Abstract
Emulsifier-free polystyrene latexes with immobilized triazolic residues have been prepared by batch emulsion copolymerization of styrene with 1,2,3-triazole-based acrylic monomers containing diethylene or triethylene glycol monomethyl ether groups DEGTz and TEGTz, respectively. The effect of monomer structure and concentration on polymerization conversion, particle size, and morphology was investigated. Particle composition was also evidenced by 1H-NMR analysis. It was found that DEGTz monomer with the shorter pegylated chain allows the formation of the smaller particles with a better control of polytriazole water soluble oligomers formation. DEGTz and TEGTz were then used in batch and shot-growth polymerization of styrene-d8 in the aim to highlight the effect of deuterium isotope substitution. It was shown that the use of deuterated styrene significantly impacts the polymerization conversion as well as particle size and shape regardless of the monomer structure. Shot-growth process disadvantaged the copolymer particle formation in favor of obtaining water soluble polymers. This was confirmed from the NMR study and from the large values of surface charge density of latexes obtained from electrophoretic mobility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the use of polymer supports for immobilization of several ligands, catalysis and probes has generated great interest for organic synthesis, biomedical, and environmental applications [1, 2]. The activity of attached reagent is directly tied to its accessibility to the active sites and is often limited due the burying of these sites inside the cross-linked supports. In this context, the development of novel supported polymers with improved selectivity, efficiency, stability, reaction kinetic, thermal, and mechanical properties is highly requested for products that fulfill customers’ requirements.
Several methods have been used to access to such polymers with controlled functionality, size, and size distribution such as emulsion polymerization, suspension polymerization, precipitation polymerization, or atom transfer radical (ATRP) and reversible addition-fragmentation chain transfer (RAFT) polymerization techniques [3,4,5]. Micro- and submicrosized particles with a broad variety of morphologies can be obtained that exhibit high surface area to volume ratio helpful for interaction with actives sites. Among these methods, emulsion polymerization has gained its popularity as an easy production process because of its numerous outstanding advantageous as good temperature control, high polymerization rate, low viscosity of the final dispersions, and easy removal of unreacted monomers. Furthermore, the use of emulsifier–free process allows to prevent the contamination of the final products by emulsifier and consequently the latex properties deterioration due to the surfactant migration particularly for coating and adhesive applications [6, 7].
For metal remediation applications, various low-molecular ligands have been immobilized on polymer matrix. Among them, those containing nitrogen, oxygen, and phosphorus atoms received particular attention due to their strong affinity for divalent transition metal cations. The most common ligands include heterocyclic amines, amidoximes, dithizone, hydroxamic acid, crown ethers, phenolics, and phosphorus-based ligands [8, 9]. The Incorporation of these ligands can be ensured onto polymer particles through different strategies as physical adsorption of chelating polymers on polymer latex supports [10], functionalization of preformed latex particles bearing suitable reactive functions and spacers with ligand moieties [11,12,13] or copolymerization of polymer particles with functional monomers containing the chelating functions [14].
This last strategy, more suitable and versatile, that enables tailoring the particles’ surface composition by simple variation of experimental conditions, was indisputably less studied probably due to the difficulty of designing suitable functional monomer using simple and efficient synthetic pathways.
The first objective of the present study was to investigate the soap-free emulsion polymerization of styrene in the presence of two chelating monomers, 1-((diethylene glycol) methyl ether)-1H-1,2,3-triazol-4-yl methyl acrylate (DEGTz) and 1-((triethylene glycol) methyl ether)-1H-1,2,3-triazol-4-yl methyl acrylate (TEGTz) by batch process. These monomers were designed and synthesized because oligoethylene glycol moieties and 1,2,3-triazole heterocycle constitutes multiple complexation sites of metal ions. The colloidal properties and surface composition of latex particles were examined using different technics as 1H-NMR, DLS, and TEM analyses.
In a second time, batch and shot-growth polymerization reactions of these monomers were carried out by using perdeuterated styrene (styrene d8) as a comonomer in the aim to highlight the influence of deuterium substitution on the size, morphology and composition of final latex particles. Although recent studies show that the physical properties of polymers can be affected by deuteration [15,16,17], very few data on isotope effects in radical reactions have been reported.
Experimental procedure
Methods
1H and13C-NMR spectra were recorded on a Brucker 300-MHz spectrometer (Brucker, Wissembourg, France). Tetramethylsilane was used as an internal reference for chemical shifts. Column chromatography separations were carried out using E-Merck silica gel (Kieselgel 60,230–400 mesh) as the stationary phase. Thin-layer chromatographies were performed on aluminum plates precoated with Merck silica gel 60F254 and visualized by means of ultraviolet fluorescence quenching.
Materials
Diethylene glycol methyl ether, triethylene glycol methyl ether, 4-toluenesulfonyl chloride (99.5%), triethylamine (99%), sodium azide, propargyl alcohol, and potassium persulfate initiator KPS (+ 99%) were purchased from Sigma-Aldrich (Tlemcen-Algeria) and used without further purification. The solvents were of commercial grade quality and were dried and distilled before use.
Styrene-d8 (98 atom% D) stabilized with 4-t butylcatechol was purchased from Aldrich (Germany). Styrene 99%, stabilized with 10–15 ppm catechol was purchased from Alfa Aesar. They were purified by washing with an aqueous solution of NaOH 1 M to remove inhibitor then three times with deionized water and stored at − 20 °C. In all polymerization procedures, deionized water was used.
Synthesis
Synthesis of O-tosyl-(ethylene glycol) methyl ether derivatives (1a–b)
In a typical procedure, (0.083 mol) of diethylene glycol methyl ether or triethylene glycol methyl ether was added to a solution of (9.54 g, 2.8 eq) sodium hydroxide dissolved in 50 mL of a mixture of water/tetrahydrofuran 1/1. The mixture was placed in an ice bath, and (15.82 g, 0.083 mol) of 4-toluene sulfonyl chloride dissolved in 50 mL of tetrahydrofuran were added dropwise during 2 h. The stirring was maintained for 2 h at 0 °C then the reaction mixture was poured into cold water and extracted three times with dichloromethane. The organic layers were dried over magnesium sulfate. The products were obtained as yellow oil after solvent evaporation and used without further purification.
-
1a: Yield (90%), 1H NMR (300 MHz, CDCl3):δ (ppm) 2.39 (s, 3H, CH3), 3.29 (s, 3H, OCH3), 3.42 (t, 2H, CH3OCH2, 3J = 5.44 Hz), 3.51 (t, 2H, CH3OCH2CH2, 3J = 5.02 Hz), 3.63 (t, 2H, OCH2CH2OSO2, 3J = 4.88 Hz), 4.11 (t, 2H, CH2OSO2, 3J = 4.88 Hz), 7.30 (d, 2H, Ar-H, 3J = 8.34 Hz), 7.74 (d, 2H,Ar-H,3J = 8.34 Hz).13C NMR (75 MHz, CDCl3) δ (ppm) 21.43(CH3), 58.75 (OCH3), 68.46 (CH2SO2), 69.32 (OCH2CH2SO2), 70.37 (CH3OCH2CH2O), 71.60 (CH3OCH2), 127.79 (Ar), 129.81(Ar), 132.76 (Ar), 144.84 (Ar).
-
1b: Yield (94%), H1NMR (300 MHz, CDCl3)
 (ppm): 2.30 (s, 3H, CH3), 3.21 (s, 3H,OCH3), 3.38 (t, 2H, CH3OCH2, 3J = 5.32 Hz), 3.46 (m, 6H, CH3OCH2CH2OCH2CH2O), 3.54 (t, 2H,OCH2CH2OSO2, 3J = 4.84 Hz), 4.02 (t, 2H,CH2OSO2, 3J = 4.84 Hz), 7.22 (d, 2H, Ar-H, 3J = 8.32 Hz), 7.65 (d, 2H, Ar-H,3J = 8.32 Hz).13C NMR (75 MHz, CDCl3)
(ppm): 2.30 (s, 3H, CH3), 3.21 (s, 3H,OCH3), 3.38 (t, 2H, CH3OCH2, 3J = 5.32 Hz), 3.46 (m, 6H, CH3OCH2CH2OCH2CH2O), 3.54 (t, 2H,OCH2CH2OSO2, 3J = 4.84 Hz), 4.02 (t, 2H,CH2OSO2, 3J = 4.84 Hz), 7.22 (d, 2H, Ar-H, 3J = 8.32 Hz), 7.65 (d, 2H, Ar-H,3J = 8.32 Hz).13C NMR (75 MHz, CDCl3)  (ppm) 21.45 (CH3), 58.77 (OCH3), 68.46 (CH2SO2), 69.28 (OCH2CH2SO2), 70.31 (CH3OCH2CH2O), 70.49 (CH3OCH2CH2OCH2CH2O), 71.72 (CH3OCH2), 127.78 (Ar), 129.78 (Ar), 132.83 (Ar), 144.78 (Ar).
(ppm) 21.45 (CH3), 58.77 (OCH3), 68.46 (CH2SO2), 69.28 (OCH2CH2SO2), 70.31 (CH3OCH2CH2O), 70.49 (CH3OCH2CH2OCH2CH2O), 71.72 (CH3OCH2), 127.78 (Ar), 129.78 (Ar), 132.83 (Ar), 144.78 (Ar).
Synthesis ofazido-(ethylene glycol) methyl ether derivatives (2a–b)
O-tosyl-(ethylene glycol) methyl ether derivative (1a–b) (0.021 mol) was added to a solution of sodium azide (2.73 g, 0.042 mol) dissolved in 10 mL of dimethylformamide. The reaction was stirred at 70 °C for 48 h under nitrogen atmosphere. The solution was extracted three times with dichloromethane and the combined organic phases were washed with water then dried under anhydrous magnesium sulfate. Solvent evaporation affords an orange liquid.
-
2a: Yield (94%), H1 NMR (300 MHz, CDCl3) δ (ppm) 3.16 (s, 3H, OCH3), 3.20 (t, 2H, CH2N3,3J = 5.22 Hz), 3.36 (t, 2H, OCH2CH2N3,3J = 5.22 Hz), 3.55 (m, 4H, CH3OCH2CH2O).13C NMR (75 MHz, CDCl3) δ (ppm) 50.49 (CH2N3), 58.86 (OCH3), 69.88 (CH3OCH2CH2O), 70.42 (OCH2CH2N3), 71.81 (CH3OCH2).
-
2b: Yield (92%), H1 NMR (300 MHz, CDCl3) δ (ppm) 3.27 (s, 3H, OCH3), 3.29 (d, 2H, CH2N3,3J = 5.26 Hz), 3.45 (t, 2H, OCH2CH2N3,3J = 5.26 Hz),3.56 (m, 8H, CH3O (CH2CH2O)2). 13C NMR (75 MHz,CDCl3)
 (ppm) 50.55 (CH2N3), 58.84 (OCH3), 69.89 (OCH2CH2N3), 70.43 (CH3OCH2CH2), 70.49 (OCH2CH2O), 70.54 (OCH2CH2O), 71.79 (CH3OCH2).
(ppm) 50.55 (CH2N3), 58.84 (OCH3), 69.89 (OCH2CH2N3), 70.43 (CH3OCH2CH2), 70.49 (OCH2CH2O), 70.54 (OCH2CH2O), 71.79 (CH3OCH2).
Synthesis of 1,2,3-triazole derivatives (3a–b)
Azido-(ethylene glycol) methyl ether derivative (2a–b) (0.013 mol) was added to a solution containing (0.72 g, 0.013 mol) of propargylalcohol, (0.25 g, 0.0013 mol) of copper iodide and few drops of triethylamine (Et3N) in 20 mL of a mixture of water/ethanol (1/1). The reaction mixture was heated at 70 °C for 48 h. The solution is then extracted three times with dichloromethane and the organic phases are dried under magnesium sulfate. After solvent evaporation, the product is obtained as oil.
-
3a: Yield (95%), H1NMR (300 MHz, CDCl3) δ (ppm) 3.27 (s, 3H, OCH3), 3.44 (t, 2H, CH3OCH2, 3J = 5.50 Hz), 3.51 (t, 2H, CH3OCH2CH2O, 3J = 5.50 Hz), 3.78 (t, 2H, OCH2CH2N, 3J = 4.96 Hz), 4.46 (t, 2H, CH2N, 3J = 4.96 Hz), 4.66 (s, 2H, CH2OH), 7.72 (s, 1H, triazole).13C NMR (75 MHz,CDCl3) δ (ppm) 50.49 (CH2N), 55.74 (CH2OH), 58.85 (OCH3), 69.32 (OCH2CH2N), 70.30 (OCH2CH2O), 71.61 (CH3OCH2), 123.33 (CHtriazole), 142.1(Ctriazole).
-
3b: Yield (90%), H1NMR (300 MHz, CDCl3) δ (ppm) 3.33 (s, 3H, OCH3), 3.50 (t, 2H, CH3OCH2,3J = 5.34 Hz), 3.55 (m, 6H, CH3OCH2CH2OCH2CH2O), 3.82 (t, 2H, OCH2CH2N3, 3J = 4.89 Hz), 4.50 (t, 2H, OCH2CH2N3, 3J = 5.17 Hz), 4.72 (s, 2H, CH2OH), 7.78 (s, 1H, triazole). 13C NMR (75 MHz,CDCl3) δ (ppm) 50.62 (CH2N), 56.10 (CH2OH), 58.93 (OCH3), 69.89 (OCH2CH2N), 70.43 (CH2OCH2CH2N), 70.49 (CH3OCH2CH2O), 70.54 (CH3OCH2CH2OCH2), 71.82 (CH3OCH2), 123.27 (CHtriazole), 147.83 (Ctriazole).
General procedure for the synthesis of monomers DEGTz and TEGTz
In a three necked round-bottomed flask placed in an ice bath was dissolved (0.19 g, 2.2 mmol) acryloyl chloride and (0.61 g, 6 mmol) triethylamine in 10 mL of dry dichloromethane under nitrogen atmosphere. When the mixture becomes homogeneous, (0.4 g, 2 mmol) of triazole derivative (3a-b) dissolved in 10 mL of dry dichloromethane was added. The mixture was heated at 40 °C for 48 h. After cooling down to room temperature the organic phase was washed successively with HCl solution 1 M, then with water and finally dried over magnesium sulfate. The solvent was evaporated and the obtained residue was purified by silica gel column chromatography (eluent: CH2Cl2/AcOEt 4/1).
-
DEGTz: Yield (60%), H1NMR (300 MHz, CDCl3) δ (ppm) 3.30 (s, 3H, OCH3), 3.45 (t, 2H, CH3OCH2, 3J = 5.01 Hz), 3.54 (t, 2H, CH3OCH2CH2,3J = 5.01 Hz), 3.81 (t, 2H, OCH2CH2, 3J = 4.91 Hz), 4.50 (t, 2H, OCH2CH2, 3J = 4.91 Hz), 5.24 (s, 2 H, CH2O), 5.81 (dd, 1H, CH=CH2, 3Jcis = 10.41 Hz, 2J = 0.76 Hz), 6.08 (dd, 1H,CH=CH2, 3Jcis = 10.41 Hz, 3Jtrans = 18.07 Hz), 6.37 (dd, 1H,CH=CH2, 3Jtrans = 18.07 Hz, 2Jgem = 0.76 Hz), 7.79 (s,1Htriazole).13CNMR (75 MHz,CDCl3) δ (ppm) 50.10 (CH2N), 57.57(OCH3), 58.82(CH2OCO), 69.20 (CH2CH2N), 70.29 (CH3OCH2CH2), 71.55 (CH3OCH2), 124.98 (CHtriazole), 127.89 (COCH=CH2), 131.36 (COCH=CH2), 142.34 (Ctriazole), 165.74 (C=O).
-
TEGTz: Yield (62%), H1NMR (300 MHz, CDCl3) δ (ppm) 3.34 (s, 3H, OCH3), 3.52 (t, 2H, CH3OCH2, 3J = 4.97 Hz), 3.59 (m, 6H, CH3OCH2CH2OCH2CH2O),3.85 (t, 2H,CH2CH2N, 3J = 5.17 Hz), 4.52 (t, 2H, OCH2N, 3J = 5.17 Hz), 5.27 (s,2H, CH2OCO), 5.82 (dd, 1H, CH=CH2, 3Jcis = 10.41 Hz,2Jgem = 1.45 Hz), 6.10 (dd, 1H,CH=CH2, 3Jcis = 10.41 Hz, 3Jtrans = 17.31 Hz), 6.40 (dd, 1H, CH=CH2,3Jtrans = 17.31 Hz, 2Jgem = 1.45 Hz), 7.83 (s, 1Htriazole). 13CNMR (75 MHz,CDCl3) δ (ppm) 50.28 (CH2N), 57.61 (OCH3), 58.93 (CH2OCO), 69.29 (OCH2CH2N), 70.39 (CH2OCH2CH2N), 70.42 (CH3OCH2CH2), 70.48 (CH3OCH2CH2OCH2), 71.83 (CH3OCH2), 125.13 (CHtriazole), 128.00 (COCH=CH2), 131.42 (COCH=CH2), 142.4 (Ctriazole), 165.92 (C=O).
Preparation of latexes
Emulsion polymerizations were carried out in a glass reactor equipped with a glass paddle agitator and a condenser. The temperature was controlled at 70 °C and the stirring rotation was kept constant (300 rpm).
Batch polymerization
The recipes of the different prepared latexes are reported in Table 1. Deionized water was introduced in a reactor and the temperature was raised to 70 °C. Styrene (for SDZ1, SDZ2, SDZ3, STZ1, STZ2 latexes) or styrene-d8 (for SdDZ, SdTZ latexes) and monomer DEGTz or TEGTz, were then charged and the stirring rate was maintained until temperature stabilization. Polymerization was initiated by introducing KPS and was allowed to proceed for 21 h.
Polystyrene and perdeuterated polystyrene homopolymers were prepared according to the same procedure without addition of acrylic monomers DEGTz and TEGTz.
Polymerization conversions were determined gravimetrically and by NMR analysis as reported in the next section. For kinetic studies, sampling of the reaction mixture at various time intervals was performed to control the polymerization conversion and the particle sizes.
Shot-growth polymerization (latexes SSdDZ and SSdTZ)
In 80 mL, a glass reactor was introduced of water then the temperature was raised to 70 °C and 3 g of styrene-d8 were charged. After thermal stabilization, 0.1 g of KPS was added. The stirring was maintained during 21 h at a stirring rate of 300 rpm. Afterward, acrylic monomer DEGTz or TEGTz (Table 2) and 0.6 g of styrene-d8 were charged and the reaction mixture was stirred during 2 h. Finally, another quantity of KPS was added and the polymerization reaction was allowed to proceed for additional 21 h.
Characterization of polymer particles
The final polymer content (g/g of latex) was determined via gravimetric method from which the overall conversion was deduced. The various latexes obtained after solvent evaporation were analyzed by 1H NMR in CDCl3 from which the polymerization conversions and the polymer compositions were deduced.
Particle size and size distribution measurements of the prepared latex particles were carried out by dynamic light scattering technique (DLS) using Zetasizer (NANO ZS, Malvern instruments). A small drop of sample was dispersed in 1 ml of deionized water. The measurement was repeated for three times. Zeta potentials of particles were determined as function of pH values using the Malvern Zetasizer via electrophoretic mobility measurements. Different pH solutions were prepared using 1 mM NaCl solution and the pH values were adjusted by 1 mM NaOH or 1 mM HCl solution using a pH-meter. The samples were diluted in the corresponding solution before measuring the electrophoretic mobility.
The morphology and sizes were also examined by transmission electron microscope TEM (Phillips CM120, CMEABG, University of Claude Bernard Lyon 1). A highly diluted aqueous latex dispersion was deposited a carbon-coated copper grid then dried at room temperature overnight before TEM imaging. The dried samples were imaged under 120 kV acceleration voltages.
Results and discussion
Synthesis
1,2,3-triazole-based acrylic monomers containing diethylene or triethylene glycol monomethyl ether groups, DEGTz and TEGTz respectively were prepared through 4-step procedure as represented in Scheme 1.
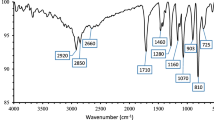
The synthetic strategy involves the reaction of diethylene glycol methyl ether or triethylene glycol methyl ether with p-toluenesulfonyl chloride followed by the conversion of the obtained tosyl compounds (1a–b) to azide derivatives (2a–b) in the presence of sodium azide in reflux of dimethylformamide. The addition of propargyl alcohol to compounds (2a–b) in the condition of copper-catalyzed 1,3-dipolar cycloaddition reaction affords the 1,2,3- triazole derivatives (3a–b). In these reaction conditions, only the 1,4-regioisomers were obtained as confirmed by 1H and 13C NMR analyses. Subsequent reaction with acryloyl chloride leads to the corresponding acrylic monomers DEGTz and TEGTz with good yield. The presence in 1H NMR spectra of the signals related to the acrylic protons at 5.81–6.38 ppm (=CH2) and 6.09 ppm (CH=), H5-triazole (7.79–7.83 ppm) and that of the terminal methoxy group (3.34–3.45 ppm) indicates that the monomers were successfully synthesized.
Styrene polymerization
Acrylic monomers DEGTz and TEGTz were compared for their efficiency in free-surfactant emulsion polymerization with styrene by using batch polymerization procedure.
Firstly, polymerization reactions have been carried out at 94/6 styrene/monomer weight ratio with KPS as initiator (latexes SDZ2 and STZ2 respectively). The analysis of the sera by 1H NMR, showed that almost total polymerization conversions have been reached for these systems instead of 86% for styrene homopolymerization (Table 3). Similar increase of polymerization conversion has been previously reported for emulsifier-free copolymerization of styrene with hydrophilic reactive monomers and was attributed to the influence of such monomers on the early step of nucleation where large number of precursor particles are formed which rapidly suffer a limited coagulation period providing mature particles [18,19,20].
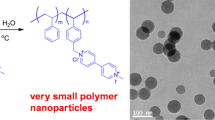
DLS analysis showed a significant decrease in mean particle diameters and polydispersity index for both investigated latexes in comparison with polystyrene latex (PS). This result suggests that these monomers act as efficient active-surface agents in emulsion polymerization process.
This feature was particularly noticeable for acrylic based triazole monomer DEGTz bearing diethylene glycol methyl ether chain where rather monodisperse particles (PDI = 0.069) with a mean diameter of 266 nm have been obtained whereas TEGTz monomer conducted to larger particles (393 nm) with slightly higher polydispersity index.
The higher efficiency of DEGTz monomer in reducing particle size in comparison to TEGTz analogous may be explained by the difference of the chemical structure of the two investigated surfactants. In TEGTz, the hydrophilic ethylene glycol chain is longer than in DEGTz. The longer ethylene glycol chain, the more it will be extending into water phase to the detriment of the hydrophobic part of the monomer that anchors into monomer droplets and latexes. Thus, DEGTz with the less hydrophilic chain will act as a better stabilizer for emulsion polymerization by means of adsorption phenomena and allows the formation of smaller particle sizes compared with the system with TEGTz. It should also generate higher number of precipitating oligo radicals during nucleation stage and prevents of particles from flocculation in the growth step. For TEGTz monomer, homopolymer formation will be more favored as confirmed by NMR analysis of polymerization recipes reported in the next section (see also Table 3).
Transmission electron microscopy was used to examine the morphology of SDZ2 and STZ2 particles (Fig. 1b, e). The investigated latex particles exhibit homogeneous spherical shape. Similar to DLS analysis, a slightly more polydisperse particles with polyelectrolytes formation were evidenced for STZ2 latex. It is however worth mentioning that particle size obtained from TEM analysis was lower than obtained from light scattering measurements reported in Table 3 probably due to the shrinking of the latex particles under the electron beam. Furthermore, since TEM analysis described the size in the dried state of the sample, whereas DLS measured the size in the hydrated state, the size measured by DLS is rather a hydrodynamic diameter and had a larger value.
Kinetic
Figure 2 presents the particle size evolution of the latex particles SDZ2 and STZ2 calculated by DLS as a function of polymerization time. The emulsion polymerization reactions of acrylic triazole monomers DEGTz and TEGTz with styrene have been carried out at in the same previously used conditions, i.e., styrene/monomer weight ratio (94/6), 6.6% solid content and 3 wt.% of KPS. Polystyrene homopolymer was prepared under the same conditions for comparison.
For all investigated systems, gradual increase of particle size during the first 240-min polymerization reaction was observed followed by a stabilization at 777, 265, and 392 nm for PS, SDZ2, and STZ2 latexes respectively.
Interestingly, SDZ2 and STZ2 latexes exhibit much lower particle sizes than polystyrene particles during the whole investigated time reaction. The particle size distribution remained narrow through the process for the both systems in the range of 0.014–0.222 and 0.097–0.184 for SDZ2 and STZ2, respectively with a limited amount of aggregated particles (coagulum).
Effect of monomer concentration
The polymerization of styrene was also conducted at various concentrations of triazolic monomers (3, 6, and 9 wt.% for DEGTz and 3 and 6 wt.% for TEGTz in comparison to styrene) with constant concentration of KPS. From Table 3, we can see that when the concentration of DEGTz or TEGTz monomer increases from 3 to 6 wt.%, there is a significant decrease in mean particle diameters (i.e., 577 to 266 nm and 643 to 393 nm for DEGTz and TEGTz respectively). Further increase on DEGTz concentration leads to larger particles (495 nm); which is assumed to be because of the enhanced particle coagulation. This behavior can be related to the increase of water-soluble oligomer contents and/or the reduction of the precipitation of oligo radicals during the nucleation stage.
Figure 1 shows TEM images of these investigated particles. By increasing triazolic monomer content, the particle morphology slightly deviated from spherical shape to irregular and more polydisperse shape with some occlusions at particle surfaces.
Such particle morphology has been already reported for emulsifier-free copolymerization of styrene with hydrophilic monomers as NIPAM [18] and acryloylpiperidine [21] and can be explained by a two-step mechanism as follows: at the beginning of polymerization process, triazolic monomer will rapidly reacts due to its favorable partitioning in aqueous phase to form polymer chains that precipitate rapidly onto unstable nuclei and coagulate to produce larger particles. When almost the major quantity of triazolic monomer polymerized, styrene starts to react by the diffusion of the monomer from the droplets and the subsequent swelling of the polytriazolic-rich particles. Due to the poor compatibility between the already formed polytriazolic polymers and styrene-rich chains, a phase separation occurs leading to the formation of occlusion at the surface of particles. As the polymerization proceeds, the polystyrene-rich phase will be predominant, expelling the most of polytriazole at the particle surface as small asperities.
Zeta potential
Zeta potential is considered as an important parameter that reflects the charge of particle surfaces and the intensity of repulsive forces among particles affect the stability of dispersion. In this study, zeta potential was measured for polystyrene latexes at different pH ranging from 3 to 11. The obtained zeta potential data are plotted in Fig. 3. One can see that zeta potential values of all investigated particles are negative ranging from − 23 to − 73 mv over the whole range of measured pH probably due the presence of sulfate functions of potassium persulfate initiator (KPS) on polystyrene particle surface. Any isoelectric point was observed for these latexes.
The evolution of zeta potentials exhibits the same Gaussian profile as a function of pH with a maximum ζ value for pH in between 8 and 9. All ζ values are found to increase with increasing DEGTZ triazolic monomer concentration (latexes SDZ1, SDZ2, and SDZ3) whereas no significant changes were observed for latex prepared with TEGTz (STZ1 and STZ2). It seems however that for similar initial monomer content, the latter latexes present slightly higher zeta potential values and particularly for pH > 6 which can be related to the presence of neutral adsorbed polytriazole polymers.
NMR study
1H NMR was used to examine the composition of polymer particles. By using D2O as solvent, only the signals related to triazole-based monomers are observed since D2O is non-solvent of the hydrophobic polystyrene core. In CDCl3, both shell and core chains were noticed that allows the determination of the composition of the polymers. Comparative NMR spectra of DEGTz monomer and corresponding homopolymer and polystyrene copolymer latex SDZ1 are given as an example in Fig. 4. The presence of resonance peaks belonging to triazolic monomer and styrene moieties is clearly evident in copolymer spectra. Moreover, we noticed the coexistence of two signals at 7.41 and 7.39 ppm that was assigned to H5-triazolic signal of polytriazole homopolymer and copolymer respectively. This was confirmed by two ways: (a) by comparing with NMR spectra of polytriazole homopolymer prepared in the same condition and (b) NMR spectra of this latex carried out in D2O instead of CDCl3 shows only one H5-triazolic signal of the water soluble pegylated triazole homopolymer (Fig. 4c).
The integral area of these two protons was used to calculate the mole ratio of copolymer/homopolymer in investigated latexes. The content of acrylic DEGTz and TEGTz moiety in copolymer particles was also estimated from 1H NMR spectra of latexes by comparing the signal of styrene moiety (7.11 or 6.60 ppm) with that of H5-triazole of copolymer. The obtained results are collected in Table 3.
It was found that the initial feed composition used to prepare polystyrene particles has significant effect on the composition of the final copolymer particles. The increasing of the initial concentration of triazolic monomer leads to higher incorporation of triazole monomer in latex particles. However, it is noticed in the same time that the formation of polytriazole water soluble oligomers was more favored particularly for monomer bearing the longer pegylated chain (TEGTz). This result is in good agreement with TEM and DLS results discussed previously.
All these results give more insight concerning the polymerization mechanism of triazole monomer under these heterogeneous conditions. In conventional emulsion polymer systems using water-insoluble monomer such as styrene, the primary reaction locus is inside the polymer particles, and aqueous-phase polymerization is usually considered to be negligible. Since pegylated triazolic acrylic monomers are “partially” soluble in water, they will probably be partitioned between both the organic and aqueous phases. We can expect that the primary reaction locus in the triazole-styrene system shifts from the particles to the aqueous phase after the hydrophobic styrene monomer has been consumed. Thereby, oligomeric radicals can be formed in the aqueous phase and will play an important role in particle nucleation and stabilization. We can expect that for DEGTz and TEGTz monomers, the water-soluble oligomers will grow in both the aqueous phase and the organic phase and will impact substantially the composition of final polymers. In aqueous phase, more triazolic sequence units in the oligomer chains will be formed owing to the higher triazolic acrylic monomer concentration there. This feature will be more pronounced for monomer TEGTz containing longer hydrophilic chain. Similar behavior was reported by Shoaf and Poehlein [22, 23] for seeded emulsion copolymerization of styrene with hydrophilic acrylic acids systems.
Perdeuterated styrene polymerization
The polymerization of DEGTz or TEGTz monomers with perdeuterated styrene was carried out under free-surfactant polymerization condition at 70 °C and 6% solid content by using KPS as initiator.
Two series of experiments have been considered. First, batch copolymerization of styrene-d8 with triazolic monomers DEGTz and TEGTz was conducted; the related latexes are named SdDZ and SdTZ, respectively (Table 4). The second series of experiments consists on shot-growth polymerization. In this case, the corresponding latexes are noted SSdDZ and SSdTZ respectively.
One can see in Table 4 that in batch condition, polymerization of perdeuterated styrene proceeds with good yield to afford monodisperse latex particles PSd in the range size of 662 nm. Copolymerization recipes SdDZ and SdTZ showed a strong decrease of the particle sizes confirming that the triazolic monomers act as efficient surface-active agents in heterogeneous polymerization by providing stability to the particles. The particle sizes achieved by DEGTz and TEGTz (370 and 660 nm for SdDZ and SdTZ latexes respectively) were however larger and slightly more polydisperse than those obtained for previously discussed polystyrene-based latexes SDZ2 and STZ2 (266 and 393 nm respectively).
The morphology and particle size distribution of these systems was also investigated by TEM analysis. Monodisperse spherical particles with a smooth surface have been obtained for perdeuterated polystyrene particles PSd (Fig. 5a). By copolymerization with triazolic monomers, the particle morphology slightly deviated from spherical shape to irregular raspberry-like shape (Fig. 5b–d). The deviation is more important for SdTZ particles where many bulges are present on the particle surfaces. This behavior is supported by the higher polydispersity index value of 0.228 calculated by DLS analysis for these particles. The particle size determined by TEM analysis was, as observed previously, smaller than that of those calculated by DLS in the range of 327 and 544 nm for SDZ2 and STZ2, respectively.
The findings of this study proved that the polymerization process and particles morphology are significantly affected by styrene deuterium substitution. Previous studies have reported the influence of the isotopic effect of deuterium substitution on the rate of free-radical polymerization for some deuterated monomers. The rate of propagation Rp was found to be dependent on the position of the deuterium and was in most cases higher for the deuterated monomer than for the H-monomer. In the case of perdeuterated styrene, Clouet and Chaffanjon [24] have shown that the deuterium substitution affects not only the propagation step of the polymerization of styrene-d8 but also the termination step. The activation energy for termination step of styrene-d8 was found to be 1.5 times higher than for styrene and an increase in termination reaction by combination was obtained for the former monomer was reported. This feature can explain in our case, the lower conversion yields and the more heterogeneous particle surface observed for polymers prepared from perdeuterated styrene in comparison with styrene based analogous (Table 3). 1H NMR study carried out for perdeuterated polystyrene recipes (Fig. 6a,b) showed lower monomer conversion for perdeuterated polystyrene particles in comparison with polystyrene analogous and higher amounts of water-soluble polymers. We can expect a higher demixing of the small polystyrene-rich domains during polymerization leading to the formation of the occlusions at particle surfaces as observed by TEM analysis.
In a second time, polymerization experiments were performed under shot-growth conditions in the aim to have a better control of the amount of triazole-based monomer copolymerized at the surface of polystyrene particles. The basis of the shot-growth technique is to polymerize the main monomer, i.e., styrene-d8, by the usual surfactant-free emulsion polymerization process until the seed latex reaches a fairly high conversion (> 60%) then the second monomer (DEGTz or TEGTz) is added and the reaction is allowed to proceed to completion.
Results as regards to particle size and distribution are reported in Table 4 (latexes SSdDZ and SSdTZ). Moderate conversion yields were obtained for both investigated acrylic monomers. Dynamic light scattering analysis showed particles with large sizes ranging from 568 and 834 nm with broad size distribution. Nevertheless, TEM micrographs of these latexes reveal more homogeneous spherical particles with smaller particles size in the range of 314 and 538 nm for SSdDZ and SSdTZ respectively but with a high tendency for aggregation. It is worth mentioning that these values are slightly lower than those obtained by batch polymerization.
By comparing NMR spectra of latexes prepared by batch and shot-growth conditions for both investigated triazolic monomer (Fig. 6), we noticed that this latter mode of polymerization promotes the formation water-soluble polymers at the detriment of the formation of copolymers (Table 4). The smoother particle surfaces observed by TEM analysis for these latexes can be the consequence of (a) the formation of less polystyrene-rich domains during polymerization process and (b) a better capture of the core particles for polymer from the aqueous phase due to the monomer shell actively polymerizing at the core particle surface. This shell is becoming entwined in the polymer matrix and unable to transfer back out of the particle. The core particles will contain also some residual monomer that will act as plasticizer. The less rigid, plasticized core particles will probably favor more regular spherical shape [25].
In this study, zeta potential was also measured for all samples at different pH, i.e., pH 3, 5, 8, 9, and 11. The resulting values are plotted according to pH in Fig. 7.
One can see that polymers prepared by shot-growth polymerization technic exhibit higher ζ values particularly in acidic media in comparison to polystyrene particles and corresponding batch polymers. This can be related to the high concentration of water-soluble polytriazolic species as showed previously in NMR study. One can expect that the triazole ring can undergo protonation reaction at acid pH values leading to positively charged triazolium groups and thus an increase of zeta potential values.
TEGTz-based latex (SdTZ or SSdTZ) exhibit higher zeta potential values in comparison with DEGTz-based particles (SdDZ or SSdDZ). This behavior was more pronounced in the case of polymers prepared in shot-growth conditions.
Conclusion
Two pegylated triazolic monomers were synthesized and used in emulsifier-free copolymerization of styrene by batch process for producing latex particles with covalently attached triazolic residues. Both investigated monomers strongly affected emulsion copolymerization rate and final particle size and surface charge density. DEGTz monomer bearing the shorter hydrophilic chain allowed the formation monodisperse particles with the smaller particle size. Particle size and surface charge density of the prepared particles were found to be dependent on monomer concentration. Owing to partially hydrophilic character of the monomers, the concomitant formation of polytriazolic homopolymer was evidenced by 1H NMR study particularly for TEGTz monomer. In addition to ionic groups originated from initiator, these polymers will probably participate on latex stability via electrostatic stabilization.
These monomers have been then successfully engaged on emulsion polymerization with styrene-d8 by batch or shot-growth processes. The use of deuterated styrene affects substantially the polymerization process and particle morphology and size.
Dealing with short-growth process conducts to higher formation of homopolytriazole particles that was evidenced by NMR analysis and through the electrophoretic mobility behavior of the triazolic functionalized latexes.
Due to the well-known chelating properties of 1,2,3-triazole heterocycle [9, 26], such triazole-styrene latex particles are potential candidate as a solid support for metal ion remediation in wastewater effluents.
References
Altava B, Burguete MI, Garcıa-Verdugo E, Luis SV (2018) Chiral catalysts immobilized on achiral polymers: effect of the polymer support on the performance of the catalyst. Chem Soc Rev 47:2722–2771. https://doi.org/10.1039/C7CS00734E
Aydın EB, Aydın M, Sezgintürk MK (2018) Highly sensitive electrochemical immunosensor based on polythiophene polymer with densely populated carboxyl groups as immobilization matrix for detection of interleukin 1β in human serum and saliva. Sensors Actuators B Chem 270:18–27. https://doi.org/10.1016/j.snb.2018.05.014
Cho YS, Shin CH, Han S (2016) Dispersion polymerization of polystyrene particles using alcohol as reaction medium. Nanoscale Res Lett 11:46. https://doi.org/10.1186/s11671-016-1261-8
Elaissari A (2003) Colloidal polymers: synthesis and characterization. CRC press
Bell CA, Smith SV, Whittaker MR, Whittaker AK, GahanL R, MonteiroM J (2006) Surface-functionalized polymer nanoparticles for selective sequestering of heavy metals. Adv Mater 18:582–586. https://doi.org/10.1002/adma.200501712
Huang C, Kobayashi H, Moritaka M, Okubo M (2017) Hollow particles are produced by the burying of sulfate end-groups inside particles prepared by emulsion polymerization of styrene with potassium persulfate as initiator in the absence/presence of nonionic emulsifier. Polym Chem 8:6972–6980. https://doi.org/10.1039/C7PY01608E
Okubo M, Kobayashi H, Huang C, Miyanaga E, Suzuki T (2017) Water absorption behavior of polystyrene particles prepared by emulsion polymerization with nonionic emulsifiers and innovative easy synthesis of hollow particles. Langmuir 33(14):3468–3475. https://doi.org/10.1021/acs.langmuir.7b00232
Trochimczuk AW, Kolarz BN (2000) Synthesis and chelating properties of resins with methylthiourea, guanylthiourea and dithiocarbamate groups. J Eur Polym 36:2359–2363. https://doi.org/10.1016/S0014-3057(00)00007-0
Lahmar H, Saidi-Besbes S, Elaissari A, Derdour A (2015) Poly(1,2,3-triazole) latex particles: synthesis and chelating properties. J Colloid Sci Biotech 4:1–7. https://doi.org/10.1166/jcsb.2015.1111
Denizli A, Kesenci K, Arica Y, Piskin E (2000) Dithiocarbamate-incorporated monosize polystyrene microspheres for selective removal of mercury ions. React Funct Polym 44:235–243. https://doi.org/10.1016/S1381-5148(99)00099-1
Dinu MV, Dragan ES (2008) Heavy metals adsorption on some iminodiacetate chelating resins as a function of the adsorption parameters. React Funct Polym 68:1346–1354. https://doi.org/10.1016/j.reactfunctpolym.2008.06.011
Trochimczuk AW (1998) Chelating resins with n-substituted diamides of malonic acid as ligands. J Eur Polym 11:1657–1662. https://doi.org/10.1016/S0014-3057(98)00018-4
Pan B, Pan B, Zhang W, Lv L, Zhang Q, Zheng S (2009) Development of polymeric and polymer-based hybrid adsorbents for pollutants removal from waters. J Chem Eng 151:19–29. https://doi.org/10.1016/j.cej.2009.02.036
Uzun L, Kara A, Tüzmen N, Karabakan A, Beşirli N, Denizli A (2006) Synthesis and characterization of poly(ethylene glycol dimethacrylate–1-vinyl-1,2,4-triazole) copolymer beads for heavy-metal removal. J Appl Polym Sci 102:4276–4283. https://doi.org/10.1002/app.24830
Jakowski J, Huang J, Garashchuk S, LuoY HK, Keum J, Sumpter BG (2017) Deuteration as a means to tune crystallinity of conducting polymers. J Phys Chem Lett 8:4333–4340. https://doi.org/10.1021/acs.jpclett.7b01803
White RP, Lipson JEG, Higgins JS (2010) Effect of deuterium substitution on the physical properties of polymer melts and blends. Macromolecules 43:4287–4293. https://doi.org/10.1021/ma902707u
Shao M, Keum J, Chen J, He Y, Chen W, Browning JF, Jakowski J, Sumpter BG, Ivanov IN, Ma YZ, Rouleau CM, Smith SC, Geohegan DB, Hong K, Xiao K (2014) The isotopic effects of deuteration on optoelectronic properties of conducting polymers. Nat Commun 5:3180. https://doi.org/10.1038/ncomms4180
Duracher D, Sauzedde F, Elaissari A, Perrin A, Pichot C (1998) Cationic amino-containing N-isopropylacrylamide styrene copolymer latex particles: 1-particle size and morphology vs. polymerization process. Colloid Polym Sci 276:219–231. https://doi.org/10.1007/s003960050232
Ni H, Du Y, Ma G, Nagai M, Om S (2001) Mechanism of soap-free emulsion polymerization of styrene and 4-vinylpyridine: characteristics of reaction in the monomer phase, aqueous phase, and their Interface. Macro 34(19):6577–6585. https://doi.org/10.1021/ma010829d
Ho KM, Li WY, Wong CH, Li P (2010) Amphiphilic polymeric particles with core-shell nanostructures: emulsion-based syntheses and potential applications. Colloid Polym Sci 288(16):1503–1523. https://doi.org/10.1007/s00396-010-2276-9
Kawaguchi H, Hoshino F, Ohtsuka K (1986). Makromol Chem Rapid Commun 7:109–114. https://doi.org/10.1002/marc.1986.030070302
Shoaf GL, Poehlein GW (1991) Kinetics of emulsion copolymerization with acrylic acids. J Appl Polym Sci 42:1213–1237. https://doi.org/10.1002/app.1991.0704205067
Shoaf GL, Poehlein GW (1991) Solution and emulsion polymerization with partially neutralized methacrylic acid. J Appl Polym Sci 42:1239–1257
Clouet G, Chaffanjon P (1990) Rate constants in the free-radical polymerization of perdeuterated styrene. J Macromol Sci Chem 27:193–212. https://doi.org/10.1080/00222339009351496
Chainey M, Hearn J, Wilkinson MC (1981) Preparation of overcoated polymer latices by a ‘shot growth’ technique. J Br Polym 13:132–136. https://doi.org/10.1002/pi.4980130310
Mokadem Z, Mekki S, Saidi-Besbes S, Agusti G, Elaissari A, Derdour A (2017) Triazole containing magnetic core-silica shell nanoparticles for Pb2+, Cu2+ and Zn2+ removal. J Arabian Chem 10:1039–1051. https://doi.org/10.1016/j.arabjc.2016.12.008
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lahmar, H., Badr, I., Kaewsaneha, C. et al. 1,2,3-triazole functionalized polystyrene and perdeuterated polystyrene chelating latexes. Colloid Polym Sci 297, 1119–1131 (2019). https://doi.org/10.1007/s00396-019-04509-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-019-04509-2


 (ppm): 2.30 (s, 3H, CH3), 3.21 (s, 3H,OCH3), 3.38 (t, 2H, CH3OCH2, 3J = 5.32 Hz), 3.46 (m, 6H, CH3OCH2CH2OCH2CH2O), 3.54 (t, 2H,OCH2CH2OSO2, 3J = 4.84 Hz), 4.02 (t, 2H,CH2OSO2, 3J = 4.84 Hz), 7.22 (d, 2H, Ar-H, 3J = 8.32 Hz), 7.65 (d, 2H, Ar-H,3J = 8.32 Hz).13C NMR (75 MHz, CDCl3)
(ppm): 2.30 (s, 3H, CH3), 3.21 (s, 3H,OCH3), 3.38 (t, 2H, CH3OCH2, 3J = 5.32 Hz), 3.46 (m, 6H, CH3OCH2CH2OCH2CH2O), 3.54 (t, 2H,OCH2CH2OSO2, 3J = 4.84 Hz), 4.02 (t, 2H,CH2OSO2, 3J = 4.84 Hz), 7.22 (d, 2H, Ar-H, 3J = 8.32 Hz), 7.65 (d, 2H, Ar-H,3J = 8.32 Hz).13C NMR (75 MHz, CDCl3)  (ppm) 21.45 (CH3), 58.77 (OCH3), 68.46 (CH2SO2), 69.28 (OCH2CH2SO2), 70.31 (CH3OCH2CH2O), 70.49 (CH3OCH2CH2OCH2CH2O), 71.72 (CH3OCH2), 127.78 (Ar), 129.78 (Ar), 132.83 (Ar), 144.78 (Ar).
(ppm) 21.45 (CH3), 58.77 (OCH3), 68.46 (CH2SO2), 69.28 (OCH2CH2SO2), 70.31 (CH3OCH2CH2O), 70.49 (CH3OCH2CH2OCH2CH2O), 71.72 (CH3OCH2), 127.78 (Ar), 129.78 (Ar), 132.83 (Ar), 144.78 (Ar).






