Abstract
To obtain multiple emulsions containing Agnique™ AE 3-2H as oil phase and Atlas™ G-5000 as emulsifier, two formulation variables were studied: the effect of the Atlas™ G-5000 concentration in emulsions containing 15 wt% Agnique™ AE 3-2H and the study of the influence of the solvent concentration maintaining the Agnique™ AE 3-2H/Atlas™ G-5000 ratio equal to 10. To assess microstructure and physical stability of these emulsions different techniques were employed, namely laser diffraction, transmitted light optical microscopy, steady-state measurements, and multiple light scattering. An increase in copolymer concentration and solvent concentration provoked a decrease in Sauter diameter but an increase in volume mean diameter and polydispersion due to droplet coalescence. Regardless of polymer concentration, all emulsions showed Newtonian behavior which led to shear thinning with increasing oil concentration. The main destabilization processes are creaming, when the concentration of polymer or solvent is low, and coalescence, when both concentrations are high.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The huge potential applications of multiple emulsions have stimulated an increasing effort to develop and optimize such complex formulations and to study their physical stability and properties. These issues are directly related to the microstructure of multiple emulsions which consist of droplets of simple emulsion dispersed in a continuous phase. The most common multiple emulsion structure is that in which a water in oil emulsion is dispersed in an aqueous continuous phase, which is known as a W/O/W multiple emulsion [1]. In these emulsions, it is possible to encapsulate a hydrophilic active ingredient in inner aqueous droplets so that safer formulations can be obtained [2]. This makes it possible to design formulations with new functional properties in which the encapsulated material can be released as a consequence of a controlled alteration in the environmental conditions such as a change in temperature, pH, or ionic strength [3].
In order to determine the release kinetic of the internal active ingredient under controlled conditions, the destabilization mechanisms of these emulsions must be known. As is the case of all types of emulsion, multiple emulsions are thermodynamically unstable systems due to the existence of large and polydispersed droplets. For this reason, they tend to suffer destabilization mechanism by creaming, flocculation, and coalescence processes [4]. Another destabilization process to be taken into account in multiple emulsions is the conversion of these into simple emulsions as a result of the osmotic gradient existing between both aqueous phases. This leads to the transportation of water molecules from the external to the internal aqueous phase. According to this, an increase in the inner droplet size takes place and subsequently, droplets break down. The development of emulsions that allow a controlled release of some active ingredients can be achieved as a result of the study of the kinetics of the different destabilization processes. In fact, the main applications of multiple emulsions are associated with their destabilization mechanisms [5,6,7,8,9,10].
In addition, future applications are related to the nature of their components. In this work, 2-ethylhexyllactate, which is a green solvent consisting of lactic esters obtained from renewable sources [11], was used as oil phase. This characteristic, among others, has made this solvent frequently used in agrochemical formulations [12,13,14,15,16]. These eco-friendly solvents make it possible to fulfill the European requirements, in which oil phases containing aromatic components are being banned from agrochemical products [11, 13].
In order to improve the physical stability of multiple emulsions, amphiphilic block copolymers are frequently used as emulsifiers. Their ability to reduce the interfacial tension leads to an increase in the stability of these emulsions. In addition, their high molecular weight makes them an attractive option since multiple emulsions can be stabilized using a smaller quantity of surfactant [17]. In this work, an A-B block copolymer, the Atlas™ G-5000, was used as emulsifier. It consists of polyalkylene glycol ether (EO-PO) with an HLB close to 17 which is suitable for the stabilization of O/W interfaces. The physical stability is improved due to the large absorption energy of the anchoring groups in the dispersed phase interfaces. This fact prevents the desorption and displacement of these macromolecules from the droplet interface [18].
Frequently, multiple emulsions are produced by methods involving two-step protocols, in which the first one produces simple emulsions, by means of a conventional rotor–stator homogenization treatment, and the second one involves the dispersion of the latter emulsion in another non-miscible continuous phase [5, 19]. Nevertheless, there are an increasing number of studies assessing the production of multiple emulsions in a single step [20,21,22,23]. For this purpose, the choice of the components for emulsion formulation is of great importance. In this way, Hong et al. [20] were able to achieve extraordinarily stable multiple emulsions containing an asymmetric copolymer by a procedure which involves a unique step based on a catastrophic inversion. These researchers found that the copolymer utilized successfully adsorbed at the interfaces with its hydrophilic blocks oriented to the aqueous phase while the hydrophobic blocks were efficiently anchored into the oil phase [20].
In previous studies, W/O/W multiple emulsions containing 2-ethylhexyl lactate have also been prepared by applying a simple one-step protocol, using a rotor–stator homogenizer [22].
The main aim of this study was to evaluate the effect of copolymer concentration while keeping the solvent concentration constant, as well as the influence of solvent concentration, maintaining the Agnique™ AE 3-2H/Atlas™ G-5000 ratio equal to 10, on the microstructure and physical stability of the resulting multiple emulsions. In this way, the potential use of these system as matrices for the future incorporation of active ingredients seem an interesting option since they were produced an only one-step protocol and containing an alone copolymer as emulsifier, and therefore, with a reduction in the ingredient costs and in the applied energy.
Materials and methods
Materials
In order to obtain multiple emulsions, 2-ethylhexyl lactate (Agnique™ AE 3-2H), cordially provided by BASF, was utilized as oil phase. Its boiling and melting points are 246 °C and − 23 °C, respectively, and its density measured at 20 °C, 940 kg/m3. Atlas™ G-5000, commercialized by Croda, whose HLB is 16.9, was used as emulsifier. Additionally, a defoaming agent, MD10 (Dow Corning), was added during the preparation process.
Preparation of emulsions
Emulsions were produced with a rotor–stator turbine (Ultraturrax T-50/G45F) applying 4000 rpm of rotational speed for 150 s. Firstly, Atlas™ G-5000 copolymer was dissolved in the necessary amount of water to complete the formulation by utilizing a magnetic stirring plate (SB 162, Stuart, Scientific Laboratory Supplies), and then the 2-ethylhexyl lactate was incorporated into the aqueous phase and the mixture homogenized with the rotor-stator device, before the addition of the defoaming agent. The nomenclature used to denote the emulsion formulations is EX/Y, where X stands for wt% oil phase and Y for wt% Atlas™ G-5000. Compositions of the studied formulations are shown in Table 1.
The emulsions obtained were characterized by means of laser diffraction analysis, optical microscopy, and steady-state viscous flow tests, 24 h after emulsion preparation. The multiple light scattering technique was used to monitor the emulsions’ stability over time.
Steady shear viscous flow measurements
Viscous flow tests were performed at 25 °C ± 0.1 °C in a rotational controlled-strain rheometer (ARES, TA Instruments), in a shear rate range of 0.1–400 s−1 using a coaxial cylinder geometry whose inner and outer diameters were 33.9 mm and 32 mm, respectively. Special surfaces and/or geometries were not necessary since, as demonstrated in a previous work, wall depletion effects were not significant [24]. To attain the steady-state regime, data were acquired after 2 min at each shear rate. Before starting the test, samples were maintained in a quiescent state for 600 s as equilibration time once placed in the measuring tool. At least 3 replicates of each measurement were carried out on fresh samples.
Globule size distribution measurements
Globule (external droplet) size distribution was determined by means of laser diffraction analysis (Mastersizer Hydro2000, Malver Instruments). Water was used as the dispersant medium. The refraction index was 1.52 for the dispersed phase and the refraction and adsorption indexes for the aqueous medium were 0.5 and 1.33, respectively. At least four replicates of each test were performed at room temperature.
Optical microscopy
Transmitted light optical microscopy, using an Axio Scope A1, Carl Zeiss equipment with a × 63 objective, was used to acquire microstructural information concerning the emulsions studied.
Multiple light scattering
A Turbiscan Lab Expert device was utilized to assess the physical stability of the emulsions obtained. Tests were performed at room temperature (~ 25 °C). Turbiscan measures backscattering and transmission as a function of sample length. However, in this work, only backscattering data are shown due to the fact that the transmission values recorded were null.
Results and discussion
Influence of Atlas G-5000 concentration on physical properties and stability of multiple emulsions containing 15 wt% Agnique™ AE 3-2H
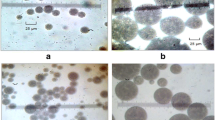
The occurrence of multiple emulsions was demonstrated by transmitted light optical microscopy (Fig. 1), which shows the confinement of W/O droplets inside larger globules.
The viscous flow curves revealed a Newtonian response for all Atlas™ G-5000 concentrations studied, with viscosity values only slightly higher than water viscosity (ranging from 2 to 4 mPa s). Results were fitted to the power law equation whose fitting parameters are exhibited in Table 2.
where η0 is the Newtonian viscosity and n is the flow index. The flow index obtained in all cases was 1, which indicated the Newtonian response of the studied emulsions. The viscosity values obtained ranged from 2 to 4 mPa s (Table 2). This behavior is typically found in diluted emulsions where the interactions among droplets are negligible [25].
The mean diameters of the globules (Sauter and volumetric diameters) are shown in Table 3. As can be observed, the values of the Sauter diameter decreased as the copolymer concentration increased, while the opposite tendency was exhibited by the volume diameter. This fact could be due to the migration of water molecules from the aqueous continuous phase to aqueous inner droplets as a consequence of an osmotic pressure gradient between both aqueous phases [5, 6, 22]. In addition, it must be considered that these globule size distributions can be affected by the occurrence of a coalescence process of inner aqueous droplets with the outer aqueous continuous phase. As reported by Schuch et al. [26], this fact may be a consequence of the existence of the hydrophilic copolymer in the continuous aqueous phase and would, therefore, be favored at high concentrations. This was supported by the globule size distributions of these emulsions (Fig. 2). As can be observed, the distribution of the emulsion containing 1.5 wt% Atlas™ G-5000 exhibited a principal peak at around 6.5 μm and a secondary peak at 60 μm. An increase in the copolymer concentration up to 3 wt% led to a division of the main peak into two peaks. One of them slightly shifted to smaller diameters (at around 3.1 μm) and the other one to higher diameters (at around 10.3 μm). In addition, this emulsion presented two maximums at greater diameters located at 56 μm and 267 μm. In the case of E15/6, the increase in the copolymer concentration caused the appearance of a main peak at 5 μm and a wide tail at higher diameters situated between 126 and 558 μm.
The evolution of the stability over time was measured by means of multiple light scattering. Figure 3a–c show BS profiles as a function of the tube length over a maximum of a 5-day period. For the emulsion with 1.5 wt% copolymer concentration (Fig. 3a), the BS value at the bottom of the container of the sample significantly decreased after 5 days, whereas the value at the top increased, reflecting a creaming-induced destabilization according to multiple light scattering theory [27]. This phenomenon could be observed by the naked eye after 5 days from the emulsion preparation. As the copolymer concentration increases, the decrease of BS value at the bottom of the measuring cell is dampened, indicating that destabilization by creaming was less significant (Fig. 3b, c). Along with this fact, a slight reduction in backscattering was observed at the top of the measuring cell, which could be attributed to a phase separation process (oiling off) as a consequence of the coalescence destabilization mechanism in the creamed phase [28].
The %BS data at a selected tube length (12.5 mm) was plotted vs. the aging time of the samples (Fig. 3d). After an induction time, the % BS showed an evolution with time as a result of the destabilization phenomena. These data were fitted to a first-order kinetic Eq. (2) [22, 29]:
where BS stands for backscattering as a function of aging time, BSe is the corresponding equilibrium value, BS0 is the initial value of backscattering, K is the first-order kinetic coefficient, and ti is the induction time.
The fitting parameters are shown in Table 4. As can be observed, an increase in the copolymer concentration significantly decreased the destabilization rate (see K value) due to creaming.
Results are in concordance with those obtained by laser diffraction. The higher the polymer concentration, the lower the interfacial tension and the Sauter mean diameters but, at the same time, the higher the coalescence, and therefore, D4,3 is higher, due to a change in osmotic pressure as a consequence of the presence of the Atlas G-5000. In addition, the higher polymer concentration implies lower Sauter mean diameters, which provide the emulsions’ higher stability against creaming.
Influence of Agnique™ AE 3-2H concentration on physical properties and stability of multiple emulsions
The influence of the oil phase fraction was evaluated by maintaining the Agnique™ AE 3-2H/Atlas™ G-5000 ratio equal to 10. Again, optical micrographs showed the existence of W/O/W multiple emulsions. The different microstructures achieved by increasing the amount of Agnique™ AE 3-2H are depicted in Fig. 4, where a decreasing size of the larger globules can be observed by increasing the oil fraction.
The viscous flow measurements revealed that the increase in the oil phase concentration led to a gradual change in the rheological behavior of the samples (Fig. 5), from Newtonian, for E15/1.5 emulsion, to shear thinning behaviors, for E30/3, E40/4, and E50/5 samples. These observations were corroborated by fitting parameters to power law model (1) (Table 5).
where η is the viscosity, η1s is the viscosity at 1 s−1, \( \overset{.}{\gamma } \) is the shear rate achieved, and n is the flow index. Coherently with the results shown in Fig. 5, η1s values increased with the oil phase fraction. In addition, emulsions with an apparent Newtonian response presented a flow index close to 1. The slightly lower flow index for sample E30/3 indicates that this oil concentration roughly represents the threshold to obtain shear thinning behavior. From this threshold concentration, the shear thinning became more pronounced (see n values in Table 5). Regarding globule size, all emulsions, excepting E50/5, exhibited bimodal size distributions as a function of the volume fraction (Fig. 6a). As can be observed, the main peak was displaced towards smaller sizes as Agnique™ AE 3-2H concentration increased, which supports the optical microscopy observations. However, the secondary maximum was also greater as the oil phase increased. In addition, the emulsion containing 50 wt% oil showed a third peak at higher sizes. This effect was again evidenced in the different evolution of D3,2 and D4,3 shown in Table 6. Thus, an increase in the oil phase fraction caused a reduction in the Sauter diameter whereas the volumetric mean diameter and span values increased. Nevertheless, although these large globules represent an important contribution to the volume fraction, their number was actually insignificant, as can be observed in Fig. 6b, where the distributions are plotted in terms of the frequency of the number of droplets.
Khopkar et al. [30] reported a study in which the influence of the dispersed phase fraction on the mean droplet size and the droplet size distribution was evaluated. In this, the authors studied emulsions containing 10 wt%, 20 wt%, 40 wt%, and 50 wt% oil concentrations and found that the emulsion with 50 wt% oil phase exhibited a greater number of small drops compared to that with 40% oil phase. However, the polydispersity of the distribution was higher for the emulsion with higher oil concentration. This fact was attributed to a greater coalescence rate, which occurred as consequence of the higher oil concentration, and the reduced speed of surfactant adsorption. These results are similar to those obtained in this investigation. Thus, the observed increase in both large droplets and the polysdispersity at greater solvent concentration could be due to a high coalescence speed related to low surfactant adsorption rate, as found by Khopkar et al. [20].
The multiple light scattering technique was employed to study the physical stability of these emulsions. As mentioned above, the emulsion E15/1.5 results shown in Fig. 3a manifested a creaming process, since a decrease in the %BS was observed at the bottom of the cell and an increase in the %BS at the top due to droplet migration from the lower to the higher part of the vial. Figure 7a shows % BS as a function of the aging time for emulsion E30/3. This figure showed the existence of several coexisting destabilization mechanisms, which is characteristic of multiple emulsions [31]. On the one hand, a decrease in BS at the bottom of the tube containing the sample as a consequence of the migration of larger globules due to creaming is clearly apparent, which leads to a clarification process in the lower part of container. On the other hand, in the upper part of the measuring cell, a drop in % BS with time can be observed. This result was a consequence of an oil phase separation resulting from a coalescence process [28].
Similar effects can be observed in Fig. 7b for the E40/4 multiple emulsion. However, the higher solvent and copolymer concentrations slowed down these phenomena, and therefore, the destabilization processes were observed at longer aging times. Finally, for emulsion E50/5 (Fig. 7c), the main effect observed was the increase in % BS in the middle of the measuring cell which indicates an increase in globule size. Regarding the multiple light scattering theory, the occurrence of this effect in samples containing droplets smaller than 0.6 μm could be attributed to a flocculation and/or coalescence phenomenon [27]. As can be observed in Fig. 6b, for this emulsion, the maximum of globule size distribution was located at around 0.4 μm. Indeed, a more important clarification was detected at the top of the measuring cell which may be related to the existence of a phase separation as a result of this coalescence phenomenon.
Figure 7d shows the %BS at a 12.5 mm tube length as a function of the aging time for the samples studied. The %BS evolves with time due to the destabilization phenomena discussed above, which were observed after an induction time. These data were again fitted to the first-order kinetic equation (Eq. 2) [22, 29] and the values of fitting parameters included in Table 7.
As can be observed in Fig. 7d, the curve shape of the curve for sample E15/1.5 was different due to the fact that, at this height (12.5 mm), the emulsion was mainly affected by a very fast clarification effect as a consequence of the migration of the globules towards the upper part of the measuring cell. Nevertheless, for the rest of the emulsions, at this tube length, the increase in globule concentration as a result of the creaming process is the predominant effect. This fact is reflected in the term (BS0-BSe) which is negative for all emulsions except for emulsion E15/1.5, as can be seen in Table 7. The kinetic coefficient, K, showed a significantly higher value for the emulsion E15/1.5, which indicated a higher destabilization rate. However, the value of this parameter was of the same order of magnitude for the rest of the samples, emulsion E50/5 showing the lowest value. Additionally, the induction time tends to increase with the oil fraction, which indicates that the destabilization inception time was higher for more concentrated emulsions.
Again, a good concordance is found between all results obtained. Emulsions with low oil concentration exhibit higher D3,2 values and as a consequence they are more unstable against creaming. Thus, an increase in dispersed phase concentration increases the viscosity, delays the adsorption of the surfactant ,and increases the coalescence.
Conclusions
The use of a hydrophilic copolymer like Atlas™ G-5000 makes it possible to obtain W/O/W multiple emulsions by following a simple one-step processing protocol. Therefore, the use of these emulsions as matrices in which could be encapsulated an active material to be liberated under determined conditions could be an interesting options in some fields such as agrochemical, food, and pharmaceutical.
The emulsions containing 15 wt% of Agnique™ AE 3-2H as oil phase exhibited Newtonian behavior with apparent viscosity values ranging between 2 and 4 mPa s. An increase in the copolymer concentration led to a reduction in the Sauter diameter and, at the same time, an increase in the mean volume diameter and span values, which is attributed to the migration of water molecules from the aqueous continuous phase and inner aqueous phase, due to an osmotic process produced by the higher concentration of hydrophilic copolymer in the inner phase, as well as to a coalescence phenomenon.
These multiple emulsions underwent several destabilization mechanisms over a time period of a few days. The multiple light scattering analysis demonstrated that the emulsions containing 1.5 and 3% Atlas™ G-5000 presented destabilization mainly caused by a creaming process while the emulsion with 6% of the copolymer was predominantly destabilized by coalescence.
An increase in oil phase fraction, by maintaining the Agnique™ AE 3-2H/Atlas™ G-5000 ratio equal to 10, caused a change in the rheological behavior, from Newtonian for 15 and 30% wt. oil, to shear thinning for 40 and 50% oil fraction. At this oil/copolymer ratio, the threshold oil concentration for obtaining shear thinning behavior is around 30%. Additionally, the laser diffraction technique also demonstrated a decrease in the Sauter diameter and an increase in the mean volume diameter and span values as oil fraction increases. This fact is, in part, a consequence of the coalescence process due to the slow adsorption rate of the copolymer. On the other hand, the multiple light scattering analysis demonstrated that emulsions with 30 and 40% wt. oil content were mainly destabilized by a creaming process, despite the fact that a slight oil layer was also observed as a result of the coalescence process, whereas for 50% oil content the main destabilization mechanism was coalescence, this being the most stable emulsion. In general, the increase in oil content slowed down the destabilization by creaming.
References
Perrin P, Prigent F, Hébraud P (2008) Structure and rheology of stable multiple emulsions. In: Aserin A (ed) Multiple emulsions: technology and applications. John Wiley and Sons Inc, New Jersey, pp 29–43
ElShafei GMS, Elsaid MM, Attia HAE, Mohammed TGM (2010) Environmentally friendly pesticides: essential oil-based w/o/w multiple emulsions for antifungal formulations. Ind Crop Prod 31:99–106
McClements DJ (2012) Advances in fabrication of emulsions with enhanced functionality using structural design principles. Curr Opin Colloid Interface Sci 17:235–245
Aserin A (2008) Introduction to multiple emulsions technology and applications: an update. In: Aserin A (ed) Multiple emulsions: technology and applications. John Wiley and Sons, Inc, Hoboken, pp xix–xxi
Matsumoto S, Inoue T, Kohda M, Ohta T (1980) An attempt to estimate stability ofthe oil layer in W/O/W emulsions by means of viscosimetry. J Colloid Interface Sci 77:564–565
Ohwaki T, Machida R, Ozawa H, Kawashima Y, Hino T, Takeuchi H, Niwa T (1993) Improvement of the stability of water-in-oil-in-water multiple emulsions bythe addition of surfactants in the internal aqueous phase of the emulsions. Int J Pharm 93:61–74
Yan J, Pal R (2001) Osmotic swelling behavior of globules of W/O/W emulsion liquidmembranes. J Membrane Sci 190:79–91
Jager-Lezer N, Terrisse L, Bruneau F, Tokgoz S, Ferreira L, Clausse D, Seiller M, Grossiord JL (1997) Influence of lipophilic surfactant on the release kinetics ofwater- ofwater-soluble molecules entrapped in a W/O/W multiple emulsions. J Control Release 45:1–13
Zhao C-X (2013) Multiphase flow microfluidics for the production of single or multiple emulsions for drug delivery. Adv Drug Deliv Rev 65:1420–1446
Jiménez-Colmenero F (2013) Potential applications of multiple emulsions in thedevelopment of healthy and functional foods. Food Res Int 52:64–74
Bigorra J, Cognis Iberia-Castellbisba (2010) Innovative solvents based on renewableraw material. In: 40th Jornadas Anuales del CED, España, pp. 1–11
Hottois D, Bruneau A, Bogaert JC, Coszach P (2010) Manufacture of lactic acid esters, in: Belgium Patent BE1017951A320100112
Merlet S, Munzenberg C, Wiethoff H (2011) Biocide compositions comprising car-bamates, in: PCT Patent WO2011060890A120110526
Mertoglu M, Mayer W, Strobel D, Berghaus R, Strathmann S (2011) Anhydrous com-position comprising a dissolved and a suspended pesticide, alkyl lactate and alcohol. In: PCT Patent WO2011098419A220110818
Höfer R, Bigorra J (2007) Green chemistry—a sustainable solution for industrial spe-cialties applications. Green Chem 9:203–212
Merlet S, Bigorra J, Raya J, Valls R, Busch S (2012) Biocide compositions comprising amides based on 2-furoic acid and its derivatives, with high solubility and improved emulsification properties. In: PCT PatentWO2012038306A120120329
Michaut F, Hébraud J, Perrin P (2003) Amphiphilic polyelectrolyte for stabilization of multiple emulsions. Polym Int 52:594–601
Tadros TF (2009) Colloids and interface science series 5: colloids in agrochemicals. WILEY-VCH Verlag CmbH & Co. KGaA, Weinheim
Magdassi S, Garti N (1986) A kinetic model for release of electrolytes from w/o/w multiple emulsions. J Control Release 3(1–4):273–277. https://doi.org/10.1016/0168-3659(86)90098-2
Hong L, Sum G, Cai J, Ngai T (2012) One-step formation of w/o/w multiple emulsions stabilized by single amphiphilic block copolymers. Lagmuir 28:2332–2336. https://doi.org/10.1021/la205108w
Lutz R (2008) Aserin a (2008) multiple emulsions stabilized by biopolymers. In: Aserin A (ed) Multiple emulsions: technology and applications. John Wiley and Sons, Inc, Hoboken
García MC, Muñoz J, Alfaro MC, Franco JM (2014) Physical characterization of multiple emulsions formulated with a green solvent and different HLB block copolymers. Colloids Surf A Physicochem Eng Asp 458:40–47
García MC, Cox P, Trujillo-Cayado L, Muñoz J, Alfaro MC (2018) Rheology, microstructural characterization and physical stability of W/α-PINENE/W emulsions formulated with copolymers. Colloids Surf A Colloids Surf A 536:125–132. https://doi.org/10.1016/j.colsurfa.2017.07.029
García MC, Muñoz J, Alfaro MC, Franco JM (2013) Assessment of wall-depletion phenomena in dilute emulsions. In: Rubio-Hernández FJ, Gómez-Merino AI, Pino C, Parras L, Campo-Deaño L, Galindo-Rosales FJ, Velázquez-Naverro JF (eds) Perspectives in fundamental and applied rheology. Grupo Español de Reología, Málaga, pp 297–301
Pons R (2000) Polymeric surfactants as emulsions stabilizers. In: Paschalis A, Björn L (eds) Amphiphilic block copolymers: self-assembly and applications. Elsevier Science B. V, Amsterdam, pp 409–422
Schuch A, Leal LG, Schuchmann HP (2014) Production of W/O/W double emulsions. Part I: visual observation of deformation and breakup of double emulsion drops and coalescence of the inner droplets. Colloids Surf A Physicochem Eng Asp 462:336–343
Mengual O, Meunier G, Cayre I, Puech K, Snabre P (1999) Characterisation of instability of concentrated dispersions by a new optical analyser: the TURBISCAN MA 1000. Colloids Surf A Physicochem Eng Asp 152:111–123
Palazolo G, Sorgentini A, Wagner J (2005) Coalescence and flocculation in o/w emulsions of native and denatured whey soy proteins in comparison with soy protein isolates. Food Hydrocoll 19:595–604
García MC, Alfaro MC, Calero N, Muñoz J (2014) Influence of polysaccharides on the rheology and stabilization of α-pinene emulsions. Carbohydr Polym 105:177–183
Khopkar A r, Fradett L, Tanguy PA (2009) Emulsification capability of a dual shaft mixer. Chem Eng Res Des 87(12):1631–1639
Grossiord JL, Seiller M (1998) Applications. In: Grossiord JL, Seiller M (eds) Multiple emulsions: structure, properties and applications. Editions de Sante´, Paris, pp 279–437
Acknowledgments
The financial support received from the Spanish Ministerio de Economía y Competitividad (MINECO) and FEDER, UE is kindly acknowledged (project CTQ2015-70700-P).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
García, M.C., Muñoz, J., Alfaro-Rodriguez, MC. et al. Formulation variables influencing the properties and physical stability of green multiple emulsions stabilized with a copolymer. Colloid Polym Sci 297, 1095–1104 (2019). https://doi.org/10.1007/s00396-019-04529-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-019-04529-y