct values were used to calculate the fold induction using the ΔΔct-method.
Induction of transient middle cerebral artery occlusion and antibody treatments
Transient middle cerebral artery occlusion (tMCAO) was induced as described [26]. Briefly, animals were anesthetized with 1 % isoflurane (30 % O2, remainder N2O) and their rectal temperature was maintained between 36.5 and 37.0 °C using a feedback-controlled heating system (Harvard Apparatus, Heidelberg, Germany). A midline neck incision was made and the left common and external carotid arteries were ligated. A microvascular clip was temporarily placed on the internal carotid artery. A silicon resin-coated nylon monofilament was introduced through a small incision into the common carotid artery and advanced to the carotid bifurcation for middle cerebral artery occlusion. Transcranial laser Doppler flowmetry (Perimed, Jarfalla, Sweden) was used to confirm a severe reduction in cerebral blood flow in the area of the cerebral cortex supplied by the MCA. Reperfusion was initiated after 45 min by monofilament removal. Laser Doppler flow changes were monitored up to 15 min after reperfusion onset. After the surgery, wounds were carefully sutured and anesthesia was discontinued. Mice were monitored until they regained consciousness and were returned to their cages.
Myeloid cells were depleted with 100-µg anti-mouse Gr-1 (Clone RB6-8C5, BioXcell, West Lebanon, USA), PMN with 200-µg anti-mouse Ly6G (Clone 1A8, BioXcell), and T-cells with 400-µg anti-mouse CD3 (Clone 17A2, BioXcell,). Antibodies were i.p. injected 24 h before and 24 h after ischemia. Control mice received 400-µg isotype control (Clone LTF-2, BioXcell) at the same time points. To inhibit PMN infiltration, 150 µg of anti-mouse VLA-4 (Clone P/S2, BioXCell) was i.v. injected at the beginning of reperfusion and 24 h later. Control mice received equal amounts of isotype.
72 h after ischemia, behavioral tests were conducted followed by transcardial perfusion with 0.9 % NaCl. Brains were removed, fresh frozen on dry ice and cut on a cryostat into 20-μm coronal sections [57] to perform immunohistochemical analysis of infarct volumes, PMN infiltration, and distance of neutrophils to vessels. Behavioral testing, including analysis and infarct volume measurements, was performed while being blinded to the experimental groups.
Animal allocation and exclusion criteria for tMCAO experiments
A total of 120 adult male C57BL/6 mice (8–10 weeks old, body weight 23–28 g) were enrolled. Based on our own previous experiences, we performed a sample size calculation (beta error level 20 %, alpha error level 5 %) prior to experiments resulting in a sample size of 12 mice per group. In the first set of studies, 24 mice were randomly assigned to 2 groups treated with isotype (n = 12) or anti-VLA-4 (n = 12). These mice were used for behavioral testing and immunohistochemical analysis of ischemic injury and neutrophil infiltration. In a second cohort of mice (n = 96), specific immune cell subsets were depleted in combination with anti-VLA-4 treatment. These mice were assigned to 8 experimental groups by balanced randomization: isotype/isotype (n = 12), isotype/anti-VLA-4 (n = 12), anti-CD3/isotype (n = 12), anti-CD3/anti-VLA-4 (n = 12), anti-Gr-1/isotype (n = 12), anti-Gr-1/anti-VLA-4 (n = 12), anti-Ly6G(1A8)/isotype (n = 12), anti-Ly6G(1A8)/anti-VLA-4 (n = 12). Mice were used for behavioral testing and determination of infarct volumes.
Exclusion criteria were defined as follows: prolonged operation time >15 min; no reperfusion after filament withdrawal; insufficient drop of blood flow (<70 %); death within 72 h after MCAO. Altogether, 16 mice (13.3 %) were excluded from the study [first experiment: n = 3 isotype and n = 3 anti-VLA-4; second experiment: n = 2 isotype/isotype, n = 2 isotype/anti-VLA-4, n = 1 anti-CD3/isotype, n = 2 anti-CD3/anti-VLA-4, n = 0 anti-Gr-1/isotype, n = 0 anti-Gr-1/anti-VLA-4, n = 1 anti-Ly6G(1A8)/isotype, n = 2 anti-Ly6G(1A8)/anti-VLA-4]. Due to technical problems (construction works in the behavioral testing facility) and animals lacking motivation, known to occur in rare cases [4], further animals had to be excluded from behavioral testing analysis [first experiment: n = 1 isotype and n = 3 anti-VLA-4; second experiment: n = 0 isotype/isotype, n = 3 isotype/anti-VLA-4, n = 5 anti-CD3/isotype, n = 5 anti-CD3/anti-VLA-4, n = 4 anti-Gr-1/isotype, n = 5 anti-Gr-1/anti-VLA-4, n = 2 anti-Ly6G(1A8)/isotype, n = 1 anti-Ly6G/anti-VLA-4].
Post-stroke functional recovery
Motor-coordination deficits were analyzed 3 days after stroke induction using the rota rod, tight rope, and corner turn tests. Behavioral tests were performed on the same animals as used for the immunohistochemistry. Animals were trained 1 day before stroke induction. Rota rod and tight rope tests were performed as described [17]. Maximum speed was achieved after 260 s, and maximum testing time was 300 s. The time until the animals dropped was registered. In the tight rope test, the maximum test time was 60 s followed by the scoring of animals from 0 (minimum) to 20 (maximum) according to a validated score. Rota rod and tight rope tests were performed twice and means were calculated. For the corner turn test, two vertical boards were attached with an angle of 30°, and each mouse was tested for the side chosen over 10 trials. Healthy animals leave the corner without side preference, stroke mice preferentially turn to the non-impaired body side [71]. The laterality index (LI) was calculated by (number of left turns – number of right turns)/10.
Determination of infarct volumes and PMN infiltration
Infarct volumes were analyzed on cresyl violet-stained sections at a distance of 800 µm within the range of +2 mm up to −4.4 mm from bregma. Sections were scanned and analyzed using ImageJ (NIH, USA). Infarct volumes were determined by application of the indirect infarct measurement as previously described [40]. As such, the non-lesioned volume of the ipsilateral hemisphere was subtracted from the total volume of the contralateral side. Edema formation is expressed as the ratio of ipsilateral hemisphere volume to contralateral volume. For assessment of PMN infiltration and quantification of vessel-PMN distance, 20-µm cryostat sections taken at the level of bregma was sequentially stained for the PMN-specific antigen Ly6G (rat anti-mouse Ly6G, clone 1A8, 1:200, BD) and the pan endothelial cell marker CD31 (rat anti-mouse CD31 PE, clone MEC 13.3; 1:100, BD). Briefly, slides were fixed with methanol/acetone followed by incubation with primary antibodies in PBS containing 0.2 % Tween-100. Primary antibody binding (Ly6G) was detected with a goat anti-rat Alexa Fluor 488 (1:500, Invitrogen, Darmstadt, Germany) followed by incubation with anti-mouse CD31 PE. PMN infiltration was quantified by counting Ly6G+ cells in 8 regions of interest (ROI, each 25.000 µm2, 4 ROI striatum, 4 ROI cortex) in 3–5 sections per animal. For quantification of PMN-vessel-distances confocal analysis was performed, acquiring Z-stacks in 6–10 ROI (210.32 × 210.32 × 10 µm, Z-distance 0.2 µm) per animal, followed by measurement of distances between single PMN and the nearest adjacent vessels. A total of 251 cells derived from 4 mice were analyzed. Histological analysis was performed while being blinded to the experimental groups.
Statistical analysis
Statistical analysis was performed using PASW Statistics 18.0 (SPSS Ltd., Quarry Bay, Hong Kong). For comparison of two groups, unpaired two-sided Student t tests were applied. Data of functional recovery and infarct volumes from combined antibody treatment experiments were analyzed by two-way ANOVA evaluating effects of depletion (anti-Gr-1, anti-CD3, 1A8, isotype control) and treatment (anti-VLA-4 and isotype control). Whenever there was a significant effect, we further determined effects between single groups by two-sided unpaired Student t tests followed by Bonferroni correction for multiple comparisons. A value of p < 0.05 was considered statistically significant.
Results
PMN infiltration pattern after focal cerebral ischemia
Permanent occlusion of the distal middle cerebral artery (pMCAO) yields a distinct small cortical infarct (Fig. 1a, inset) which is ideal for intracranial microscopy, especially in areas of the ischemic core, penumbra, (areas labeled 1 and 2, Fig. 1a) and beyond (area labeled 3, Fig. 1a). Using CX3CR1-eGFP mice [29], where microglia appear as green cells in the brain, we found that these zones were distinguishable by different morphologies of resident microglia (Fig. 1b1–3, seen in all experiments with permanent cerebral ischemia and in line with the common literature). Cells most close to the ischemic core presented with activated ameboid morphology, higher motility of cytoplasmic processes, and increased diameter of somata (Fig. 1b1,2, c1 and supplemental movie 1). The farther away from the core the more resting the morphology appeared (Fig. 1b3, c3 and supplemental movie 1). The quantification showed significant differences in the behavior (motility of membrane processes) and appearance (diameter of somata) of microglia within and outside of the ischemic penumbra (Fig. 1d, e).
PMN infiltrates the brain parenchyma after pMCAO. a Toluidine-blue staining of a coronal section shows an ischemic lesion in the cortex 24 h after focal cerebral ischemia and the averaged lesion size of 6 mice. The inset bar graph shows the average infarct volume of this model (mean + SEM). Areas with different distances to the affected brain are indicated by broken lines. b 1–3 Images captured during two-photon in vivo imaging of CX3CR-1-eGFP mice 24 h after pMCAO with respect to the area 1–3 indicated in a. Next to the ischemic core in area 1 (b 1) microglia are swollen and show ameboid morphology. The area 2 (b 2) displays the zone of transition from ameboid to more ramified morphology of microglia and in area 3 (b 3) only ramified microglia are present. c 1/2 Different morphological appearance of microglia in area 1 (swollen and in ameboid shape) or 3 (ramified) of the penumbra. d The analyses of the velocity of microglial cytoplasmic processes (distance of retraction and extension over time) indicate a significantly higher motility of cytoplasmic processes of ameboid microglia in area 1 in the vicinity of the ischemic core compared to microglial cells in area 3 (mean + SEM of 9 cells quantified from 3 independent experiments, *p < 0.05). e Quantification of the size of microglial somata in area 1 and 3 (mean + SEM of 18 cells quantified from 3 independent experiments *p < 0.05). f Quantification (mean + SEM of 3 independent experiments, 3 animals per time point) of brain infiltrated Gr-1high/eGFP-positive cells per punch biopsy at 3, 6, 24, 48 and 72 h after ischemia by FACS analysis resulted in a peak at 48 h. g Image shows infiltrated PMN (green cells) within the brain parenchyma 24 h after ischemia surrounding a vessel (asterisk) in a depth of 530 μm from the cortical surface by confocal microscopy of fixed tissue. h 1/h 2 Images represent area 1 and 2 (depicted in a) in Lys-eGFP mice 24 h after ischemia and clearly show an intra-parenchymal presence of eGFPbright cells. i Illustration of the coexistence of PMN (inset with *) as well as microglia (inset with #) in double transgenic mice (CX3CR1-EGFP/Lys-EGFP) in the same focal plane of imaging. j In CX3CR1-eGFP mice, very few green cells (asterisk) roll on the inflamed endothelium of the infarcted brain while many unlabeled cells (arrowheads) are present. k Representative quantification of adhered eGFP+ and eGFP− cells in CX3CR-1-eGFP mice after ischemia. Scale bars: b 1, b 2 10 μm, b 3 30 μm; c 1/3 20 µm; g 100 µm; h 1, h 2, 50 μm; i 50 μm; i*, i # 10 μm; j 30 μm
We next addressed the time-dependent infiltration of eGFP-expressing PMN [25] after pMCAO in LysM-eGFP mice. We found the infiltration of PMN into the brain parenchyma to start 3 h after focal ischemia, with a peak after 24–48 h (Fig. 1f). PMNs were enriched in close proximity to the ischemic core 24 h after focal cerebral ischemia up to a depth of >500 μm below the cortical surface (Fig. 1g). We found two overlapping areas adjacent to the ischemic core (Fig. 1a, areas labeled 1 and 2) with detectable PMN association. In the first area close to the ischemic core (area 1, Fig. 1a), infiltrated motile PMNs were positioned within the parenchyma and a slow rolling, adhesion or sometimes extravasation of PMN through blood vessels, as well as active migration in the brain was observed (Fig. 1h1; supplemental movie 2). Mean migratory speeds of PMN in brain tissue were 10 µm/min (supplemental Fig. 5), thus corresponding to values measured for PMN in inflamed skin [36]. In the second area (area 2, Fig. 1a), low amounts of either rolling or freely floating PMN were seen in the blood, and only very few PMN in the brain. (Fig. 1h2; supplemental movie 3). Farther away from the ischemic core (area 3, Fig. 1a), we could neither detect PMN in the parenchyma nor attached to the endothelium (supplemental movie 4).
To verify that we were imaging within the cortical parenchyma, and not in the overlying meninges, we performed two-photon microscopy in double transgenic mice (CX3CR1-eGFP/Lys-eGFP), thereby visualizing microglia as well as PMN simultaneously. Although both cell types were green, PMN could be easily differentiated from microglia based on the different morphology and almost complete lack of cell migration in the latter. We and others [9, 48] showed, that in CX3CR1-eGFP mice, cells of microglial morphology exclusively reside in the brain parenchyma, but not in blood vessels or meninges. A deep Z-stack into the brain of a CX3CR1-eGFP mouse using 2-photon microscopy also demonstrated a prominent second harmonics signal, indicating the presence of extracellular matrix such as collagen, in the focal plane of the meninges which sharply dropped off, when the Z-level reached deeper areas of the brain tissue. At the same time, the morphology of green cells changed from ameboid/roundish to microglial (supplemental movie 15). Importantly, our analyses always demonstrated the co-localization of microglia and PMN in the same focal plane within double transgenic animals (Fig. 1i) and proved that the imaging depth was within the cortical parenchyma, and not above. In addition, PMN invaded the brain tissue deeply, as demonstrated by measuring the distance of individual cells to the nearest blood vessel in intravital 2-photon movies (supplemental Fig. 6a), or by ex vivo analysis of brain slices using immunofluorescence and confocal microscopy (supplemental Fig. 6b–d).
Since eGFP+ cells in Lys-eGFP mice are not selectively PMN but also contain other myeloid cells including monocytes [19], we analyzed the appearance of eGFP+ cells in CX3CR1-eGFP mice. In these mice mainly monocytes and some NK cells express eGFP while PMNs are not labeled [29]. CX3CR1+ monocytes can also crawl on endothelial surfaces [2, 62]. After pMCAO we very rarely detected CX3CR1-eGFP+ cells attached to and rolling on the endothelium in the inflamed brain area (Fig. 1j/k and supplemental movie 5), in contrast to the high amount of Lys-eGFP+ cells as described above. Moreover, we observed a significantly higher number of unlabeled cells associated with the endothelium, which were detectable as block outs in the rhodamine-dextran labeled blood (Fig. 1j/k and supplemental movie 5). Thus, the Lys-eGFP+ cells which attached at the endothelium after pMACO were highly enriched in PMN rather than monocytes.
These findings indicated that under local ischemia PMN rolled on inflamed blood vessels, infiltrated the brain parenchyma, and migrated within. Microglia reacted with morphological adaptations to the environmental changes in the brain and this response was directly associated with the distance to the ischemic core.
Physical interaction between microglia and PMN in the ischemic area
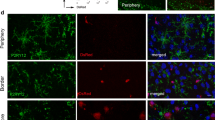
Previously we had observed interactions of microglia and PMN which led to the phagocytosis of dead and live PMN by microglia under conditions of oxygen and glucose deprivation [47]. The co-localization of both cell types in inflamed brain regions (Fig. 1i) suggested that physical interactions of the cells could also be observed in vivo. Indeed, a number of infiltrating PMN could be observed to touch local microglia. PMN contact induced rapid and pronounced morphological alterations of microglia. Several microglia could co-operate and form flat net-like membrane protrusions appearing to trap associated PMN and hindering their further migration (Fig. 2; supplemental movie 6). This was reminiscent of the interactions observed before in vitro. Importantly, morphological changes observed under ischemic conditions in the absence of PMN (Fig. 1b and supplemental movie 1) could clearly be distinguished from PMN-induced alterations. Whether PMNs were also phagocytosed could not be clarified in these experiments, but at least they were frequently observed to be captured at sites of microglial cytoplasmic processes. Thus, microglia establish long-lived physical contacts with PMN upon the stroke-related brain invasion of PMN in vivo.
Microglia interact with infiltrated PMN after pMCAO. Intravital two-photon microscopy was performed 24 h after pMCAO of double transgenic mice (arrowheads PMN, arrows microglia). The panel shows the reaction of microglia on infiltrated PMN (white tracks indicate the migration path of PMN). Within the white square, the PMNs were trapped by microglial protrusions and net-like structures (supplemental movie 6). Similar events were observed in 3 mice
Rapid response of PMN within thrombotic lesions
One way of causing stroke is the embolic or thrombotic clogging of a blood vessel accompanied by the activation of its endothelium. It is likely, but has not been investigated, that PMN will detect and respond to such perturbations of the blood circulation. To study the very first responses of circulating PMN towards endothelial activation and thrombotic clogging, we set additional lesions in blood vessels within the two different infarct areas by irradiating the local endothelia with the imaging laser for different amounts of time. Varying the duration of laser impact allowed to control whether the outcome was only endothelial activation or also formation of a solid thrombus. Importantly, endothelia were not punctured with the laser beam, so that there was no direct blood leakage into the brain. True hemorrhage induced with more intense laser-power provoked clearly visible leakage of the vessel tracer (supplemental Fig. 1 and supplemental movie 7). Endothelial activation in the area most close to the ischemic core led to immediate rolling of circulating PMN at the irradiated site (Fig. 3a; supplemental movie 8). This interaction also resulted in multiple PMN firmly adhering to the affected endothelial spot. Importantly, any contacts of PMN were highly localized to the laser-activated spot and did not occur at sites away from that region (Fig. 3a). Also microglia immediately sensed the endothelial activation and responded to this insult with the rapid extension of cytoplasmic processes from the abluminal side (Fig. 3a and supplemental movie 8). This behavior was similar to the microglial responses observed before where it, however, occurred in response to laser-induced blood vessel disruption and vessel leakage [9, 48]. The initial recruitment of PMN on laser-activated endothelium led to the accumulation of additional cells in a self-enhancing process (Fig. 3b). This could finally lead to local impairment of blood flow and disruption of the blood–brain barrier with subsequent massive infiltration of PMN into the brain (Fig. 3b and supplemental movie 9). After intravascular thrombus formation, PMNs were able to detect the presence of thrombotic vessel clogging from a distance, and were even able to crawl against the blood stream to the site of injury (Fig. 4 and supplemental movie 10). Beyond the areas associated with the ischemic core, and in control mice without ischemic brain lesions, we could not induce PMN recruitment by laser-induced endothelial activation (supplemental Fig. 2 and supplemental movie 11). Thus, the ischemia-induced tissue damage and environmental changes provided a stimulus able to recruit PMN to activated local endothelia followed by tissue infiltration.
PMNs adhere, accumulate and infiltrate the brain parenchyma after initiation of a thrombus next to the ischemic core. Intravital two-photon microscopy was performed 24 h after pMCAO. a A laser-induced vascular lesion (flash) without leakage of intravascular dye (mild vessel irritation) was followed by endovascular accumulation of PMN at the lesion site (arrows) as well as microglia reaction by sending of thin processes to the outer site of the lesioned vessel (broken line). b A more severe vascular lesion was induced with subsequent thrombus formation (circular broken line, supplemental movie 6). The images show a vessel with initially rolling PMN (before thrombus formation; broken line arrow shows the direction of blood flow), thereafter one observes a shift from rolling to adhering PMN, accumulation of PMN at the thrombus site and subsequently a pronounced infiltration of PMN into the brain parenchyma (exit site of PMN marked by blue broken line). Real time of the experiments is indicated in the Figs. (supplemental movie 8). Scale bar: a, b 20 μm. Similar events were observed in 6 mice
PMNs are recruited rapidly to the endothelium after thrombus formation. Intravital two-photon microscopy was performed 24 h after pMCAO. a Vessel without rolling or adhering PMN before laser-induced lesioning. After the induction of a thrombotic lesion by laser irradiation after 5 min (flash) its development over time is shown (in higher magnification the thrombotic lesion is seen in b). The thrombus formation results in a recruitment of PMN at the thrombus but also on the surface of the endothelium in a non-related vessel (white arrows). c This image shows in higher magnification the accumulation of PMN at the lesioned site. d Images illustrate that recruitment of PMN to the thrombotic lesion is also achieved by crawling against the blood flow (direction indicated by broken line arrow). The depicted cell (white dotted circle) crawled 40 μm against the blood flow (white line) (supplemental movie 10). Scale bar: a, b, c 20 μm; d 10 μm. Similar events were observed in 6 mice
VLA-4 expression in PMN
The beta 1 integrin VLA-4 (very-late-antigen 4, a CD29/CD49d) is expressed on the surface of leukocytes in mice as well as in humans and is responsible for their tethering, initial rolling, and firm adhesion on blood vessels [38], which are crucial steps before extravasation. Flow cytometric analysis showed that murine PMN expressed moderate levels of VLA-4 (CD49d and CD29). This was true for circulating blood PMNs as well as for isolated bone marrow cells and could be confirmed by qPCR. Furthermore, the levels of VLA-4 on isolated cells were rapidly increased by proinflammatory stimuli (Fig. 5a). VLA-4 expression was also detected on infiltrated cells in the ischemic hemisphere in mice (data not shown). Human cells also showed VLA-4 expression on PMN (Supplemental Fig. 3). However, there was no alteration in the VLA-4 level on circulating PMNs after stroke, neither in mice nor in humans (data not shown).
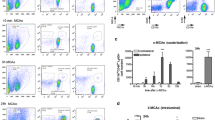
Anti-VLA-4 treatment reduces adherence of PMN and inhibits PMN infiltration 24 h after focal ischemia. a Left Expression of CD29 and CD49d (black bar) compared to isotype control (gray bar) in Blood PMNs (gated on neutrophils via FSC/SSC) or isolated BM-PMNs (gated on single cells). Right: Fold induction of mRNA level for CD29 and CD49d in isolated BM-PMNs after stimulation with either PMA (white), LPS (light gray) or fMLP (dark gray) for 30 min at 37 °C. VLA-4 protein and mRNA are expressed on/in PMN and can be quickly upregulated on the mRNA level by the inflammatory stimuli LPS and PMA, while fMLP triggering leads to a small reduction. b PMNs in blood vessels of mice 24 h after induction of pMCAO were observed by intravital two-photon microscopy. Quantification of PMN numbers per vessel was subdivided into adhesive (white bar) or slow rolling (gray bar) cells 30 min pre- and 30 min post anti-VLA-4 treatment (*p < 0.05 pre- vs. post-treatment, n = 3 animals/condition). The intravenous application of anti-VLA-4 antibody resulted in a significant shift from adhesion to slow rolling. c Percent adhesive PMN per vessel as function of time. The arrow indicates the time point of intravenous injection of the anti-VLA-4 antibody and illustrates the decreased number of adherent eGFPbright cells after the treatment. Representative intravital two-photon microscopy images are shown in d (supplemental movie 12). e LysM-eGFP mice were treated intravenously with 150 μg of anti-VLA-4 antibody immediately after pMCAO and 150 μg 6 h later. The infiltration of PMN was assessed 24 h later by FACS analysis (**p < 0.01 isotype vs. anti-VLA-4, n = 3 animals/bar). Anti-VLA-4 treatment resulted in a significant reduction of brain infiltrated PMN. Scale bar: d 50 μm. Data are mean + SEM of 3 independent experiments
Anti-VLA-4 treatment abrogates vascular adhesion of PMN
We next wanted to test, whether VLA-4-blockade would interfere with PMN infiltration into ischemic brain tissue. Therefore, we injected anti-CD49d intravenously 30 min after onset of two-photon microscopy of Lys-eGFP mice, which had been subjected to pMCAO 24 h earlier. This setup allowed the direct investigation of the effect of VLA-4 blockade on individual PMN before and after antibody delivery. VLA-4 blockade led to a rapid and significant reduction of PMN numbers per vessel. Furthermore, most remaining cells shifted their endothelial interaction from adhesion to slow rolling (Fig. 5b). This occurred within 10 min after the injection of anti-VLA-4 (Fig. 5c/d and supplemental movie 12). Thus, VLA-4-blockade immediately and effectively blocks PMN interaction with endothelia activated by ischemic events in affected brain areas.
Anti-VLA-4 treatment reduces the cerebral infiltration of PMN
Since VLA-4 strongly interfered with individual PMN function in vivo, we investigated whether anti-VLA-4 was able to inhibit PMN infiltration into ischemic brain systemically. We thus applied anti-CD-49d twice, immediately after pMCAO onset and again 6 h later in Lys-eGFP mice. 24 h later, the brain was isolated and examined by FACS for the number of eGFP-expressing Gr-1high cells. We found significantly fewer infiltrated PMN in the anti-VLA-4 treated group compared to the isotype control. Interestingly, in control animals there was an almost selective PMN recruitment to the ipsilateral, but not the contralateral hemisphere (Fig. 5e), further confirming that PMNs were able to sense the neuronal inflammation. Thus, VLA-4-blockade interfered with PMN infiltration of inflamed brain.
Anti-VLA-4 reduces infarct size and results in better neuronal outcome
We next asked whether VLA-4-blockade had consequences on the functional outcome of stroke. Therefore, we induced the transient occlusion of the proximal MCA (tMCAO), because in our hands, this model causes bigger infarct sizes than the pMCAO (40 mm3 compared to 20 mm3 in pMCAO) and also more distinctive neurological deficits, with the consequence that the neuroprotective effects could be more credibly assessed. Furthermore, reperfusion after transient occlusion causes the circulating PMN to be exposed to a large inflamed vascular bed, providing a bigger challenge for any therapy aiming at the inhibition of PMN entry [55].
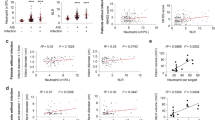
After tMCAO anti-VLA-4 or control antibodies were applied intravenously at the beginning of reperfusion and again 24 h later. All mice performed behavioral tests before determination of the infarct volumes. Importantly, all these tests showed a significantly better neurological outcome in the anti-VLA-4 group compared to control (Fig. 6a–c). Furthermore, we also found a significantly reduced infarct volume in the anti-VLA-4 group (Fig. 6d). In addition, we observed significantly fewer infiltrated PMN in the injured brain of mice treated with anti-VLA-4 compared to controls (Fig. 6e). Thus, VLA-4-blockade resulted in strongly reduced infarct size as well as significantly fewer neurological deficits 3 days after tMCAO. These results are in line with published data [39].
Anti-VLA-4 treatment improves neurological outcome and reduces infarct volume. a Rota rod, b tight rope and c corner turn test of mice receiving isotype IgG or anti-VLA-4 treatment. The different behavioral tests illustrate the significantly better neurological outcome at day 3 after experimental stroke of mice treated with VLA-4 antibody. d Quantification of infarct volumes by cresyl violet staining demonstrates the significantly reduced infarct size in the anti-VLA-4 treated group. e Representative images of a brain section stained for Ly6G (green) and nuclei (blue). The number of Ly6G+ cells in two brain areas (4 ROI/region) of mice after isotype or anti-VLA-4 treatment is quantified. Scale bar: 50 µm. Data in a, b and c are mean + SD and data in d, e are mean + SEM (8–11 animals per group, e 4 animals per group, 4 ROI per brain area in each animal). *p < 0.05 compared with isotype IgG treatment
Anti-VLA-4-mediated improvement of stroke defects functions mainly by blocking the entry of PMN, not T-cells
Since T-cells are also well known to be inhibited from brain entry via VLA-4- blockade [39, 54] we had to clarify which cell type was primarily responsible for the observed behavioral improvement following VLA-4-blockade (Fig. 6). Hence, we performed a second set of studies, either depleting myeloid cells or T-cells by the use of anti-Gr-1 or anti-CD3, respectively. Both antibodies are known to deplete the relative cell types after systemic injection [35, 60]. To test for synergistic effects of the depleting antibodies with anti-VLA-4 on stroke outcome, the injection of the depleting antibodies was performed either alone or in combination with anti-VLA-4 (Fig. 7a). The depletion efficiency of cells was followed by blood analysis and found to be routinely >60 % for CD3 and almost 100 % for anti-Gr-1. All mice performed behavioral tests 3 days after tMCAO before their brains were investigated for infarct volumes.
VLA-4 inhibition synergistically reduces infarct volume in addition to T-cell but not myeloid cell depletion. a Experimental protocol of cell depletion and anti-VLA-4 treatment. Specifications of antibodies and concentrations used are given in the “Materials and methods” section. b Rota rod, c tight rope and d corner turn test of mice receiving isotype IgG or mice depleted for PMN (anti-Gr-1) or T-cells (anti-CD3) that additionally received isotype IgG or anti-VLA-4 antibody treatment. Behavioral tests were performed 72 h post 45 min MCAO. e Infarct volumes of the same mice as shown in b–d determined by cresyl violet staining 72 h post 45 min MCAO. Data are mean + SD [5–10 (b–d)/10–12 (e) animals per group]. *p < 0.05
We found the same extent of neurological improvement in the anti-Gr-1-treated group as with anti-VLA-4 alone. The combined treatment of anti-VLA-4 and anti-Gr-1 had no further effect (Fig. 7b–d). In contrast anti-CD3 treatment alone failed to induce neurological improvement compared to the control, whereas the combination of anti-CD3 with anti-VLA-4 restored the neurological performance to the same level as seen with anti-Gr-1 (Fig. 7b–d). These data indicated that anti-VLA-4 promoted its behavioral effects primarily via blocking the entry of myeloid cells rather than T-cells to the infarcted brain.
However, the analysis of the infarct volume showed a different picture. In accordance with published literature [32], we indeed found effective infarct volume reduction by anti-CD3 treatment, which was even significantly more profound than the reduction with anti-Gr-1 (Fig. 7e). Furthermore, the infarct volume was significantly more reduced by anti-CD3 and anti-VLA-4 co-injection, while anti-VLA-4 showed no synergistic effects with anti-Gr-1 (Fig. 7e). Thus, T-cells and myeloid cells together promote the development of the stroke-related infarct volume, while only immigrating myeloid cells mediate the behavioral dysfunction associated with the ischemic condition. To exclude the possibility of effects by brain swelling, we calculated volume ratios of ipsi- and contralateral hemispheres. This demonstrated that exclusive CD3-depletion reduced edema formation, whereas other cell types and blockade of VLA-4 did not induce any changes (Supplemental Fig. 7). Therefore, discrepancies between behavioral outcome and infarct volume for depletion of T-cells cannot simply be explained by edema formation in these mice.
The injection of anti-Gr-1 does not selectively deplete PMN, but can also remove monocyte/macrophage types of cells. In contrast, depletion of Ly6G-positive cells via the antibody 1A8 is considered neutrophil specific [68]. We thus finally tested the importance of PMN for the observed effects on stroke by depleting with 1A8 rather than anti-Gr-1. Intravital microscopy of Lys-eGFP mice injected with depleting doses of 1A8 showed an almost complete lack of eGFP+ cells rolling on inflamed endothelium in the brain or invading the ischemic tissue (Fig. 8a–c and supplemental movies 13 and 14). Furthermore, 1A8-depletion before stroke-onset was as efficient in ameliorating the outcome of behavioral tests as the depletion with anti-Gr-1 (Fig. 8d–g). This finding further supporting the notion that from all myeloid cells, especially PMN, are important for the induction of morbidity associated with stroke.
PMNs are the main myeloid cell type responsible for behavioral impairment after experimental stroke. a Lys-eGFP mice were injected with isotype antibody or depleting doses of the Ly6G-specific antibody 1A8 (3 mice per group). Subsequently, mice were subjected to pMCAO and analyzed by intracranial 2-photon microscopy. Presence of high numbers of green cells on the endothelial surface or the brain parenchyma after isotype treatment and almost complete lack of green cells after 1A8 depletion. b Quantification of adherent cells and c infiltrated cells in these mice from intravital movies (representative analysis from 3 independent experiments). d Experimental protocol of cell depletion and anti-VLA-4 treatment. Specifications of antibodies and concentrations used are given in the “Materials and methods” section. e Rota rod, and f tight rope test of treated mice and G analysis of infarct volume after PMN or control depletion after 45-min tMCAO. Data are mean + SD (9–11 animals per group). *p < 0.05. Scale bar is 20 µm
Discussion
The infiltration process of PMN has only recently become amenable for direct observation by intravital microscopy. Since then PMN invasion into Leishmania infected skin [52] or necrotic liver tissue has been documented [43]. In brain, the invasion of PMN in virus-infected meninges was shown [31], but until now the inflammatory responses associated with stroke remained unexplored. We have shown the rapid response of circulating PMN to endothelial activation associated with the ischemic insult. PMN rolling followed by firm adhesion and finally transmigration into the brain parenchyma was only observed close to the ischemic penumbra, and not in regions outside. The rolling speed we observed was very low so that even with frame rates of 1 per 5 s, we could observe continuous movements of neutrophils. This is reminiscent of the lowest rolling speeds observed before in ~20 % of all leukocytes rolling on TNFα-stimulated cremaster muscle [49]. Thus, the endothelium in this zone seems to be alerted by the primary insult and the little additional irritation induced by laser irradiation tips the balance towards an immediate PMN response.
The fact that even more distant blood vessels are still sensitive to additional triggering was striking. This might explain why the zone of PMN infiltration is typically larger than the actual infarcted region [39]. This is the first time that PMN extravasation was observed directly in association with stroke, and is also well in line with other histological studies that demonstrated large neutrophilic infiltrates within the brain tissue [21, 37, 39, 58]. Interestingly, these findings are distinct from a recent study that unexpectedly found stroke-related PMN infiltrates strictly associated with perivascular basement membranes of the neurovascular unit, but never in the main parenchyma [18]. In the light of our direct imaging data, these findings are difficult to reconcile and probably rely on the slightly different time points analyzed for PMN infiltration in the said study.
Microglia showed a clear tendency to obtain an activated ameboid shape in the vicinity of the ischemic core that gradually regressed to the typical ramified morphology at a distance. Interestingly, the state of endothelial sensitivity to laser radiation correlated with this morphological pattern of microglia. At sites of more activated microglial shape endothelia were still activatable, while at sites with resting microglial shape the endothelia were resistant to laser activation. Thus, while the definition of the penumbra currently only looks at the physiological state of neurons and blood hypoperfusion in the area, our data suggest that it should also contain an immunological definition defined by the zone with activated microglial morphology and PMN infiltration.
Traditionally, concepts on the role of perivascular cells in damage to the abluminal side of the blood–brain barrier focused on pericytes and astrocytes [24]. Our data also suggest a prominent role of microglia. They obviously sense the endothelial activation state induced by the ischemia and immediately react by sending cytoplasmic processes. In contrast to previous studies [9, 48], this even happened in the absence of endothelial leakage. It is currently unclear whether the endothelial activation alone or the binding of PMN to the luminal side was inducing a signal that could be detected on the abluminal side. Also the nature of this signal has not been investigated. A likely candidate would be ATP released by the stressed tissues that could be sensed by microglia via P2Y receptors [9]. Maybe the same signal from the abluminal side could also induce the adherence and transmigration of PMN, which were shown to respond to ATP as well [43].
Furthermore, we provide the first in vivo proof of the physical interaction between microglia and invading PMN. PMN contact induced morphological alterations of microglia which were then able to trap PMN. Although PMN uptake by microglia could not be unequivocally proven here, the interactions were still highly reminiscent of the microglia-PMN contacts we had observed earlier in situ [47]. While it is well known that macrophages can ingest dead or dying cells [20], our study for the first time hints to the ability of microglia to phagocytose live immune cells also in vivo. The ultimate proof of successful phagocytosis would require an animal model where the two cellular partners are differentially stained without being “touched”, e.g., by antibodies. We showed that microglia can be directly protective for neurons under ischemic conditions [46]. Microglial phagocytosis of invading PMN might be another mechanism to exert such a protection which culminates in the immune privileged condition of the CNS. It remains to be shown, whether other invading immune cells such as T-cells can be targeted by microglia, what the molecular mechanisms behind this process are, and whether this interaction is directly neuroprotective in vivo.
The ability of PMN to sense thrombotic clogging, even if it was happening in a blood vessel not initially used by the circulating cell, was striking. Obviously, a thrombotic plug leads to an area of activated endothelium that can also affect unrelated blood vessels. The ability of PMN to migrate towards a thrombotic plug, even against the counter flow within an unrelated blood vessel, probably explains why PMNs reach the brain in numbers 10 times higher than any lymphocyte [39], although they are 3–6 times less frequent in mouse blood [64]. The unique ability of PMN to migrate against intense levels of shear stress has recently been shown to depend on sticky membrane slings, which PMN use to prepare their own path in front of them [61]. Unfortunately, the resolution in our intravital movies could not resolve sling-like structures.
Finally, we can confirm an important role of VLA-4 for PMN entry into the brain. We show that anti-VLA-4 almost immediately depleted slow rolling and adhering PMN from the endothelial surface. Formally, by using anti VLA-4 we only blocked CD49d, which is the alpha4 integrin chain of VLA-4. Since the α4 integrin chain can also pair with the β7-chain to form LPAM-1, our inhibitory effects could principally also be mediated by blockade of this receptor. However, the likelihood for this is rather low since the expression of β7 on neutrophils is minimal [51] and the main target organs for LPAM-1-mediated recruitment are mucosal tissues of the gastrointestinal tract, but not the CNS [22]. Thus, the inhibition of neutrophil entry into the inflamed brain by antibodies against CD49d in our model very likely acts by inhibition of VLA-4.
This dramatically reduced the immigration of PMN and other CD45+ cells. In addition, the size of brain lesions was significantly reduced, and behavioral tests were maintained at almost normal levels. This observation appears to differ from a recent study that could not find a protective effect of anti-VLA-4 treatment in experimental stroke [37]. However, in that study only 30-min ischemia led to a much smaller infarct than our experiments, which could perhaps not be reduced further. In addition, in contrast to our model, the stroke did not induce larger cortical lesions which are associated with neurological deficits. Thus, the contribution of inflammation to secondary brain injury may have been more limited than in our study. Also the application scheme of blocking antibodies was very different from ours. These aspects could explain the observed differences in outcome.
We show that the anti-VLA-4-mediated neuroprotection is mainly driven by inhibition of PMN infiltration into the ischemic brain. Monocytes/macrophages seem to be of less importance, at least in the early phase of the insult. In addition, our data suggest that T-cell contribution for behavioral dysfunction at an early time point after stroke is minimal, while their effect on infarct volume is large, indicating that lesion size alone is likely insufficient as evidence for neuroprotection. Indeed, numerous rodent studies failed to find a close correlation between histological and behavioral outcomes [13, 63, 65]. In Particular, studies assessing the role of different immune cell subsets frequently reveal that simple correlation cannot be taken as given. E.g., Rag1−/− mice reconstituted with B cells demonstrate significantly reduced infarct volumes by 60 %, whereas functional improvement in the grip test was only slightly, but not significantly improved [33]. Similarly, CD8 T-cell deficient mice demonstrate a significant reduction of infarct volumes by 70 % which does not correlate with significantly reduced neurological deficit scores [69]. Among many factors which could account for the lack of correlation, the distribution of cell death within defined regions may be a more important determinant of behavioral function than the size of infarction [12]. Infarct size is unlikely to be able to detect deficits in the rota rod and corner turn tests when applying distal MCAO [4] which mainly affects cortical areas, where the striatum is spared [63]. This indicates that specific brain structures like the caudate/putamen, which is still injured in T-cell-depleted mice, might be decisive for performance in the motor-coordination tests applied in this study. In addition, histological limitations might explain our results. As such, infarct volume measurement via cresyl violet allows for the delineation of the boundaries of necrotic tissues. However, diffuse morphological changes within non-MCA territory cannot be identified [1], thus, it cannot be excluded that some damaged areas of the brain might be nonfunctional without being necrotic. Hence, neurobehavioral deficits that we describe after peripheral T-cell depletion might be related to a transient dysfunction of the neurons without any alteration detectable with cresyl violet staining.
Our data suggest that the initial brain lesion, as such, does not massively influence the behavioral performance of the animals if the penumbra is kept free of infiltrating PMN. Therefore, the inflammatory situation is certainly essential for evoking the neurotoxic potential of the invaders. Resting PMN did not have any neurotoxic activity in the absence of ischemic pre-lesioning in brain slices, in contrast to the neuronal destruction they induced in its presence [47], and in electrophysiological studies only LPS-activated PMN significantly reduced long-term potentiation (data not shown). Early activation of PMN in ischemic brain tissue might be caused by the rapid release of danger-associated molecular patterns (DAMP), which by engagement of their receptors on immune cells can activate the inflammasome through the NLRP3 pathway, leading to secretion of IL-1b [30, 42, 59]. The regulation of IL-1b processing and secretion has been studied extensively in monocytes/macrophages, however, in the recent past several reports have also unraveled the importance of NLRP3 in neutrophil function and associated inflammation in different experimental models such as intracerebral hemorrhage [3, 27, 41].
We are aware that our observation concerning behavioral outcome after anti-CD3 treatment appears to be in contrast to previous studies using T-cell deficient mouse models [33, 39], which might be explained by the fact that an antibody treatment is never as complete as the genetic deficiency of a cell type. However, the fact that peripheral ablation of T-cells strongly reduces infarct volumes without improving functional outcomes suggests that peripheral T-cells might be acting at the brain-vasculature interface, possibly by binding to platelets and endothelial cells causing microvascular dysfunction [70], rather than via a direct impairment of neuronal function. Indeed, reduced adherent platelets were observed in the ischemic brain of CD4 and CD8 T-cell deficient, but not in neutropenic mice [69]. A more detailed analysis was performed by Kleinschnitz et al. [32] demonstrating that specifically regulatory T-cells induce microvascular dysfunction by increased interaction with the ischemic brain endothelium via LFA-1/ICAM-1 interactions. Considering the different proposed pathogenic mechanisms of T-cells and PMN, combined with the divergent behavioral outcomes, we hypothesize that the ablation of peripheral T-cells, which could result in the protection of the cerebral microvasculature, combined with inhibition of early infiltration of PMN and its direct interactions in the brain parenchyma, are essential for functional improvement and protection of brain tissue injury after stroke. A recent study supports this concept of a lethal collaboration between peripheral T-cells and brain-invasive PMN [21]. The authors reported that T-cell-derived inflammatory cytokines induced the production of PMN-recruiting chemokines by astrocytes. Together with our study, this work demonstrates a close relationship between PMN and T-cells and supports our conclusion that both cell types are crucial in the development of postischemic brain injury.
Thus, providing patients suffering from acute stroke with T-cell depleting and VLA-4 blocking reagents might be a concept worth testing clinically. Antibodies with both functions are available for human use and are efficient as therapy of diabetes mellitus [25] or multiple sclerosis [56]. Furthermore, as opposed to PMN depletion the transient blockade of their function with anti-VLA-4 has no negative consequences on the immune defence [39], and also transient T-cell depletion does not have major side effects [25]. In contrast to the thrombolysis therapy, where time consuming prior diagnostics are mandatory, the application of VLA-4 blockade or anti-CD3 could be given already within the emergency ambulance because it would not influence cerebral hemorrhage. Considering that the restoration of blood flow by intravenous thrombolysis or intra-arterial interventions remains the method of choice in stroke therapy, the additional blockade of PMN entry and reduction of ischemic brain injury by T-cell depletion could be a promising add-on approach. This might save precious time in the struggle to rescue neurons that have survived the primary ischemic attack.
References
Aronowski J, Samways E, Strong R, Rhoades HM, Grotta JC (1996) An alternative method for the quantitation of neuronal damage after experimental middle cerebral artery occlusion in rats: analysis of behavioral deficit. J Cereb Blood Flow Metab 16:705–713
Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F (2007) Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317:666–670
Bakele M, Joos M, Burdi S, Allgaier N, Poschel S, Fehrenbacher B, Schaller M, Marcos V, Kummerle-Deschner J, Rieber N, Borregaard N, Yazdi A, Hector A, Hartl D (2014) Localization and functionality of the inflammasome in neutrophils. J Biol Chem 289:5320–5329
Balkaya M, Krober JM, Rex A, Endres M (2013) Assessing post-stroke behavior in mouse models of focal ischemia. J Cereb Blood Flow Metab 33:330–338
Barone FC, Hillegass LM, Price WJ, White RF, Lee EV, Feuerstein GZ, Sarau HM, Clark RK, Griswold DE (1991) Polymorphonuclear leukocyte infiltration into cerebral focal ischemic tissue: myeloperoxidase activity assay and histologic verification. J Neurosci Res 29:336–345
Beray-Berthat V, Croci N, Plotkine M, Margaill I (2003) Polymorphonuclear neutrophils contribute to infarction and oxidative stress in the cortex but not in the striatum after ischemia-reperfusion in rats. Brain Res 987:32–38
Choi JJ, Wang S, Tung YS, Morrison B III, Konofagou EE (2010) Molecules of various pharmacologically-relevant sizes can cross the ultrasound-induced blood-brain barrier opening in vivo. Ultrasound Med Biol 36:58–67
Connolly ES Jr, Winfree CJ, Springer TA, Naka Y, Liao H, Yan SD, Stern DM, Solomon RA, Gutierrez-Ramos JC, Pinsky DJ (1996) Cerebral protection in homozygous null ICAM-1 mice after middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest 97:209–216
Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8:752–758
del Zoppo GJ (2010) Acute anti-inflammatory approaches to ischemic stroke. Ann N Y Acad Sci 1207:143–148
del Zoppo GJ, Becker KJ, Hallenbeck JM (2001) Inflammation after stroke: is it harmful? Arch Neurol 58:669–672
DeRyck M, Van Reempts J, Duytschaever H, Van Deuren B, Clincke G (1992) Neocortical localization of tactile/proprioceptive limb placing reactions in the rat. Brain Res 573:44–60
DeVries AC, Nelson RJ, Traystman RJ, Hurn PD (2001) Cognitive and behavioral assessment in experimental stroke research: will it prove useful? Neurosci Biobehav Rev 25:325–342
Dinkel K, Dhabhar FS, Sapolsky RM (2004) Neurotoxic effects of polymorphonuclear granulocytes on hippocampal primary cultures. Proc Natl Acad Sci 101:331–336
Dirnagl U (2004) Inflammation in stroke: the good, the bad, and the unknown. Ernst Schering Res Found Workshop. pp 87–99
Dirnagl U, Iadecola C, Moskowitz MA (1999) Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22:391–397
Doeppner TR, Bretschneider E, Doehring M, Segura I, Senturk A, Acker-Palmer A, Hasan MR, Elali A, Hermann DM, Bahr M (2011) Enhancement of endogenous neurogenesis in ephrin-B3 deficient mice after transient focal cerebral ischemia. Acta Neuropathol 122:429–442
Enzmann G, Mysiorek C, Gorina R, Cheng YJ, Ghavampour S, Hannocks MJ, Prinz V, Dirnagl U, Endres M, Prinz M, Beschorner R, Harter PN, Mittelbronn M, Engelhardt B, Sorokin L (2013) The neurovascular unit as a selective barrier to polymorphonuclear granulocyte (PMN) infiltration into the brain after ischemic injury. Acta Neuropathol 125:395–412
Faust N, Varas F, Kelly LM, Heck S, Graf T (2000) Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood 96:719–726
Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K (2010) Development of monocytes, macrophages, and dendritic cells. Science 327:656–661
Gelderblom M, Weymar A, Bernreuther C, Velden J, Arunachalam P, Steinbach K, Orthey E, Arumugam TV, Leypoldt F, Simova O, Thom V, Friese MA, Prinz I, Holscher C, Glatzel M, Korn T, Gerloff C, Tolosa E, Magnus T (2012) Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood 120:3793–3802
Gorfu G, Rivera-Nieves J, Ley K (2009) Role of beta7 integrins in intestinal lymphocyte homing and retention. Curr Mol Med 9:836–850
Heinel LA, Rubin S, Rosenwasser RH, Vasthare US, Tuma RF (1994) Leukocyte involvement in cerebral infarct generation after ischemia and reperfusion. Brain Res Bull 34:137–141
Hermann DM, Elali A (2012) The abluminal endothelial membrane in neurovascular remodeling in health and disease. Sci Signal 5:re4
Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA (2002) Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 346:1692–1698
Herz J, Hagen SI, Bergmuller E, Sabellek P, Gothert JR, Buer J, Hansen W, Hermann DM, Doeppner TR (2014) Exacerbation of ischemic brain injury in hypercholesterolemic mice is associated with pronounced changes in peripheral and cerebral immune responses. Neurobiol Dis 62:456–468
Inoue Y, Shirasuna K, Kimura H, Usui F, Kawashima A, Karasawa T, Tago K, Dezaki K, Nishimura S, Sagara J, Noda T, Iwakura Y, Tsutsui H, Taniguchi S, Yanagisawa K, Yada T, Yasuda Y, Takahashi M (2014) NLRP3 regulates neutrophil functions and contributes to hepatic ischemia-reperfusion injury independently of inflammasomes. J Immunol 192:4342–4351
Jordan JE, Zhao ZQ, Vinten-Johansen J (1999) The role of neutrophils in myocardial ischemia-reperfusion injury. Cardiovasc Res 43:860–878
Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR (2000) Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 20:4106–4114
Junger WG (2011) Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol 11:201–212
Kim JV, Kang SS, Dustin ML, McGavern DB (2009) Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature 457:191–195
Kleinschnitz C, Kraft P, Dreykluft A, Hagedorn I, Gobel K, Schuhmann MK, Langhauser F, Helluy X, Schwarz T, Bittner S, Mayer CT, Brede M, Varallyay C, Pham M, Bendszus M, Jakob P, Magnus T, Meuth SG, Iwakura Y, Zernecke A, Sparwasser T, Nieswandt B, Stoll G, Wiendl H (2013) Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood 121:679–691
Kleinschnitz C, Schwab N, Kraft P, Hagedorn I, Dreykluft A, Schwarz T, Austinat M, Nieswandt B, Wiendl H, Stoll G (2010) Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood 115:3835–3842
Köhler A, De Filippo K, Hasenberg M, van den Brandt C, Nye E, Hosking MP, Lane TE, Männ L, Ransohoff RM, Hauser AE, Winter O, Schraven B, Geiger H, Hogg N, Gunzer M (2011) G-CSF mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood 117:4349–4357
Kummer U, Zengerle U, Pischel J, Trautmann B, Mailhammer R, Sidell N (2001) Increased in vivo mitogenicity of anti-TCR/CD3 monoclonal antibody through reduced interaction with Fcgamma receptors. Immunol Lett 75:153–158
Lämmermann T, Afonso PV, Angermann BR, Wang JM, Kastenmüller W, Parent CA, Germain RN (2013) Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498:371–375
Langhauser F, Kraft P, Gob E, Leinweber J, Schuhmann MK, Lorenz K, Gelderblom M, Bittner S, Meuth SG, Wiendl H, Magnus T, Kleinschnitz C (2014) Blocking of alpha4 integrin does not protect from acute ischemic stroke in mice. Stroke 45:1799–1806
Ley K, Laudanna C, Cybulsky MI, Nourshargh S (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7:678–689
Liesz A, Zhou W, Mracsko E, Karcher S, Bauer H, Schwarting S, Sun L, Bruder D, Stegemann S, Cerwenka A, Sommer C, Dalpke AH, Veltkamp R (2011) Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain 134:704–720
Lin TN, He YY, Wu G, Khan M, Hsu CY (1993) Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke 24:117–121
Ma Q, Chen S, Hu Q, Feng H, Zhang JH, Tang J (2014) NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann Neurol 75:209–219
Magnus T, Wiendl H, Kleinschnitz C (2012) Immune mechanisms of stroke. Curr Opin Neurol 25:334–340
McDonald B, Pittman K, Menezes GB, Hirota AA, Slaba I, Waterhouse CCM, Beck PL, Muruve DA, Kubes P (2010) Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330:362–366
Miljkovic-Lolic M, Silbergleit R, Fiskum G, Rosenthal RE (2003) Neuroprotective effects of hyperbaric oxygen treatment in experimental focal cerebral ischemia are associated with reduced brain leukocyte myeloperoxidase activity. Brain Res 971:90–94
Murikinati S, Juttler E, Keinert T, Ridder DA, Muhammad S, Waibler Z, Ledent C, Zimmer A, Kalinke U, Schwaninger M (2010) Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. FASEB J 24:788–798
Neumann J, Gunzer M, Gutzeit HO, Ullrich O, Reymann KG, Dinkel K (2006) Microglia provide neuroprotection after Ischemia. FASEB J 20:714–716
Neumann J, Sauerzweig S, Rönicke R, Gunzer F, Dinkel K, Ullrich O, Gunzer M, Reymann KG (2008) Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes—a new mechanism of CNS immune privilege. J Neurosci 28:5965–5975
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308:1314–1318
Norman KE, Anderson GP, Kolb HC, Ley K, Ernst B (1998) Sialyl Lewis(x) (sLe(x)) and an sLe(x) mimetic, CGP69669A, disrupt E-selectin-dependent leukocyte rolling in vivo. Blood 91:475–483
O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW (2006) 1,026 experimental treatments in acute stroke. Ann Neurol 59:467–477
Pereira S, Zhou M, Mocsai A, Lowell C (2001) Resting murine neutrophils express functional alpha 4 integrins that signal through Src family kinases. J Immunol 166:4115–4123
Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, Lawyer P, Fay MP, Germain RN, Sacks D (2008) In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 321:970–974
Petrault O, Ouk T, Gautier S, Laprais M, Gele P, Bastide M, Bordet R (2005) Pharmacological neutropenia prevents endothelial dysfunction but not smooth muscle functions impairment induced by middle cerebral artery occlusion. Br J Pharmacol 144:1051–1058
Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354:899–910
Prestigiacomo CJ, Kim SC, Connolly ES Jr, Liao H, Yan SF, Pinsky DJ (1999) CD18-mediated neutrophil recruitment contributes to the pathogenesis of reperfused but not nonreperfused stroke. Stroke 30:1110–1117
Pucci E, Giuliani G, Solari A, Simi S, Minozzi S, Di PC, Galea I (2011) Natalizumab for relapsing remitting multiple sclerosis. Cochrane Database Syst Rev:CD007621
Reitmeir R, Kilic E, Kilic U, Bacigaluppi M, Elali A, Salani G, Pluchino S, Gassmann M, Hermann DM (2011) Post-acute delivery of erythropoietin induces stroke recovery by promoting perilesional tissue remodelling and contralesional pyramidal tract plasticity. Brain 134:84–99
Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J (2008) MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke 39:1121–1126
Rubartelli A (2014) DAMP-mediated activation of NLRP3-inflammasome in brain sterile inflammation: the fine line between healing and neurodegeneration. Front Immunol 5:99
Stegemann S, Dahlberg S, Kröger A, Gereke M, Bruder D, Henriques-Normark B, Gunzer M (2009) Increased susceptibility for superinfection with Streptococcus pneumoniae during influenza virus infection is not caused by TLR7-mediated lymphopenia. PLoS One 4:e4840
Sundd P, Gutierrez E, Koltsova EK, Kuwano Y, Fukuda S, Pospieszalska MK, Groisman A, Ley K (2012) ‘Slings’ enable neutrophil rolling at high shear. Nature 488:399–403
Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ (2009) Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325:612–616
van der Staay FJ, Augstein KH, Horvath E (1996) Sensorimotor impairments in rats with cerebral infarction, induced by unilateral occlusion of the left middle cerebral artery: strain differences and effects of the occlusion site. Brain Res 735:271–284
von Vietinghoff S, Ley K (2008) Homeostatic regulation of blood neutrophil counts. J Immunol 181:5183–5188
Wahl F, Allix M, Plotkine M, Boulu RG (1992) Neurological and behavioral outcomes of focal cerebral ischemia in rats. Stroke 23:267–272
Wang Q, Tang XN, Yenari MA (2007) The inflammatory response in stroke. J Neuroimmunol 184:53–68
Wang X, Feuerstein GZ (2004) The Janus face of inflammation in ischemic brain injury. Acta Neurochir Suppl 89:49–54
Wojtasiak M, Pickett DL, Tate MD, Londrigan SL, Bedoui S, Brooks AG, Reading PC (2010) Depletion of Gr-1+ , but not Ly6G+ , immune cells exacerbates virus replication and disease in an intranasal model of herpes simplex virus type 1 infection. J Gen Virol 91:2158–2166
Yilmaz G, Arumugam TV, Stokes KY, Granger DN (2006) Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation 113:2105–2112
Yilmaz G, Granger DN (2008) Cell adhesion molecules and ischemic stroke. Neurol Res 30:783–793
Zhang L, Schallert T, Zhang ZG, Jiang Q, Arniego P, Li Q, Lu M, Chopp M (2002) A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods 117:207–214
Acknowledgments
We thank Susanne v. Kenne for excellent technical assistance. For help with flow cytometry, we thank Stefanie Holze and Jenny Schneeberg, and the Imaging Center Essen (IMCES) for help with imaging. This work was supported by the German Research foundation (DFG, SFB 854 to M.G. and K.R. as well as SPP1468 “Immunobone” to M.G. and HE3173/2-1 and HE3173/3-1 to D.M.H.), a DZNE intersite project on vascular dementia to K.R. and the Mercator Research Center Ruhr (An-2011-0081 to JH).
Author information
Authors and Affiliations
Corresponding author
Additional information
J. Neumann, M. Riek-Burchardt, J. Herz, D. M. Hermann, K. G. Reymann and M. Gunzer contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
401_2014_1355_MOESM1_ESM.tif
Supplementary material 1 (TIFF 2573 kb) Supplemental Fig. 1.Leakage of rhodamine dextran from the blood vessel after laser damage. The first two images (from left) show a small bleeding (arrows) after irritation of the bloodvessel by enhanced laser exposure. The last two images demonstrate a spontaneous bleeding (no enhanced, focussed laser beam at this vessel part) due to a much higher laser exposure than normally applied. (Supplemental movie 7) Scale bar: 20 µm
401_2014_1355_MOESM2_ESM.tif
Supplementary material 2 (TIFF 1281 kb) Supplemental Fig. 2 Lack of recruitment of PMN in mice without experimental ischemic injury. The first image represents the basal condition of blood flow in the brain of healthy Lys-eGFP mice before vessel irritation by enhanced laser exposure. The flash shows the localisation of the irritation. The second image shows no recruitment of eGFPbright cells towards the endothelium. To induce further damage, the same location was irritated by laser again. This led to a coagulation of the vessel, which is displayed by arrows in the third image. Overall a recruitment of eGFPbright cells was not observed during the whole procedure (Supplemental movie 11). Scale bar: 20 µm
401_2014_1355_MOESM3_ESM.tif
Supplementary material 3 (TIFF 974 kb) Supplemental Fig. 3 FACS analysis of the expression of VLA-4 on PMN in the blood of a human donor. The data are representative of 2 healthy volunteers as well as 2 patients (24 h after onset of stroke) without showing differences in the VLA-4 expression between both groups
401_2014_1355_MOESM4_ESM.tif
Supplementary material 4 (TIFF 584 kb) Supplemental Fig. 4 FACS analysis of the expression of GFP in PMN and macrophages of Lys-eGFP mice. Blood of Lys-eGFP mice was labeled with anti-Ly6G PE and anti-Gr-1 APC. Leukocytes were gated by forward/sideward scatter and are shown in the 1A8-PE and Gr-1-APC Plot. The 1A8bright/Gr-1high population (neutrophils, gate 1) also expresses eGFP at a very high level (histogram on the right). All Gr-1medium/1A8negative cells (monocytes/macrophages, gate 2) express ~1 log less eGFP (histogram on the right). Gr-1negative cells (other myeloid cells, lymphocytes) are also negative for eGFP (not shown)
401_2014_1355_MOESM5_ESM.tif
Supplementary material 5 (TIFF 1507 kb) Supplemental Fig. 5 Analysis of the migration speed of PMN in the brain of mice. PMN migration within the penumbra 24h after pMCAO was recorded by in vivo 2-photon microscopy in Lys-eGFP mice in a depth of 100µm below the meninges. Movies were analyzed by semi-automated single cell tracking using IMARIS. PMN display a mean speed of 10 ± 6.1 µm per min. Data are shown as mean values ± SD from 3 independent experiments
401_2014_1355_MOESM6_ESM.tif
Supplementary material 6 (TIFF 473 kb) Supplemental Fig. 6 Analysis of the distance of brain-resident PMN from blood vessels. a The distance between PMN and nearby vessels was measured using Z-stacks from in vivo recordings by 2-photon microscopy in Lys-eGFP mice 24h after pMCAO in 3 independent experiments. We found different PMN populations which are either next to the endothelium (0µm), less than 10µm away (mean 7.2± 3 µm) or more than 10µm away (mean 18.2 ± 4.6 µm) from the nearest blood vessel. b Representative images of a brain section stained for Ly6G (green), CD31 (red) and nuclei (blue) showing infiltrated and vessel-associated PMN in ischemic brain tissue 72h post 45 minutes tMCAO. c the distance between neutrophils and anti-CD31-stained vessels was measured in confocal images (6-10 ROI/animal) of stainings shown in b. A total of 251 cells out of 4 animals was analysed. d single values of distances for cells > 10 µm are shown and summarized as mean ± SD. Scale bar: 50 µm. ***p < 0.001
401_2014_1355_MOESM7_ESM.tif
Supplementary material 7 (TIFF 3502 kb) Supplemental Fig. 7 Analysis of edema formation after immune cell subset depletion combined with anti-VLA-4 treatment after 45 minutes tMCAO. The ratio of ipsilateral hemisphere volumes to contralateral volumes was calculated as a measure of edema formation for the indicated experimental groups. Treatment paradigms are given in Fig. 7a and 8d. Data are mean + SD (9-12 animals per group). *p < 0.05
Supplementary material 8 (AVI 195 kb) Supplemental Movie 1 Microglial morphology and motility in response to cerebral ischemia. The movie shows two-photon intravital microscopy of microglia (CX3CR-1-eGFP mice) distant (left side) and more closely positioned to the ischemic core (right side) 24h after pMCAO. Please note the profound membrane motility of the cells close to the ischemic core as opposed to cells more distant that are relatively quiet. Real time of the experiment is shown in minutes. The scale bar is 20 µm
Supplementary material 9 (MP4 181 kb) Supplemental Movie 1 Microglial morphology and motility in response to cerebral ischemia. The movie shows two-photon intravital microscopy of microglia (CX3CR-1-eGFP mice) distant (left side) and more closely positioned to the ischemic core (right side) 24h after pMCAO. Please note the profound membrane motility of the cells close to the ischemic core as opposed to cells more distant that are relatively quiet. Real time of the experiment is shown in minutes. The scale bar is 20 µm
401_2014_1355_MOESM10_ESM.avi
Supplementary material 10 (AVI 1730 kb) Supplemental Movie 2 Rolling, adhering and extravasation of PMN after cerebral ischemia. The movie shows two-photon intravital microscopy of a bloodvessel in the vicinity of the ischemic core (corresponding to Fig. 1a; Area 1) in Lys-eGFP mice. The tracks indicate extravasation of three PMN into the brain parenchyma. Real time of the experiment is shown in minutes. The scale bar is 20 µm
401_2014_1355_MOESM11_ESM.mp4
Supplementary material 11 (MP4 1695 kb) Supplemental Movie 2 Rolling, adhering and extravasation of PMN after cerebral ischemia. The movie shows two-photon intravital microscopy of a bloodvessel in the vicinity of the ischemic core (corresponding to Fig. 1a; Area 1) in Lys-eGFP mice. The tracks indicate extravasation of three PMN into the brain parenchyma. Real time of the experiment is shown in minutes. The scale bar is 20 µm
Supplementary material 12 (AVI 1282 kb) Supplemental Movie 3 The movie illustrates a slightly enhanced rolling of PMN visualized by two-photon intravital microscopy in Lys-eGFP mice in an area not in the immediate vicinity to the ischemic core (corresponding to Fig. 1a; Area 2). Real time of the experiment is shown inminutes. The scale bar is 50 μm
Supplementary material 13 (MP4 1251 kb) Supplemental Movie 3 The movie illustrates a slightly enhanced rolling of PMN visualized by two-photon intravital microscopy in Lys-eGFP mice in an area not in the immediate vicinity to the ischemic core (corresponding to Fig. 1a; Area 2). Real time of the experiment is shown inminutes. The scale bar is 50 μm
401_2014_1355_MOESM14_ESM.avi
Supplementary material 14 (AVI 524 kb) Supplemental Movie 4 Morphology of microglia and endothelial interaction of PMN beyond the infarcted area. The movie shows two-photon intravital microscopy in double transgenic mice (Lys-M-eGFP/ CX3CR-1-eGFP) (Fig. 1a; Area 3) normal microglial morphology and no enhanced interaction of PMN (PMN only detectable as green stripes due to their high velocity freely within blood vessels) with the endothelium. Real time of the experiment is shown in minutes. The scale bar is 20 µm
401_2014_1355_MOESM15_ESM.mp4
Supplementary material 15 (MP4 480 kb) Supplemental Movie 4 Morphology of microglia and endothelial interaction of PMN beyond the infarcted area. The movie shows two-photon intravital microscopy in double transgenic mice (Lys-M-eGFP/ CX3CR-1-eGFP) (Fig. 1a; Area 3) normal microglial morphology and no enhanced interaction of PMN (PMN only detectable as green stripes due to their high velocity freely within blood vessels) with the endothelium. Real time of the experiment is shown in minutes. The scale bar is 20 µm
401_2014_1355_MOESM16_ESM.avi
Supplementary material 16 (AVI 416 kb) Supplemental Movie 5 Absence of green cells interacting with the inflamed endothelium after stroke in CX3CR1-eGFP mice. Two- photon intravital microscopy was performed within CX3CR1-eGFP transgenic mice 24h after ischemia. In the movie the blue track shows the migration path of only one detected eGFP+ cell rolling on the inflamed endothelium of the infarcted brain. In contrast numerous other cells roll and adhere on the inflamed endothelium (black shadows within the vessel) which do not express eGFP and therefore do not represent CX3CR-1+ macrophages. Real time of the experiment is shown in minutes. The scale bar is 30 µm
401_2014_1355_MOESM17_ESM.mp4
Supplementary material 17 (MP4 405 kb) Supplemental Movie 5 Absence of green cells interacting with the inflamed endothelium after stroke in CX3CR1-eGFP mice. Two- photon intravital microscopy was performed within CX3CR1-eGFP transgenic mice 24h after ischemia. In the movie the blue track shows the migration path of only one detected eGFP+ cell rolling on the inflamed endothelium of the infarcted brain. In contrast numerous other cells roll and adhere on the inflamed endothelium (black shadows within the vessel) which do not express eGFP and therefore do not represent CX3CR-1+ macrophages. Real time of the experiment is shown in minutes. The scale bar is 30 µm
401_2014_1355_MOESM18_ESM.avi
Supplementary material 18 (AVI 948 kb) Supplemental Movie 6 Microglia cooperate to trap invading PMN. Two-photon intravital microscopy was performed within double transgenic mice (Lys-M-eGFP/ CX3CR-1-eGFP) 24h after ischemia. The movie illustrates the migration of PMN. After contact microglia send multiple membrane protrusions towards PMN and cooperate with other microglia to form net-like structures (white square). Please note, that many PMN can be seen entering a net, but very few appear to leave it again. The tracks show the migration path of PMN to the site of trapping. Real time of the experiment is shown in minutes. The scale bar is 10 µm
401_2014_1355_MOESM19_ESM.mp4
Supplementary material 19 (MP4 939 kb) Supplemental Movie 6 Microglia cooperate to trap invading PMN. Two-photon intravital microscopy was performed within double transgenic mice (Lys-M-eGFP/ CX3CR-1-eGFP) 24h after ischemia. The movie illustrates the migration of PMN. After contact microglia send multiple membrane protrusions towards PMN and cooperate with other microglia to form net-like structures (white square). Please note, that many PMN can be seen entering a net, but very few appear to leave it again. The tracks show the migration path of PMN to the site of trapping. Real time of the experiment is shown in minutes. The scale bar is 10 µm
401_2014_1355_MOESM20_ESM.avi
Supplementary material 20 (AVI 442 kb) Supplemental Movie 7 Blood vessel leakage after heavy laser damage. Two-photon intravital microscopy in CX3CR1-eGFP transgenic mice shows bleeding after an irritation of the blood vessel by enhanced laser exposure and thereafter a spontaneous intense bleeding due to a repetitive exposure to a much higher laser exposure than normally applied. After the vessel disruption the rhodamine-dextrane immediately leaks into the surrounding tissue. Real time of the experiment is shown in minutes. The scale bar is 20 µm
401_2014_1355_MOESM21_ESM.mp4
Supplementary material 21 (MP4 434 kb) Supplemental Movie 7 Blood vessel leakage after heavy laser damage. Two-photon intravital microscopy in CX3CR1-eGFP transgenic mice shows bleeding after an irritation of the blood vessel by enhanced laser exposure and thereafter a spontaneous intense bleeding due to a repetitive exposure to a much higher laser exposure than normally applied. After the vessel disruption the rhodamine-dextrane immediately leaks into the surrounding tissue. Real time of the experiment is shown in minutes. The scale bar is 20 µm
401_2014_1355_MOESM22_ESM.avi
Supplementary material 22 (AVI 2542 kb) Supplemental Movie 8 PMN and microglia sense endothelial activation after mild laser irradiation. Two-photon intravital microscopy was performed within double transgenic mice (Lys-M-eGFP/ CX3CR-1-eGFP) 24h after ischemia in area 2 (Fig. 1a). The movie shows the free flowing and slight endothelial interaction of PMN before and the immediate accumulation of PMN and the reaction of microglia (membrane protrusions) after short laser irradiation. Please note the specific localization of PMN only at the site of laser irradiation (arrow). Real time of the experiment is shown in minutes. The scale bar is 40 µm
401_2014_1355_MOESM23_ESM.mp4
Supplementary material 23 (MP4 2545 kb) Supplemental Movie 8 PMN and microglia sense endothelial activation after mild laser irradiation. Two-photon intravital microscopy was performed within double transgenic mice (Lys-M-eGFP/ CX3CR-1-eGFP) 24h after ischemia in area 2 (Fig. 1a). The movie shows the free flowing and slight endothelial interaction of PMN before and the immediate accumulation of PMN and the reaction of microglia (membrane protrusions) after short laser irradiation. Please note the specific localization of PMN only at the site of laser irradiation (arrow). Real time of the experiment is shown in minutes. The scale bar is 40 µm
Supplementary material 24 (AVI 11438 kb) Supplemental Movie 9 Adhering and extravasation of PMN after thrombus formation induced by severe laser irradiation. Two-photon intravital microscopy was performed within double transgenic mice (Lys-M-eGFP/ CX3CR-1-eGFP) 24h after ischemia (corresponding to area 1 in Fig. 1a). The movie shows rolling and transiently adhering PMN before laser irradiation and thereafter at the beginning the thrombus formation, followed by a massive accumulation of PMN, the physical breakdown of the blood brain barrier (around 53 min) and finally a tremendous extravasation of PMN. Real time of the experiment is shown in minutes. The scale bar is 30 µm
Supplementary material 25 (MP4 11418 kb) Supplemental Movie 9 Adhering and extravasation of PMN after thrombus formation induced by severe laser irradiation. Two-photon intravital microscopy was performed within double transgenic mice (Lys-M-eGFP/ CX3CR-1-eGFP) 24h after ischemia (corresponding to area 1 in Fig. 1a). The movie shows rolling and transiently adhering PMN before laser irradiation and thereafter at the beginning the thrombus formation, followed by a massive accumulation of PMN, the physical breakdown of the blood brain barrier (around 53 min) and finally a tremendous extravasation of PMN. Real time of the experiment is shown in minutes. The scale bar is 30 µm
401_2014_1355_MOESM26_ESM.avi
Supplementary material 26 (AVI 5786 kb) Supplemental Movie 10 Rapid recruitment of PMN to the endothelium after thrombus formation and crawling against the blood flow. Two-photon intravital microscopy was performed within double transgenic mice (Lys-M-eGFP/ CX3CR-1-eGFP) 24 h after ischemia (in a zone corresponding to area 2 in Fig. 1a). The movie illustrates the free flowing of PMN before and the inflammatory response after thrombus formation. Please note the rapid recruitment of PMN to a vessel segment which is located beneath the thrombus and PMN which crawl against the bloodstream to the lesioned site. The tracks show the migration path along the vessel wall to the thrombus. Please also note the profound morphological changes of most microglia visible within the brain parenchyma after onset of thrombus formation. Real time of the experiment is shown in minutes. The scale bar is 20 µm
401_2014_1355_MOESM27_ESM.mp4
Supplementary material 27 (MP4 5886 kb) Supplemental Movie 10 Rapid recruitment of PMN to the endothelium after thrombus formation and crawling against the blood flow. Two-photon intravital microscopy was performed within double transgenic mice (Lys-M-eGFP/ CX3CR-1-eGFP) 24 h after ischemia (in a zone corresponding to area 2 in Fig. 1a). The movie illustrates the free flowing of PMN before and the inflammatory response after thrombus formation. Please note the rapid recruitment of PMN to a vessel segment which is located beneath the thrombus and PMN which crawl against the bloodstream to the lesioned site. The tracks show the migration path along the vessel wall to the thrombus. Please also note the profound morphological changes of most microglia visible within the brain parenchyma after onset of thrombus formation. Real time of the experiment is shown in minutes. The scale bar is 20 µm
401_2014_1355_MOESM28_ESM.avi
Supplementary material 28 (AVI 1833 kb) Supplemental Movie 11 No recruitment of PMN in the absence of stroke. Two- photon intravital microscopy was performed in Lys-eGFP transgenic mice without previous ischemia. The first part of the movie represents the basal condition in Lys-eGFP mice before vessel irritation by enhanced laser exposure. The white arrow displays the site of enhanced laser exposure in part two of the movie. In part three, the same location was irritated by laser again to induce further damage which finally led to a coagulation of the vessel (black arrow). Overall a recruitment of PMN was not observed during the whole procedure. Real time of the experiment is shown in minutes. The scale bar is 20µm
401_2014_1355_MOESM29_ESM.mp4
Supplementary material 29 (MP4 1823 kb) Supplemental Movie 11 No recruitment of PMN in the absence of stroke. Two- photon intravital microscopy was performed in Lys-eGFP transgenic mice without previous ischemia. The first part of the movie represents the basal condition in Lys-eGFP mice before vessel irritation by enhanced laser exposure. The white arrow displays the site of enhanced laser exposure in part two of the movie. In part three, the same location was irritated by laser again to induce further damage which finally led to a coagulation of the vessel (black arrow). Overall a recruitment of PMN was not observed during the whole procedure. Real time of the experiment is shown in minutes. The scale bar is 20µm
401_2014_1355_MOESM30_ESM.avi
Supplementary material 30 (AVI 1665 kb) Supplemental Movie 12 Impact of anti-VLA-4 treatment on endothelial interaction of PMN. Two-photon intravital microscopy was performed in Lys-eGFP-mice 24h after ischemia. The movie shows on the left side the adherence and rolling of PMN in the vicinity to the ischemic core (corresponding to area 1 in Fig. 1a). The right side illustrates the PMN-endothelium interaction in the same area 15 minutes after anti-VLA-4 treatment. Real time of the experiment is shown in minutes. The scale bar is 30 µm
401_2014_1355_MOESM31_ESM.mp4
Supplementary material 31 (MP4 1611 kb) Supplemental Movie 12 Impact of anti-VLA-4 treatment on endothelial interaction of PMN. Two-photon intravital microscopy was performed in Lys-eGFP-mice 24h after ischemia. The movie shows on the left side the adherence and rolling of PMN in the vicinity to the ischemic core (corresponding to area 1 in Fig. 1a). The right side illustrates the PMN-endothelium interaction in the same area 15 minutes after anti-VLA-4 treatment. Real time of the experiment is shown in minutes. The scale bar is 30 µm
401_2014_1355_MOESM32_ESM.avi
Supplementary material 32 (AVI 397 kb) Supplemental Movie 13 PMN interact with the inflamed endothelium and invade the parenchyma of the brain after isotype treatment and pMCAO. Two-photon intravital microscopy was performed in Lys-eGFP transgenic mice 24h after pMCAO. Before induction of cerebral ischemia mice had been treated with isotype antibody. The movie shows a number of PMN (eGFP+ cells) interacting with the inflamed endothelium (adherence and rolling) as well as PMN migrating within the parenchyma and in the tissue around the blood vessels. Real time of the experiment is shown in minutes. The scale bar is 20 µm
401_2014_1355_MOESM33_ESM.mp4
Supplementary material 33 (MP4 377 kb) Supplemental Movie 13 PMN interact with the inflamed endothelium and invade the parenchyma of the brain after isotype treatment and pMCAO. Two-photon intravital microscopy was performed in Lys-eGFP transgenic mice 24h after pMCAO. Before induction of cerebral ischemia mice had been treated with isotype antibody. The movie shows a number of PMN (eGFP+ cells) interacting with the inflamed endothelium (adherence and rolling) as well as PMN migrating within the parenchyma and in the tissue around the blood vessels. Real time of the experiment is shown in minutes. The scale bar is 20 µm
401_2014_1355_MOESM34_ESM.avi
Supplementary material 34 (AVI 1421 kb) Supplemental Movie 14 Lack of PMN interacting with the inflamed endothelium and invading the brain after 1A8 treatment and pMCAO. Two-photon intravital microscopy was performed in Lys-eGFP transgenic mice 24h after pMCAO. Before induction of cerebral ischemia mice had been treated with depleting doses of 1A8 antibody. The movie illustrates very few PMN (eGFP+ cells) interacting with inflamed endothelium or migrating in the parenchyma and in the tissue around the blood vessels. Real time of the experiment is shown in minutes. The scale bar is 20 µm
401_2014_1355_MOESM35_ESM.mp4
Supplementary material 35 (MP4 1393 kb) Supplemental Movie 14 Lack of PMN interacting with the inflamed endothelium and invading the brain after 1A8 treatment and pMCAO. Two-photon intravital microscopy was performed in Lys-eGFP transgenic mice 24h after pMCAO. Before induction of cerebral ischemia mice had been treated with depleting doses of 1A8 antibody. The movie illustrates very few PMN (eGFP+ cells) interacting with inflamed endothelium or migrating in the parenchyma and in the tissue around the blood vessels. Real time of the experiment is shown in minutes. The scale bar is 20 µm
Supplementary material 36 (AVI 5632 kb) Supplemental Movie 15 Microglial morphology of CX3CR1-eGFP cells is associated with a Z-level below the focal plane of the meninges. A 2-Photon microscopy in-situ Z-stack of a CX3CR1-eGFP brain was reconstructed with 3D animation via IMARIS. The meninges were identified by their SHG signal (grey) and embedded blood vessels by PECAM1 immunostaining (red). The brain itself shows no SHG signal, but bright staining of capillaries (red). CX3CR1-GFP positive microglia (green) are found in the whole brain starting beneath the level of the meninges, whereas macrophages/monocytes (green round cells) are exclusively found attached to the meninges. Immunofluorescence staining of blood vessels: 10µg/mouse PE-anti-PECAM1 (BD, Cat: 553373) were intravenously injected. 20min after injection the mouse was perfused with PBS to remove unbound antibodies. The skull was opened and the brain was directly imaged in 37°C warmed PBS. 2-Photon setup: for imaging the CX3CR1-GFP mouse brain the 2-Photon laser was tuned to 960nm for GFP. For the ensuing simultaneous detection of PE and SHG an OPO was used at 1.100 nm excitation with SHG detection at 550nm
Supplementary material 37 (MP4 7867 kb) Supplemental Movie 15 Microglial morphology of CX3CR1-eGFP cells is associated with a Z-level below the focal plane of the meninges. A 2-Photon microscopy in-situ Z-stack of a CX3CR1-eGFP brain was reconstructed with 3D animation via IMARIS. The meninges were identified by their SHG signal (grey) and embedded blood vessels by PECAM1 immunostaining (red). The brain itself shows no SHG signal, but bright staining of capillaries (red). CX3CR1-GFP positive microglia (green) are found in the whole brain starting beneath the level of the meninges, whereas macrophages/monocytes (green round cells) are exclusively found attached to the meninges. Immunofluorescence staining of blood vessels: 10µg/mouse PE-anti-PECAM1 (BD, Cat: 553373) were intravenously injected. 20min after injection the mouse was perfused with PBS to remove unbound antibodies. The skull was opened and the brain was directly imaged in 37°C warmed PBS. 2-Photon setup: for imaging the CX3CR1-GFP mouse brain the 2-Photon laser was tuned to 960nm for GFP. For the ensuing simultaneous detection of PE and SHG an OPO was used at 1.100 nm excitation with SHG detection at 550nm
Rights and permissions
About this article
Cite this article
Neumann, J., Riek-Burchardt, M., Herz, J. et al. Very-late-antigen-4 (VLA-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke. Acta Neuropathol 129, 259–277 (2015). https://doi.org/10.1007/s00401-014-1355-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-014-1355-2