Abstract
Poly[1,5-bis(N-pyrrolyl)pentane] (PBNPP) has been designed as a new addition to the modified polypyrrole family. This study entails the introduction of a pentane spacer between two pyrrole moieties and subsequently their oxidative polymerization under ambient conditions. The newly designed monomer and its polymer were fully characterized by FT-IR. The structural and optical behaviors were comparatively explored with reference to polypyrrole (Ppy), which was again prepared under same conditions. SEM was employed to visualize the morphology of PBNPP and Ppy comparatively. The data demonstrated the morphology of the PBNPP as spherical beads with a greater extent of surface porosity compared to Ppy, which possesses a compact granular form. The structural parameters were explored by XRD, where both possess crystallites of smaller size. Thermal behaviors were analyzed by TGA and DSC, where the newly designed polymer showed greater thermal stability and more plasticizing trend as compared to Ppy. Optical and HOMO-LUMO attributes have been characterized using UV-Vis spectrophotometer and diffused reflectance spectroscopy (DRS). The PBNPP demonstrated greater molar absorption and lesser HOMO-LUMO gap as compared to Ppy. The PBNPP was proposed as a suitable candidate for photoconductivity compared with the Ppy. Thus, the polymer produced from the modified pyrrole is thermally more stable, has high plasticizing character, reflects greater optically sensitivity, and possesses ease of electronic transition compared to its counterpart.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The versatile character of Ppy has been the focus of a diverse range of research across the globe. It is known for its thermally viable [1], environmentally stable [2], and biocompatible [3] properties as well as it being a reliable sensor [4]. It has been used as actuators [5], anticorrosive agents [6], enzyme immobilizers, and in biomaterials [7]. Ppy has also been intensively studied as a nanocomposite material with reference to graphene [8], carbon nanotubes, and other metal nanostructures [8]. It has been modified to improve its structural, mechanical, optical, and electrical characteristics. These modifications of Ppy include C/N-alkylation [9, 10], backbone functionalization, co-polymerization, and metal chelation [11]. Ppy can be processed into thin films, powders, wires, and tube-like structures dependent upon application. Every aspect of synthesis, modification, and post-processing proved beneficial to improve its structural, morphological, and behavioral outcomes. Studies showed it to be a good electric and electronic material due to the highest conductivity and dielectric behavior [12, 13]. Recent works with reference to Ppy are highly focused in application for smart plastics, flexible super capacitors, and electrode materials for Li Ions batteries [14, 15].
The versatile nature of Ppy was further enriched by introducing an alkyl spacer between two pyrrole rings which linked their N-termini and converted them into N-functionalized bispyrrolyl moieties. Thus, a new modified form of pyrrole was synthesized and polymerized, in order to highlight the effects of this spacer introduction on the morphology, structure, thermal, and optical behavior. It was further compared with pristine Ppy prepared and analyzed under identical conditions. The newly synthesized material demonstrated uniform bead-like globular structures, which could be excellently exploited for enzyme immobilization, catalytic support, and as gas sensor. It has further demonstrated the enhanced thermal resistance and plasticization character. Finally, its optical properties were investigated using UV-Vis and diffused reflectance spectroscopy, which showed its greater molar absorbance and smaller HOMO-LUMO gap. Thus, this newly synthesized polymer added new scientific data to the horizon of Ppy and paved the way for further exploring new modes of synthesis, functionalization, and processing for different applications such as actuators, anticorrosive agents, electric, and electronic materials.
Experimental
Materials
The materials used are as follows: Pyrrole (BDH Chemicals.99%, UK), NaOH, FeCl3.6H2O (BDH Chemical LTD, UK), NaH (Sigma Aldrich, Germany), 1-5 dibromopentane (Merk, 99.99%, Germany), Conc. HCl, sodium sulfate, THF (analytical solvent, Sigma Aldrich Germany), DMF (analytical solvent, Sigma Aldrich, Germany), n-Hexane (analytic solvent, Lab-Scan, Thailand), methanol (analytic solvent, Sigma Aldrich, Germany), ethanol (98%, Merk, Germany), acetone (analytical reagents, Sigma Aldrich, Germany), and distilled H2O.
Methods
Synthesis of poly[1,5-bis(N-pyrrolyl)pentane (PBNPP) was achieved by synthesizing 1,5-bis (N-pyrrolyl)pentane monomers and subsequently its polymerization. The method is described in two steps, as outlined below.
Synthesis of 1,5-bis(N-pyrrolyl)pentane
1.75 ml (0.01 molar) pyrrole was mixed in 4 ml tetrahydrofuran (THF) and added 0.06 M sodium hydride. It was kept in ice bath for half hour and added 1,5-dibromo pentane (0.005 M). The progress of reaction was monitored by thin layered chromatography (TLC). Reaction was completed within 4 h, and the reaction mixture was poured into cold water, neutralized and extracted in ethyl acetate. The organic layer was further agitated using sodium sulfate (Na2SO4) as a dehydrating agent to completely remove the water. The product separated into the organic layer was concentrated under vacuo. The product was impurity free.
Synthesis of poly[1,5-bis(N-pyrrolyl)pentane] (PBNPP)
The newly synthesized monomer 1,5-bis(N-pyrrolyl)pentane was successfully polymerized in methanol using FeCl3 as oxidizing agent. The obtained monomer “1,5-bis(N-pyrrolyl)pentane” was mixed in 5 ml FeCl3 solution by 1:4 molar ratio for further reaction. The reaction was allowed to proceed for 4 h. TLC was used to monitor the consumption of monomer for the growth of polymer. Finally, the product in form of black powder was filtered and washed several times with distilled water to remove all impurities. It was dried overnight for further characterization.
Under similar conditions, PPy has been prepared for comparative study.
Characterization
Formation of bispyrrolyl and its polymer was characterized by Fourier-transform infrared spectroscopy (FT-IR) (Affinity 1, MIRacle 10, Shimadzu, Japan). Morphology of the synthesized PBNPP and PPy was characterized using SEM (FE-SEM TESCAN MIRA3MU, France). Structural information was obtained using X-ray diffraction (XRD) data using a Bruker D-8 discoverer X-ray diffractometer manufactured in the USA. The source of X-ray was Cu Kα (λ = 1.5408 Å), and the tube voltage and current were 40 kV and 40 mA, respectively. Thermal resistance of polymer was analyzed by thermogravic analysis (TGA) using Q 50 V6.2 BUILD 187; TGA instrument and Tg was measured by DSC Q100 V8.2 BUILD 268, both manufactured in the USA. Optical properties were measured under, Shimadzu 1800, Japan, UV-Vis spectrophotometer. Band gap of as-obtained polymer was analyzed by Perkin Elmer UV-Vis diffused reflectance spectrophotometer lambda 650 instrument, USA.
Results and discussion
The synthesis of 1,5-bis (N-pyrrolyl)pentane and its polymer; poly[1,5-bis(N-pyrrolyl)pentane], designated as BNPP and PBNPP, respectively were characterized by FT-IR, and their spectra were compared with that of pyrrole. There was a simple functional group conversion from cyclic amine to N-alkylated amine as shown in Scheme 1, where two pyrroles loss their H and linked with terminal C. The synthesized monomer “BNPP” was polymerized similar to pyrrole as reported elsewhere [16]. The FT-IR spectra of modified BNPP and PBNPP are shown in Fig. 1a, b. According to Fig. 1a, the disappearance of a band in the region from 3500 to 3100 cm−1 and an appearance of a band at 2926 cm−1 are indicating the replacement of an N–H bond by an N–C(aliphatic) bond [17, 18]. The stretching vibrations appearing at 2926 cm−1 band indicate the CH2 attributed to the incorporation of the n-pentane space [19]. The band at 2850 cm−1 is due to the C–H from the pyrrole ring. The absorption band at 1319 cm−1 is due to C–N stretching. The absorption at 1630 cm−1 is characteristic of pyrrole ring vibrations [20]. These IR bands confirm the synthesis of 1,5-bis[(N-pyrrolyl)pentane] had been successful. The ring and chain bending out of plane are reflected at 775 cm−1 and 721 cm−1, respectively [21]. It further confirmed the presence of pentane spacer in pyrrole moieties. The FT-IR spectrum obtained supported the theory that the conversion of pyrrole into BNPP had been successful.
Similarly, Fig. 1b shows the FT-IR spectra for the PBNPP, where all corresponding bands are much broader. The aromatic pyrrole and aliphatic pentane bands were present at 2840 cm−1 and 2910 cm−1 (respectively), which are almost identical to those observed in Fig. 1a. The aromatic ring symmetric and anti-symmetric stretching appeared at 1650 cm−1 and 1540 cm−1 respectively together with a shoulder band spreading from 1340 to 1440 cm−1 which is due to N–C stretching. The CH deformations appeared in the range of 1000 cm−1 to 1100 cm−1. The broader band at 900 cm−1 is due to the out-of-plane bending of the aromatic ring due to varying number of repeat units in as-produced chain length. Finally, the band observed at 780 cm−1 is the characteristic for the polymerization of the pyrrole as reported elsewhere [16, 22].
The morphology of the polymer was compared in Fig. 2a, b. Figure 2a shows the morphology of Ppy, which is exactly as reported by Sahoo et al. and Omastová et al. [23, 24] where Ppy was synthesized under the same conditions. Figure 2b shows the morphology of then PBNPP, prepared under identical conditions. There is a notable change in the morphology of the polymer when converted into PBNPP from Ppy. The introducing of the n-pentane spacer produced well-defined globular form with minimal appearance of the Ppy-like granules. It has also possessed small pores which may make it an important material for adsorption, storing, and sensing of different gasses like NH3, CO, and NO2 [22, 24, 25]. It may also hold potential in the clinical detection of uric acid, urea, and glucose etc. in comparison with Ppy that have been applied for such applications [26]. The as globular structure observed may also possess greater plasticizing behavior which could be used for further modification to make a highly refined membrane for selective exchange.
The structural change produced in converting the Ppy into PBNPP was analyzed using XRD, and the data is shown in Fig. 3. The material which was produced under identical conditions is amorphous, but possesses crystallite of different sizes. Herein, average chain separation is calculated using Eq. 1 [27].
According to Eq. 1, S is the average chain separation, λ is the X-ray wavelength, and θ is the diffraction angle at the maximum intensity in the graph. The average chain separation of PBNPP and Ppy is found as 2.30 nm and 2.49 nm respectively. Similarly, average crystallite size in the amorphous polymer was found through the Scherrer formula given in Eq. 2 [28].
where D is crystallite size, θ is the diffraction angle at maximum peak intensity, β is the full width with half maximum in radians, and k is the shape factor with value of 0.89. The average crystallite sizes were deduced for PBNPP and Ppy as 8.4 nm and 14 nm respectively. These values demonstrated that a greater extent of amorphous character was present in the PBNPP as compared to the Ppy. The XRD data supported this notion that the globular morphology is more amorphous as it has been seen in many bead forms of polymer [29].
Thermal stability analysis of the PBNPP from the modified pyrrole monomer is shown in Fig. 4 and is compared with the reported data achieved under identical conditions. The sample was heated from 20 to 650 °C at the rate of 5 °C/min. According to Fig. 4, the polymer weight loss which occurred between 260 and 600 °C was not sharp. It can be postulated that there may be some polymer chains in granular form on the surface of the globular structure, and hence, these would be degraded first. Furthermore, varying chain lengths also produce similar thermograph. According to the study of Cassignol et al. and Sahoo et al., pure Ppy synthesized under similar conditions showed weight loss at 180 °C with no stability in any region observed before complete degradation [23, 30]. Similar results have also been shown elsewhere [22]. The improvement of thermal resistance is attributed to the crosslinking factor, which is expressed in Scheme 2. The crosslinking factor might have developed aromatic π–π stacking to some pyrrole units and hence increased their thermal stability. Therefore, formation of different chain lengths, granular to globular morphology, and π–π stacking effect limited to some pyrrole units have enhanced significant thermal stability which only has been achieved when Ppy modified into PBNPP by introducing the pentane spacer.
The effect of plasticizing temperature on change of morphology of Ppy from granular to globular in PBNPP was investigated using differential scanning calorimetry (DSC) noting their Tg values. The values are recorded at the similar rate give in TGA analysis, i.e., 5 °C/min. The comparative data in plot form is given in Fig. 5. Ppy demonstrated its plasticizing temperature at 135 °C, which is in accordance, based on morphology to many published data as [16]. The Ppy analog PBNPP produced a globular morphology, which is less tightly packed and possesses greater voids. Therefore, it showed greater plasticizing behavior by demonstrating a reduced Tg value, i.e., at 117 °C. This may be further reduced for absolute globular structures and for larger globules. Therefore, study is still open for further exploration to relate plasticizing effect by producing new globular size.
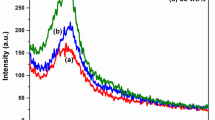
The optical behavior of PBNPP was compared with Ppy, and the data is shown in Fig. 6a, b. PBNPP and Ppy were dispersed in reagent grade ethanol using a sonicator. The stable dispersion was analyzed under UV-Vis spectrophotometer. The dominant shoulder in the case of Ppy is reflected at 400 nm, which is purely benzenoid behavior [31]. PBNPP possessed a λmax at 500 nm, which is quite red shifted. The modification of Ppy into PBNPP did not change the benzenoid configuration, but the red shifting depicts that the pyrrole rings in the PBNPP may have developed π–π stacking resulting in the decreased excitation energy.
The electronic transitions and band gap, i.e., Eg, is measured using differential reflectance spectroscopy (DRS) and is shown in Fig. 7. The values for Ppy and PBNPP appeared at 643 nm and 654 nm respectively. According to Eq. 3, given below, these can be converted to electron volts (eV) [27].
The calculations showed the band gap of Ppy and PBNPP is 1.9 eV and 1.8 eV respectively. Therefore, the newly synthesized polymer is expected to be suitable for use as a semiconducting material.
Conclusions
Modification of pyrrole into 1,5-bis(N-pyrrolyl)pentane resulted in a desirable molecule possessing a preferable morphological appearance in the polymeric form. Such transformation leads to the production of globular form rather than granular. The globular microstructure revealed porosity which ensures high potential for the anchoring of catalysts, enzymes, or other biomolecules of interest. Additionally, it showed improved thermal resistance and plasticizing character as compared to Ppy, which guaranteed the durability. It demonstrated increased optical molar absorption and decreased the band gap for electronic transitions as characterized by UV-visible spectrophotometer and DRS. This property is essential for making it a suitable candidate for photo-electric devices. It possessed reasonable crystallite size which predicated the crystalline transformation by optimizing polymerization conditions. The PBNPP is a new polymer that is widening the application of polypyrroles across multiple sectors. Further studies focusing on application-specific uses will elucidate the full potential of this exciting polymer.
References
Deng F, Min L, Luo X, Wu S, Luo S (2013) Visible-light photocatalytic degradation performances and thermal stability due to the synergetic effect of TiO 2 with conductive copolymers of polyaniline and polypyrrole. Nanoscale 18:8703–8710
Yan H, Kurogi K, Mayama H, Tsujii K (2005) Environmentally stable super water-repellent poly (alkylpyrrole) films. Angew Chem Int Ed 22:3453–3456
Zha Z, Deng Z, Li Y, Li C, Wang J, Wang S, Qu E, Dai Z (2013) Biocompatible polypyrrole nanoparticles as a novel organic photoacoustic contrast agent for deep tissue imaging. Nanoscale 10:4462–4467
Rahman MA, Kumar P, Park D-S, Shim Y-B (2008) Electrochemical sensors based on organic conjugated polymers. Sensors 1:118–141
Zheng W, Alici G, Clingan PR, Munro BJ, Spinks GM, Steele JR, Wallace GG (2013) Polypyrrole stretchable actuators. Angew Chem Int Ed 1:57–63
Hua W (2015) Preparation and corrosion performance of polypyrrole film. Surf Technol 3:111–115
Ekanayake EM, Preethichandra DM, Kaneto K (2007) Polypyrrole nanotube array sensor for enhanced adsorption of glucose oxidase in glucose biosensors. Biosens Bioelectron 1:107–113
Yang Y, Wang C, Yue B, Gambhir S, Too CO, Wallace GG (2012) Electrochemically synthesized polypyrrole/graphene composite film for lithium batteries. Adv Energy Mater 2:266–272
Delabouglise D, Roncali J, Lemaire M, Garnier F (1989) Control of the lipophilicity of polypyrrole by 3-alkyl substitution. J Chem Soc Chem Commun 8:475–477
Jang KS, Lee H, Moon B (2004) Synthesis and characterization of water soluble polypyrrole doped with functional dopants. Synth Met 3:289–294
Chebil S, Miodek A, Ambike V, Sauriat-Dorizon H, Policar C, Korri-Youssoufi H (2013) Polypyrrole functionalized with new copper complex as platform for His-tag antibody immobilization and direct antigen detection. Sensor Actuat B-Chem 185:762–770
Kim YD, Song IC (2002) Electrorheological and dielectric properties of polypyrrole dispersions. J Mater Sci 23:5051–5055
Saafan S, El-Nimr M, El-Ghazzawy E (2006) Study of dielectric properties of polypyrrole prepared using two different oxidizing agents. J Appl Polym Sci 6:3370–3379
Guo J, Song H, Liu H, Luo C, Ren Y, Ding T, Khan MA, Young DP, Liu X, Zhang X (2017) Polypyrrole-interface-functionalized nano-magnetite epoxy nanocomposites as electromagnetic wave absorbers with enhanced flame retardancy. J Mater Chem C 22:5334–5344
Xue M, Li F, Chen D, Yang Z, Wang X, Ji J (2016) High-oriented polypyrrole nanotubes for next-generation gas sensor. Adv Mater 37:8265–8270
Ahmad Z, Choudhary MA, Mehmood A, Wakeel R, Akhtar T, Rafiq MA (2016) Synthesis of polypyrrole nano/microspheres using cobalt (III) as an oxidizing agent and its ammonia sensing behavior. Macromol Res 7:596–601
Qiu T, Xie H, Zhang J, Zahoor A, Li X (2011) The synthesis of Ag/polypyrrole coaxial nanocables via ion adsorption method using different oxidants. J Nanopart Res 3:1175–1182
Ahmad Z, Saeed B, Akhtar T, Ahmad J (2016) Synthesis and polymerization of 1,5-bis(N-carbazolyl)pentane with its structural and behavioral highlights. Polym Bull. https://doi.org/10.1007/s00289-019-02797-w
Jang BN, Wilkie CA (2004) A TGA/FTIR and mass spectral study on the thermal degradation of bisphenol A polycarbonate. Polym Degrad Stab 3:419–430
Kaya İ, Aydın A (2012) A new approach for synthesis of electroactive phenol based polymer: 4-(2, 5-di (thiophen-2-yl)-1H-pyrrol-1-yl) phenol and its oxidative polymer. Prog Org Coat 2-3:239–249
Macit H, Sen S, Sacak M (2005) Electrochemical synthesis and characterization of polycarbazole. J Appl Polym Sci 3:894–898
Zhang J, Wang S, Xu M, Wang Y, Xia H, Zhang S, Guo X, Wu S (2009) Polypyrrole-coated SnO2 hollow spheres and their application for ammonia sensor. J Phys Chem C 5:1662–1665
Sahoo NG, Jung YC, So HH, Cho JW (2007) Polypyrrole coated carbon nanotubes: synthesis, characterization, and enhanced electrical properties. Synth Met 8-9:374–379
Omastová M, Trchová M, Pionteck J, Prokeš J, Stejskal J (2004) Effect of polymerization conditions on the properties of polypyrrole prepared in the presence of sodium bis (2-ethylhexyl) sulfosuccinate. Synth Met 2:153–161
Radhakrishnan S, Paul S (2007) Conducting polypyrrole modified with ferrocene for applications in carbon monoxide sensors. Sensor Actuat B-Chem 1:60–65
Çete S, Yaşar A, Arslan F (2006) An amperometric biosensor for uric acid determination prepared from uricase immobilized in polypyrrole film. Artif Cell Blood Sub 3:367–380
Bhadra S, Kim NH, Rhee KY, Lee JH (2009) Preparation of nanosiz polyaniline by solid state polymerization and determination of crystal structure. Polym Int 58:1173–1180
Chiu M-Y, Jeng U-S, Su C-H, Liang KS, Wei K-H (2008) Simultaneous use of small- and wide-angle X-ray technique to analyze nanometerscale phase separation in polymer heterojunction cell. Adv Mater 20:2573–2578
Li J, Mulder T, Vorselaars B, Lyulin AV, Michels M (2006) Monte Carlo simulation of uniaxial tension of an amorphous polyethylene-like polymer glass. Macromolecules 22:7774–7782
Cassignol C, Olivier P, Ricard A (1998) Influence of the dopant on the polypyrrole moisture content: effects on conductivity and thermal stability. J Appl Polym Sci 8:1567–1577
Jayamurugan P, Ponnuswamy V, Ashokan S, Jayaprakash R, Ashok N, Guna K, Mariappan R (2014) DBSA doped polypyrrole blended with Poly (4-styrenesulfonic acid) by mechanical mixing. Mater Sci-Poland 4:648–651
Acknowledgments
The higher education commission of Pakistan is thanked for providing resources for polymer modification project, and ORIC Mirpur University of Science and Technology (MUST) is gratefully acknowledged for channelizing the resources to develop new materials.
Funding
We have not received any formal funding for this project. It was completed mainly using the Departmental Resources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmad, Z., Bibi, S., Mushtaq, S. et al. Designing the poly[1,5-bis(N-pyrrolyl)pentane as a new horizon of polypyrrole paradigm with its structural and optical highlights. Colloid Polym Sci 297, 1437–1443 (2019). https://doi.org/10.1007/s00396-019-04574-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-019-04574-7